Abstract
The reduction pathway of nitrate (NO3−) and nitrite (NO2−) to nitric oxide (NO) contributes to regulating many physiological processes. To examine the rate and extent of dietary nitrate absorption and its reduction to nitrite, we supplemented rat diets with Na15NO3-containing water (1 g/L) and collected plasma, urine and several tissue samples. We found that plasma and urine showed 8.8- and 11.7-fold increases respectively in total nitrate concentrations in 1-day supplementation group compared to control. In tissue samples—gluteus, liver and eyes—we found 1.7-, 2.4- and 4.2-fold increases respectively in 1-day supplementation group. These increases remained similar in 3-day supplementation group. LC–MS/MS analysis showed that the augmented nitrate concentrations were primarily from the exogenously provided 15N-nitrate. Overall nitrite concentrations and percent of 15N-nitrite were also greatly increased in all samples after nitrate supplementation; eye homogenates showed larger increases compared to gluteus and liver. Moreover, genes related to nitrate transport and reduction (Sialin, CLC and XOR) were upregulated after nitrate supplementation for 3 days in muscle (Sialin 2.3-, CLC1 1.3-, CLC3 2.1-, XOR 2.4-fold) and eye (XOR 1.7-fold) homogenates. These results demonstrate that dietary nitrate is quickly absorbed into circulation and tissues, and it can be reduced to nitrite in tissues (and likely to NO) suggesting that nitrate-enriched diets can be an efficient intervention to enhance nitrite and NO bioavailability.
Similar content being viewed by others
Introduction
The roles of nitric oxide (NO) in various physiological processes have been studied since the 1980s1,2 and the three classes of nitric oxide synthase (NOS) enzymes were considered the main source of NO generation until nitrite (NO2−) was identified as a precursor of NO following nitrate (NO3−) ingestion in the mid-90s3,4. This finding began to change the older view that both nitrite and nitrate ions were largely inert end products of NO oxidation and opened a new field of research on the nitrate-nitrite-NO reduction pathway as an alternative to the classic NOS pathways. More recently it has been shown that nitrate absorbed from dietary sources such as beetroot and green leafy vegetables can be reduced to nitrite by commensal bacteria in the oral cavity5 and to some extent by mammalian enzymes (i.e., xanthine oxidoreductase, XOR)6,7. Thereafter, nitrite can be further reduced to NO by several mechanisms utilizing deoxyhemoglobin8, deoxymyoglobin9,10, several molybdenum containing enzymes11 and non-enzymatic mechanism12.
Numerous pre-clinical and clinical studies have shown that dietary nitrate supplementation can have beneficial effects in maintaining cardiovascular health and improving exercise performance, presumably by enhancing NO bioavailability13,14. Previous reports from rodent studies showed that providing animals with nitrite or nitrate in their diets significantly changed the endogenous concentrations of these ions in blood and organs such as heart, liver and skeletal muscle and modulated platelet function15,16,17,18. We also demonstrated in healthy people that increased nitrate ingestion via beetroot juice significantly elevated nitrate and nitrite concentrations in blood and skeletal muscle19. The transport of nitrate is known to be mediated in certain tissues by a specific transporter, sialin20 and also by chloride channels (CLC)21,22. Thus, the increase of nitrate and nitrite ions in internal organs upon nitrate ingestion may be affected by these anion transporters as well as various reductive pathways. However, dynamics of NO metabolism in mammals can be affected by the endogenous NOS pathways as well as the nitrate-nitrite-NO reduction pathways since oxidation of NOS-derived NO (and nitrite) can contribute to the systemic changes of nitrite and nitrate concentrations23.
We and others have examined the influences of diet and NOS on alterations of nitrate and nitrite concentrations in blood and tissues of animals by using low nitrate/nitrite diets, NOS inhibitors or a NOS-deficient mouse line24,25,26,27. Although these studies clearly demonstrated that both NOS and the nitrate-nitrite-NO reduction pathways modulate the concentrations of nitrate and nitrite ions in the circulation and internal organs, the information on the extent of contribution of each pathway to NO generation has not been precisely examined. To distinguish NO molecules that are produced after nitrite ingestion from those produced endogenously, Hughan et al. used 15N isotope and reported that the oral administration of Na15NO2 increased Hb15NO in healthy people28. In other 15N tracer studies, 15N-labeled nitrate, nitrite or arginine was administered and the levels of 15N species in plasma or urine samples were followed29,30 or whole zebrafish was used to determine the enrichment of 15N-labeled nitrate and nitrite31. However, the detailed information on the concentrations of 15NO3− or 15NO2− in various organs after 15N nitrate ingestion has not been available until now.
In the present study, we explored the dynamic changes of nitrate and nitrite concentrations in plasma, skeletal muscle, liver and eye of rats supplemented with Na15NO3-enriched water at baseline and at 1 or 3 days. Our rationale for the choice of these particular organs is that skeletal muscle (gluteus), is a nitrate storage organ32 and one of the beneficial effects of dietary nitrate is to enhance exercise performance33, while liver has relatively lower concentrations of nitrate but is abundant in Mo-containing enzymes such as XOR that are directly involved in nitrate reduction into nitrite and NO. In more recent report, we also showed that porcine ocular tissues contain considerable amount of nitrate and nitrite ions and exhibit nitrate reduction activities34, so eye was of our interest when examining nitrate metabolism. To evaluate the contribution of dietary nitrate on the concentrations of endogenous nitrate and nitrite, we measured the percent of 15NO3− and 15NO2− by LC–MS/MS in various tissue samples and also analyzed the absolute concentrations of nitrate and nitrite ions using a chemiluminescence method.
Materials and methods
Animals and nitrate supplementation
All animal procedures were approved by NIDDK Animal Care and Use Committee (protocol number K049-MMB-20) and carried out according to recommendations in the Guide for the Care and Use of Laboratory Animals of NIH. This animal study is reported in accordance with ARRIVE guidelines. The Wistar rats (250 ± 50 g, both male and female) were supplied by Envigo (Indianapolis, IN) and housed in a 12 h light/dark cycle environment with access to food (standard rat diet NIH07) and tap water ad libitum for 7 days before subjected to experiments. Rats were randomly divided into 3 groups and the investigators could not be blinded to the rat groups due to supplement preparation steps. Sample size of rat groups were determined based on previous reports35,36. The control group received tap water and the treatment groups received tap water containing Na15NO3 (1 g/L, ≥ 98 atom% 15N, Sigma, St. Louis, MO) for either 1 or 3 days. We analyzed the nitrite content in the Na15NO3 bottle using a chemiluminescence method and it was less than 0.01%.
Sample collections
Rats were enclosed in an anesthesia box and deeply anesthetized using 5% isoflurane mixture with air. Anesthetized animals were placed on the pad in supine position and anesthesia continued through a nose cone. The thoracic cavity was opened and ~ 9–10 ml of blood was collected by cardiac puncture, representing about two-thirds of total blood volume for a rat of this size. Heparinized syringes were used for blood collection. Immediately after withdrawal, blood was centrifuged (17,000 g, 5 min) to obtain plasma. Animals were perfused using heparin-containing saline (Heparin Sodium, 2 USP units/ml, Hospira, Lake Forest, IL) to flush the remaining blood out of tissues. Perfusion continued until no blood was detected in outgoing saline and liver was significantly discolored. Samples from liver, gluteus muscles and eyes were then collected, placed into microcentrifuge tubes and immediately frozen on dry ice. All samples were stored at − 80 °C until analysis.
Determination of total nitrite and nitrate concentrations
Nitrite and nitrate levels in all samples were measured using a standard chemiluminescence method as described previously37. Plasma and urine samples were mixed with cold methanol 1:2 (sample:methanol, vol/vol) for deproteinization, then centrifuged for 30 min (17,000 g, 4 °C, AccuSpinMicroR, Fisher Scientific, Pittsburgh, PA). The supernatant was collected and injected into the nitric oxide analyzer (NOA, Sievers, Model 280i NO analyzer, Boulder, CO) using helium as the carrier gas. Vanadium chloride or tri-iodide solution was used for nitrate or nitrite analysis respectively. All other tissue samples were weighed, mixed with water (dilution 1:5 sample:water, vol/vol) and homogenized using GentleMacs (Miltenyi Biotec Inc, Auburn, CA). Samples were centrifuged at 17,000 g for 30 min and the supernatant was collected. At this point, part of the supernatant was processed for LC–MS/MS analysis (see below) and the rest was used for NOA. NOA samples were deproteinized by dilution with cold methanol 1:2 (sample:methanol, vol/vol), then centrifuged for 30 min (17,000 g, 4 °C) to obtain clear supernatant.
Preparation of samples for LC–MS/MS
To measure nitrate content by LC–MS/MS, nitrate ions in all samples were first reduced to nitrite enzymatically by bacterial nitrate reductase from Aspergillus niger (N7265, Sigma-Aldrich, St. Louis, MO, USA) as previously described38,39 with some modification. Briefly, sample (20 µl) was mixed with nitrate reductase (0.1U/ml) and NADPH (100 µM) and incubated for 2 h at room temperature. Then nitrite ions in samples were derivatized with 2,3-diaminonaphthalene (DAN, D2757, Sigma-Aldrich) for 30 min at 37 °C to yield 2,3-naphthotriazole (NAT). NaOH (58 mM) was added to terminate the reaction. For measuring nitrite content only, samples (50 µl) were directly subjected to DAN derivatization.
Determination of 15NO3 − or 15NO2 − percent by LC–MS/MS
High-performance liquid chromatography (HPLC) grade solvents and LC–MS modifiers were purchased from Sigma-Aldrich (St. Louis, MO, USA). Detection and quantification were achieved by ultra-performance liquid chromatography—tandem mass spectrometry (UPLC-MS/MS) utilizing a Thermo Scientific Vanquish UPLC with a Thermo Scientific Altis triple quadrupole mass spectrometer, heated electrospray ionization (HESI-II) in positive ion mode (3500 V). 50 µl of sample was mixed with 200 µl of acetonitrile (ACN), vortexed for 5 min and then centrifuged at 4 °C, 17,000 g for 15 min. The supernatant was transferred to an LC–MS vial for analysis. Injection volume was 1 µl. A Waters Cortecs T3 column, 2.1 × 100 mm, 1.6 µm column was maintained at 35 °C. Solvent A: H2O with 0.1% formic acid (FA) and Solvent B: ACN with 0.1% FA. The flow rate was 250 µl/min, the gradient was 25% B at 0 min for 0.25 min, increasing to 65% B at 5 min, further increasing to 90% B at 5.5 min, remained 90% B until 7.5 min, then decreased to 25% B at 8 min. The total running time was 10 min. Samples were analyzed in triplicates. Quantitation of 14NAT and 15NAT were based on multiple reaction monitoring (MRM) transitions m/z, 170.062 → 115.042 and 171.062 → 115.042, respectively. The result was based on the percentage ratio of 15NAT/(14NAT + 15NAT).
Analysis of gene expression by quantitative polymerase chain reaction (qPCR)
Total RNA from rat tissues (gluteus muscle, liver and eye, 30-40 mg each) was purified by using RNeasy fibrous tissue mini kit (Qiagen, Germany), then 1 µg of total RNA was used to synthesize cDNA by High-Capacity cDNA reverse transcription kit (Applied Biosystems, MA). For qPCR measurements, 2 µl of the synthesized cDNA, which was pre-diluted with water (1:20 ratio), was mixed with PowerUp™ SYBR™ Green master mix (Applied Biosystems, MA, USA) and primers (0.2 µM). qPCR was carried out on QuantStudio™ 7 Flex real-time PCR system (Applied Biosystems, MA). The glyceraldehyde-3-phosphate dehydrogenase (gapdh) gene was used as a reference gene to calculate relative quantification by the 2−ΔΔCt method.
The primer sequences used for qPCR were as follows: sialin forward (ACACTCTGCCCCCGTAAAAG) and reverse (TCTGTGTGACGATGTAGCCG), clc1 forward (ACAATGCCCACCCAACACA) and reverse (GTCCTCATCCAAGCTGTCCA), clc2 forward (AGATTGTCCAGGTGATGCGG) and reverse (TGAACTGTCCAAAGCCAGGG), clc3 forward (CGTGTCCCGGTGAAGCTC) and reverse (CAGCTGCTCAGACTCCATGT), xor forward (TGACTGCGGATGAGTTGGTC) and reverse (AAGCTTGGTCCCACATAGCC), gapdh forward (CCGCATCTTCTTGTGCAGTG) and reverse (ATGAAGGGGTCGTTGATGGC).
Statistical analysis
Values represent average ± standard deviation. Statistical significance of results was tested using the one-way ANOVA. * denotes p < 0.05.
Results
To assess the influence of dietary nitrate delivered via drinking water on the concentrations of nitrate and nitrite in various tissues of rats, we provided animals with 15N-labeled nitrate water (Na15NO3, 1 g/L) for 0, 1 or 3 days. The total amount of nitrate and nitrite in plasma, urine and tissue samples (gluteus muscle, liver and whole eye) was measured by a standard chemiluminescence method40,41. Nitrate concentrations increased significantly in all samples of 1-day supplementation group compared to the baseline of control group (no treatment) and remained elevated in 3-day supplementation group (Fig. 1A). The fold change of nitrate concentrations in plasma and urine was 8.8- and 11.7-fold increase respectively compared to control and other tissue samples, gluteus muscle, liver and eye homogenates, also exhibited a significantly raised nitrate concentrations in 1-day supplementation group compared to control (1.7-, 2.4- and 4.2-fold increase respectively, Fig. 1B). These changes remained similar in all these sample tissues in 3-day supplementation group.
Total nitrate concentrations in rat tissues. (A) Tissue samples were homogenized by a rotary homogenizer, then centrifuged (17,000 g, 30 min) after mixing with methanol for deproteinization40. The supernatant was used for nitrate measurement by a standard chemiluminescence method with vanadium chloride. Data are plotted as average ± standard deviation (n = 4–6, * p < 0.05). (B) The change in each organ at 1-day and 3-day Na15NO3 supplementation (1 g/L) relative to control.
Since nitrate ions can be reduced to nitrite ions by several mechanisms once it is absorbed into blood and tissues, we measured the concentrations of nitrite in tissue samples as well as in plasma and urine (Fig. 2A). In plasma, gluteus muscle and liver samples, there was a significant rise in nitrite concentrations after 1- and 3-day supplementation, while eye and urine showed an increase after 1-day supplementation and it was statistically significant increase after 3-day supplementation. In fold change analysis, plasma showed the biggest increases (2.3- and 2.1-fold increase at 1-day and 3-day supplementation respectively) but eyes (1.6- and 1.9-fold increase), liver (1.4- and 1.6-fold increase) and gluteus muscle (1.4- and 1.4-fold increase) also exhibited notable increases in nitrite concentrations (Fig. 2B). Tissue samples suggested further small increases at 3-day compared to 1-day supplementation.
Total nitrite concentrations in rat tissues. (A) Tissue samples were homogenized by a rotary homogenizer40, then centrifuged (17,000 g, 30 min) after mixing with methanol for deproteinization. The supernatant was used for nitrite content measurement using a standard chemiluminescence method with triiodide. Data are plotted as average ± standard deviation (n = 4 ~ 6, * p < 0.05). (B) The change in each organ at 1-day and 3-day Na15NO3 supplementation (1 g/L) relative to control.
Although it is reasonable to presume that the supplemented nitrate via drinking water is responsible for the elevation of endogenous nitrate and nitrite concentrations as shown in Figs. 1 and 2, it is important to confirm the direct contribution of dietary nitrate to the concentrations of nitrate and nitrite in tissues to distinguish it from the NOS-derived nitrite and nitrate formation. To precisely analyze how much nitrate and nitrite is coming from the supplemented 15N-labeled nitrate, we measured 15N-labeled nitrate and nitrite content by LC–MS/MS. Figures 3A and 4A show the percent of 15N nitrate and nitrite respectively in three tissue samples as well as plasma and urine. In 1-day supplementation group, 76% of total plasma nitrate was 15N nitrate and 85% of total urine nitrate was 15N nitrate and these were remained very similar in 3-day supplementation group. Gluteus muscle, liver and eye homogenates also showed a remarkable increase in 15N nitrate percent (15, 14 and 45% respectively in 1-day supplementation group) (Fig. 3A). The percent of 15N nitrite was also significantly elevated in all tissue samples after 15N nitrate supplementation and plasma had the highest percent of 15N nitrite (47 and 44% at 1-day and 3-day supplementation). Among tissue samples, eye had higher 15N nitrite levels (24 and 20% at 1-day and 3-day supplementation) than gluteus muscle (4.8% at both 1-day and 3-day supplementation) and liver (15 and 20% at 1-day and 3-day supplementation) (Fig. 4A). Figures 3B and 4B show the proportion of 15N nitrate and nitrite respectively in control and 3-day supplementation group.
Relative enrichment of 15N-nitrate in tissues after dietary intervention analyzed by LC–MS/MS. (A) 15N-labeled nitrate content was measured by LC–MS/MS. All nitrate ions in samples were first reduced to nitrite by bacterial nitrate reductases (Aspergillus niger). Then nitrite derivatization by DAN was performed to yield NAT. The result was based on the percentage ratio of 15NAT/(14NAT + 15NAT). Data are plotted as average ± standard deviation (n = 4–6, * p < 0.05). (B) The proportion of 15N-labeled nitrate in control and 3-day Na15NO3 supplementation group is shown.
Relative enrichment of 15N-nitrite in tissues after dietary intervention analyzed by LC–MS/MS. (A) 15N-labeled nitrite content was measured by LC–MS/MS. Nitrite derivatization by DAN was performed to yield NAT. The result was based on the percentage ratio of 15NAT/(14NAT + 15NAT). Data are plotted as average ± standard deviation (n = 4–6, * p < 0.05). (B) The proportion of 15N-labeled nitrite in control and 3-day Na15NO3 supplementation group is shown.
To examine the expression changes in genes related to nitrate transport and reduction pathway, we performed a qPCR assay with gluteus muscle, liver and eye homogenates (Fig. 5). We assessed the expression levels of sialin, CLC (nitrate transporters) and XOR (nitrate reductase). In gluteus muscle, there was a significant increase in sialin, CLC1 (skeletal muscle-specific isoform of CLC), CLC3 and XOR expression levels in 3-day supplementation group. We observed a trend of increases in sialin, CLC2 and CLC3 in eye homogenates and a significant rise in XOR expression levels in 1-day and 3-day supplementation group. However, in liver, there was no change in these genes after nitrate supplementation.
Gene expression analysis. Total RNA was isolated from tissue samples (gluteus skeletal muscle, liver and eye) of control, 1- and 3-day Na15NO3 supplementation group and used for cDNA synthesis. The levels of mRNA were determined by qPCR and relative quantification was calculated by the 2−ΔΔCt method using gapdh as a reference gene. Data are plotted as average ± standard deviation (n = 3–6, * p < 0.05).
Discussion
Since the beneficial effects of dietary nitrate as NO source are well documented, particularly in enhancing cardiovascular health and exercise performances42,43, it is evident that the contribution of diet to NO homeostasis plays a big role in various physiological processes. Importantly, the endogenous NOS requires oxygen for its activity when producing NO, but the nitrate-nitrite-NO reduction pathway can be augmented under hypoxic environment44, which makes it a valuable alternative to the other NO generation mechanism. To elucidate the physiological importance and direct contribution of dietary nitrate to the concentrations of nitrate and nitrite as NO source, we employed 15N-labelled nitrate salts in drinking water to trace both 15N nitrate and 15N nitrite in several different tissues. Although there have been a few studies where stable isotopes were used to detect 15N nitrate and nitrite in body fluids such as plasma and urine, more detailed information on the levels of these isotopes in major organ samples after consuming 15N-labelled nitrate salts is not available so far. To our knowledge, this is the first study that shows the distribution of both 15N nitrate and nitrite in plasma, urine and tissues from rats supplemented with 15N-labelled nitrate salts and provides direct evidence of the reductive pathway for NO generation mediated by dietary nitrate in mammalian tissues. It also provides a short glimpse into the interesting complexity of nitrate uptake dynamics into internal organs, with a somehow unexpected speed of dietary nitrate influx into these tissues. In principle, isotope studies will allow to follow nitrate uptake kinetics into various organs and evaluate similarities/differences between various tissues.
In our recent studies in rats, we identified skeletal muscle as a nitrate-enriched organ compared to liver or blood and showed that nitrate, stored in skeletal muscle, could be utilized to produce nitrite and NO after exercise7,32. Consistent with previous results, we show in the current study that skeletal muscle (in particular, gluteus) contained higher nitrate concentrations (27.5 nmol/g) than other tissues (liver and eye, 8.1 and 12.5 nmol/g respectively) and plasma (12.5 nmol/g) at baseline (Fig. 1A). However, this nitrate concentration gradient, from muscle to plasma and other organs, in the steady state did not persist after nitrate supplementation. Nitrate concentrations were much higher in plasma (105.1 nmol/g) than other tissues (47.9, 19.8 and 51.8 nmol/g in gluteus, liver and eye respectively) except urine (1245.0 nmol/g) in 1-day supplementation group and the same pattern remained in 3-day supplementation group. Likely, high concentrations of nitrate in the circulation are important to distribute these ions to specific places where needed. Urine was previously identified as an important route to excrete excess nitrate out of body. It was shown that approximately 60% of administered nitrate is excreted in the urine in 24–48 hours45 and we also observed very high concentrations of nitrate in urine samples both at 1-day (1245.0 nmol/g) and 3-day (1693.3 nmol/g) supplementation. It is noteworthy and somewhat surprising that eyes absorbed nitrate ions more efficiently than gluteus muscle and liver when comparing relative changes after nitrate supplementation (Fig. 1B). Interestingly, recent papers showed an inverse association of dietary nitrate intake with primary open-angle glaucoma and age-related macular degeneration suggesting that a higher nitrate consumption may lower the risk of getting such ocular diseases46,47. While NO signaling governed by NOS was reported to be involved in the regulation of ocular blood flow and pressure48,49,50, very little information is currently available regarding the effects of nitrate and nitrite as NO precursors in eye physiology. To elucidate potential contributions of nitrate to NO pathways in the eye, we previously demonstrated that porcine ocular tissues, particularly cornea and sclera, possess the ability to reduce nitrate to nitrite suggesting that a functional nitrate-nitrite-NO pathway exists in the eye34. In that study, we postulated that lacrimal glands absorb nitrate from blood and secret it into the eye where it can be reduced to nitrite and NO by several mechanisms and this function of lacrimal glands seems to resemble well-known roles of salivary glands on concentrating and secreting nitrate into the oral cavity. In the present study, we measured nitrite and nitrate concentrations in whole eye homogenates after 15N nitrate supplementation to extend our knowledge on nitrate metabolism in the eye. We showed that eye homogenates exhibited 1.9-fold increase in absolute nitrite concentrations while gluteus and liver showed 1.4- and 1.6-fold increase in 3-day supplementation group (Fig. 2B) and more importantly, the 15N nitrite content at 3-day supplementation was 20.1, 19.6 and 4.8% in eye, liver and gluteus respectively (Fig. 3B). The difference between eye and other tissues was even more notable in 15N nitrate content (Fig. 3A). In 3-day supplementation group, 44.3% of total nitrate was 15N nitrate in the eye, while 16.9 and 10.9% was 15N nitrate in gluteus and liver. This suggests that eyes are more sensitive to taking dietary nitrate ions up and have ability to metabolize it. However, more detailed study is required to understand the precise mechanism by which nitrate ions are utilized and play a role in eye physiology and perhaps the pathophysiology of some ocular diseases.
The 15N nitrite detected in tissue samples could be derived from two sources, namely the reduction from supplemented 15N nitrate in situ or transport from the circulation which was already reduced from nitrate in the oral cavity or in other tissues. Although we could not estimate the extent of contribution by each source in the tissue samples, these results demonstrate that the substantial amount of dietary nitrate can be reduced to nitrite in the body and be utilized in specific tissues or re-distributed according to the needs.
We previously showed that the concentrations of several proteins related to nitrate metabolism, such as XOR and sialin, changed in rat skeletal muscle upon perturbation of nitrate supply18. Additionally, we and others show that knock-out mice, specifically myoglobin and eNOS knock-out mice, have higher expression levels and activities of XOR51,52. Although oral commensal bacteria are thought to be the main contributor to nitrate reduction to nitrite upon dietary nitrate consumption, several molybdenum-containing enzymes were suggested to be able to reduce nitrate, particularly XOR was shown to function as a nitrate reductase in mammalian tissues6. We also demonstrated that the inhibition of XOR almost completely prevented nitrate reduction to nitrite in rat skeletal muscle homogenates7. However, it is also important to note that there are significant differences between human and rodents in nitrate reduction capacity by commensal bacteria53,54. This suggests that mammalian enzymes such as XOR might be more important in reducing nitrate and nitrite in rodents than it is in humans, at least in some tissues. To examine whether XOR expression levels were altered by nitrate administration, we performed a qPCR analysis and observed a significant increase of XOR gene in gluteus muscle and eye in 3-day supplementation group (Fig. 4). Interestingly, we noted an increase in expression levels of nitrate transporter, sialin and CLC as well in gluteus and eye, although the difference was not statistically significant in the eye (Fig. 4). These results suggest that there is a positive feedback loop between nitrate and its transport system which can eventually augment the nitrate-nitrite-NO pathway in these tissues.
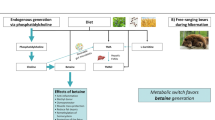
In summary, we analyzed both absolute concentrations and 15N percent of nitrate and nitrite ions after Na15NO3 administration in drinking water and showed that nitrate supplementation led to avid uptake of nitrate in rodent tissues, such as muscle, liver and eye and the conversions of nitrate to nitrite occur in these tissues. Our study is the first to estimate the rate and extent of absorbed exogenous nitrate and its reduction to nitrite in major rodent organs by using 15N-labeled nitrate salts and LC–MS/MS analysis. A summary in Fig. 6 shows the effect of nitrate supplementation on distribution of nitrate and nitrite ions and gene expression in tissues as discussed in this study. These results demonstrate that supplying nitrate via drinking water is an efficient way of increasing nitrate and nitrite concentrations in tissues and is likely to contribute to improving NO bioavailability in these tissues.
Effects of dietary nitrate supplementation on distribution of nitrate and nitrite ions and gene expression levels in tissues. 1: Na15NO3 (1 g/L) supplementation for 3 days via drinking water lead to significant increases of 15N nitrate contents in rat tissues and plasma. Unabsorbed 15N nitrate is excreted in the urine. 2: Endogenous nitrate reductases, such as XOR, can reduce 15N nitrate to 15N nitrite in tissues and cause a rise in 15N nitrite concentrations. Other Mo-containing enzymes include aldehyde oxidase (AO), sulfite oxidase (SO) and mitochondrial amidoxime reducing component (mARC). 3: Na15NO3 (1 g/L) supplementation for 3 days induced an upregulation of nitrate transporter (Sialin) and nitrate reductase (XOR) genes in eye and gluteus muscle.
Data availability
The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request.
References
Moncada, S., Radomski, M. W. & Palmer, R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem. Pharmacol. 37, 2495–2501. https://doi.org/10.1016/0006-2952(88)90236-5 (1988).
Ignarro, L. J. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J. Physiol. Pharmacol. 53, 503–514 (2002).
Benjamin, N. et al. Stomach NO synthesis. Nature 368, 502. https://doi.org/10.1038/368502a0 (1994).
Lundberg, J. O., Weitzberg, E., Lundberg, J. M. & Alving, K. Intragastric nitric oxide production in humans: Measurements in expelled air. Gut 35, 1543–1546 (1994).
Govoni, M., Jansson, E. A., Weitzberg, E. & Lundberg, J. O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19, 333–337. https://doi.org/10.1016/j.niox.2008.08.003 (2008).
Jansson, E. A. et al. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat. Chem. Biol. 4, 411–417. https://doi.org/10.1038/nchembio.92 (2008).
Piknova, B., Park, J. W., Kwan Jeff Lam, K. & Schechter, A. N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 55–56, 54–61. https://doi.org/10.1016/j.niox.2016.03.005 (2016).
Cosby, K. et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9, 1498–1505. https://doi.org/10.1038/nm954 (2003).
Shiva, S. et al. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 100, 654–661. https://doi.org/10.1161/01.RES.0000260171.52224.6b (2007).
Rassaf, T. et al. Nitrite reductase function of deoxymyoglobin: Oxygen sensor and regulator of cardiac energetics and function. Circ. Res. 100, 1749–1754. https://doi.org/10.1161/CIRCRESAHA.107.152488 (2007).
Millar, T. M. et al. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 427, 225–228 (1998).
Carlsson, S., Wiklund, N. P., Engstrand, L., Weitzberg, E. & Lundberg, J. O. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide 5, 580–586. https://doi.org/10.1006/niox.2001.0371 (2001).
Gee, L. C. & Ahluwalia, A. Dietary nitrate lowers blood pressure: Epidemiological, pre-clinical experimental and clinical trial evidence. Curr. Hypertens. Rep. 18, 17. https://doi.org/10.1007/s11906-015-0623-4 (2016).
Jones, A. M., Thompson, C., Wylie, L. J. & Vanhatalo, A. Dietary nitrate and physical performance. Annu. Rev. Nutr. 38, 303–328. https://doi.org/10.1146/annurev-nutr-082117-051622 (2018).
Bryan, N. S. et al. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 104, 19144–19149. https://doi.org/10.1073/pnas.0706579104 (2007).
Raat, N. J. et al. Dietary nitrate and nitrite modulate blood and organ nitrite and the cellular ischemic stress response. Free Radic. Biol. Med. 47, 510–517. https://doi.org/10.1016/j.freeradbiomed.2009.05.015 (2009).
Park, J. W., Piknova, B., Huang, P. L., Noguchi, C. T. & Schechter, A. N. Effect of blood nitrite and nitrate levels on murine platelet function. PLoS ONE 8, e55699. https://doi.org/10.1371/journal.pone.0055699 (2013).
Park, J. W., Thomas, S. M., Schechter, A. N. & Piknova, B. Control of rat muscle nitrate levels after perturbation of steady state dietary nitrate intake. Nitric Oxide 109–110, 42–49. https://doi.org/10.1016/j.niox.2021.03.003 (2021).
Wylie, L. J. et al. Human skeletal muscle nitrate store: Influence of dietary nitrate supplementation and exercise. J. Physiol. 597, 5565–5576. https://doi.org/10.1113/JP278076 (2019).
Qin, L. et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 109, 13434–13439. https://doi.org/10.1073/pnas.1116633109 (2012).
Fahlke, C., Beck, C. L. & George, A. L. Jr. A mutation in autosomal dominant myotonia congenita affects pore properties of the muscle chloride channel. Proc. Natl. Acad. Sci. U. S. A. 94, 2729–2734. https://doi.org/10.1073/pnas.94.6.2729 (1997).
Srihirun, S. et al. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 94, 1–8. https://doi.org/10.1016/j.niox.2019.10.005 (2020).
Kapil, V. et al. The noncanonical pathway for in vivo nitric oxide generation: The nitrate-nitrite-nitric oxide pathway. Pharmacol. Rev. 72, 692–766. https://doi.org/10.1124/pr.120.019240 (2020).
Bryan, N. S., Calvert, J. W., Gundewar, S. & Lefer, D. J. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic. Biol. Med. 45, 468–474. https://doi.org/10.1016/j.freeradbiomed.2008.04.040 (2008).
Carlstrom, M. et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 107, 17716–17720. https://doi.org/10.1073/pnas.1008872107 (2010).
Milsom, A. B., Fernandez, B. O., Garcia-Saura, M. F., Rodriguez, J. & Feelisch, M. Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxid Redox Signal 17, 422–432. https://doi.org/10.1089/ars.2011.4156 (2012).
Gilliard, C. N. et al. Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 75, 1–7. https://doi.org/10.1016/j.niox.2018.01.010 (2018).
Hughan, K. S. et al. Conjugated linoleic acid modulates clinical responses to oral nitrite and nitrate. Hypertension 70, 634–644. https://doi.org/10.1161/HYPERTENSIONAHA.117.09016 (2017).
Hu, C. W. et al. (15)N-labelled nitrite/nitrate tracer analysis by LC-MS/MS: Urinary and fecal excretion of nitrite/nitrate following oral administration to mice. Free Radic. Biol. Med. 143, 193–202. https://doi.org/10.1016/j.freeradbiomed.2019.08.005 (2019).
Siervo, M., Jackson, S. J. & Bluck, L. J. In-vivo nitric oxide synthesis is reduced in obese patients with metabolic syndrome: Application of a novel stable isotopic method. J. Hypertens. 29, 1515–1527. https://doi.org/10.1097/HJH.0b013e3283487806 (2011).
Axton, E. R. et al. Treatment with nitrate, but not nitrite, lowers the oxygen cost of exercise and decreases glycolytic intermediates while increasing fatty acid metabolites in exercised zebrafish. J. Nutr. 149, 2120–2132. https://doi.org/10.1093/jn/nxz202 (2019).
Piknova, B. et al. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 47, 10–16. https://doi.org/10.1016/j.niox.2015.02.145 (2015).
Coggan, A. R. et al. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 48, 16–21. https://doi.org/10.1016/j.niox.2014.08.014 (2015).
Park, J. W. et al. Potential roles of nitrate and nitrite in nitric oxide metabolism in the eye. Sci. Rep. 10, 13166. https://doi.org/10.1038/s41598-020-69272-9 (2020).
Arifin, W. N. & Zahiruddin, W. M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 24, 101–105. https://doi.org/10.21315/mjms2017.24.5.11 (2017).
Serdar, C. C., Cihan, M., Yucel, D. & Serdar, M. A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. (Zagreb) 31, 010502. https://doi.org/10.11613/BM.2021.010502 (2021).
Piknova, B., Park, J. W., Cassel, K. S., Gilliard, C. N. & Schechter, A. N. Measuring nitrite and nitrate, metabolites in the nitric oxide pathway, in biological materials using the chemiluminescence method. J. Vis. Exp. https://doi.org/10.3791/54879 (2016).
Chao, M. R. et al. Urinary nitrite/nitrate ratio measured by isotope-dilution LC–MS/MS as a tool to screen for urinary tract infections. Free Radic. Biol. Med. 93, 77–83. https://doi.org/10.1016/j.freeradbiomed.2016.01.025 (2016).
Li, H., Meininger, C. J. & Wu, G. Rapid determination of nitrite by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 746, 199–207. https://doi.org/10.1016/s0378-4347(00)00328-5 (2000).
Park, J. W. et al. Preparation of rat skeletal muscle homogenates for nitrate and nitrite measurements. J. Vis. Exp. https://doi.org/10.3791/62427 (2021).
Piknova, B. & Schechter, A. N. Measurement of nitrite in blood samples using the ferricyanide-based hemoglobin oxidation assay. Methods Mol. Biol. 704, 39–56. https://doi.org/10.1007/978-1-61737-964-2_4 (2011).
Jones, A. M. et al. Dietary nitrate and nitric oxide metabolism: Mouth, circulation, skeletal muscle, and exercise performance. Med. Sci. Sports Exerc. 53, 280–294. https://doi.org/10.1249/MSS.0000000000002470 (2021).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167. https://doi.org/10.1038/nrd2466 (2008).
DeMartino, A. W., Kim-Shapiro, D. B., Patel, R. P. & Gladwin, M. T. Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol. 176, 228–245. https://doi.org/10.1111/bph.14484 (2019).
Wagner, D. A., Schultz, D. S., Deen, W. M., Young, V. R. & Tannenbaum, S. R. Metabolic fate of an oral dose of 15N-labeled nitrate in humans: Effect of diet supplementation with ascorbic acid. Cancer Res. 43, 1921–1925 (1983).
Gopinath, B. et al. Association of dietary nitrate intake with the 15-year incidence of age-related macular degeneration. J. Acad. Nutr. Diet 118, 2311–2314. https://doi.org/10.1016/j.jand.2018.07.012 (2018).
Kang, J. H. et al. Association of dietary nitrate intake with primary open-angle glaucoma: A prospective analysis from the nurses’ health study and health professionals follow-up study. JAMA Ophthalmol. 134, 294–303. https://doi.org/10.1001/jamaophthalmol.2015.5601 (2016).
Meyer, P., Flammer, J. & Luscher, T. F. Endothelium-dependent regulation of the ophthalmic microcirculation in the perfused porcine eye: Role of nitric oxide and endothelins. Invest. Ophthalmol. Vis. Sci. 34, 3614–3621 (1993).
Nathanson, J. A. & McKee, M. Alterations of ocular nitric oxide synthase in human glaucoma. Invest. Ophthalmol. Vis. Sci. 36, 1774–1784 (1995).
Polak, K. et al. Altered nitric oxide system in patients with open-angle glaucoma. Arch. Ophthalmol. 125, 494–498. https://doi.org/10.1001/archopht.125.4.494 (2007).
Park, J. W., Piknova, B., Dey, S., Noguchi, C. T. & Schechter, A. N. Compensatory mechanisms in myoglobin deficient mice preserve NO homeostasis. Nitric Oxide 90, 10–14. https://doi.org/10.1016/j.niox.2019.06.001 (2019).
Peleli, M. et al. Enhanced XOR activity in eNOS-deficient mice: Effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radic. Biol. Med. 99, 472–484. https://doi.org/10.1016/j.freeradbiomed.2016.09.004 (2016).
Hyde, E. R. et al. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic. Biol. Med. 77, 249–257. https://doi.org/10.1016/j.freeradbiomed.2014.09.017 (2014).
Montenegro, M. F. et al. Profound differences between humans and rodents in the ability to concentrate salivary nitrate: Implications for translational research. Redox Biol. 10, 206–210. https://doi.org/10.1016/j.redox.2016.10.011 (2016).
Funding
Open Access funding provided by the National Institutes of Health (NIH). This work was supported by intramural NIDDK/NIH grant ZIA DK 025104-15 to Alan N. Schechter.
Author information
Authors and Affiliations
Contributions
A.N.S., B.P. and J.W.P. designed the study. B.P. and J.W.P. collected samples with an assistance from S.M.T. and K.J.T-S. P.J.W. and H.C. performed LC–MS/MS. S.U. performed qPCR. J.W.P. and B.P. analyzed the data and prepared figures. J.W.P., B.P. and A.N.S. wrote the manuscript and all authors discussed and contributed to the reviewing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Alan N. Schechter is listed as a co-inventor on several patents issued to the National Institutes of Health for the use of nitrite salts for the treatment of cardiovascular diseases. He receives royalties based on NIH licensing of these patents for clinical development but no other compensation. These arrangements do not affect his adherence to Scientific Reports journal policies. All other authors declare they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, J.W., Piknova, B., Walter, P.J. et al. Distribution of dietary nitrate and its metabolites in rat tissues after 15N-labeled nitrate administration. Sci Rep 13, 3499 (2023). https://doi.org/10.1038/s41598-023-28190-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28190-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.