Abstract
Loss of muscle mass occurs rapidly during critical illness and negatively affects quality of life. The incidence of clinically significant muscle wasting in critically ill patients is unclear. This study aimed to assess the incidence of and identify predictors for clinically significant loss of muscle mass in this patient population. This was a single-center observational study. We used ultrasound to determine the rectus femoris cross-sectional area (RFcsa) on the first and seventh day of ICU stay. The primary outcome was the incidence of significant muscle wasting. We used a logistic regression model to determine significant predictors for muscle wasting. Ultrasound measurements were completed in 104 patients. Sixty-two of these patients (59.6%) showed ≥ 10% decreases in RFcsa. We did not identify any predictor for significant muscle wasting, however, age was of borderline significance (p = 0.0528). The 28-day mortality rate was higher in patients with significant wasting, but this difference was not statistically significant (30.6% versus 16.7%; p = 0.165). Clinically significant muscle wasting was frequent in our cohort of patients. Patient age was identified as a predictor of borderline significance for muscle wasting. The results could be used to plan future studies on this topic.
Trial registration: ClinicalTrials.gov NCT03865095, date of registration: 06/03/2019.
Similar content being viewed by others
Introduction
Acute skeletal muscle wasting and weakness is an important medical problem of critically ill patients1. The muscle mass diminishes rapidly during the early phase of a critical illness2. Muscle wasting is a result of reduced physical activity, increased protein breakdown, and decreased protein synthesis3,4. Loss of muscle mass is associated with poor clinical outcomes. Patients who develop muscle wasting have higher risk of intensive care unit (ICU) acquired weakness5, increased length of ICU stay2 and loss of muscle mass during the first week of ICU stay is associated with increased 60-day mortality6. Weakness and fatigue are persisting symptoms which have a negative impact on the quality of life of critical illness survivors7,8.
The identification of predictors for muscle wasting may help to identify the population we have to focus on to improve quality of care and patient-oriented outcomes. However, to the best of our knowledge, only a few published studies were designed to identify predictors for muscle wasting9,10.
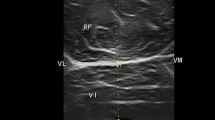
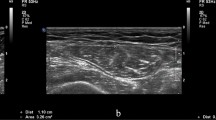
One of the critical aspects of identifying the predictors for muscle wasting is the correct assessment of muscle mass. This can be challenging in ICU patients because the procedures commonly used for assessing muscle mass, such as manual muscle testing, nerve conduction studies, dual X-ray absorptiometry, computed tomography or magnetic resonance imaging, are rarely useful in critical care settings.ICU patients typically have limited ability to cooperate and follow commands, require trained staff, require invasive procedures, or require patient transport from the ICU. Several studies have documented the use of ultrasonography to estimate muscle quantity and quality at the bedside and have compared these results to measurements of quadriceps muscle thickness in critical care settings11,12,13. Puthucheary et al. proposed the use of the rectus femoris cross-sectional area (RFcsa) as a replacement for measurements of muscle thickness14. In contrast to measurements of muscle atrophy based on muscle thickness, changes in RFcsa are directly correlated with changes in muscle strength in critically ill septic patients15.
Decrease of RFcsa ≥ 10% is considered as significant and sufficient to affect muscle function14,16,17. However, the incidence of significant muscle wasting in critically ill adult patients has been poorly reported to date. We believe that the information about incidence of muscle wasting is importantfor devising optimal patient management strategies in the ICU14,17.
Thus, this study aimed to (1) assess the incidence of significant loss of muscle mass in critically ill adult patients; (2) to assess quantitative changes in muscle mass; and (3) to identify any predictors for muscle wasting.
Results
Patients
A total of 1293 patients was screened during the study period. Of these, 186 patients were initially enrolled based on the inclusion and exclusion criteria. An ultrasound examination was performed to document initial RFcsa within 24 h of ICU admittance. Ultrasound measurements were not performed on day 7 in 82 of these patients due to mortality, transfer to another hospital, and other reasons (Fig. 1), providing us with data from 104 patient cases for analysis. Figure 1 presents the flow diagram for this study. The broad admission categories included medical (n = 41; 39.4%), surgical (n = 3; 2.9%), multiple trauma (n = 44; 42.3%), and neurosurgical (n = 16; 15.4%). Patient characteristics are outlined in Table 1.
Measurement of rectus femoris cross-sectional area
The mean RFcsa (95% CI) on day 1 was 2.638 (2.286–2.990) cm2 for the group of patients with RFcsa measurements that decreased by ≥ 10% and 2.333 (1.967–2.699) for the group that exhibited a < 10% decrease in RFcsa. The mean (95% CI) RFcsa on day 7 was 1.986 (1.683–2.289) cm2 versus 2.355 (2.001–2.710) cm2 in the groups exhibiting decreases of ≥ 10% and < 10%, respectively. These results are shown in Table 2.
Outcomes
We evaluated 104 patients in this study. Sixty-two of these patients (59.6%) exhibited a ≥ 10% decrease in RFcsa between day 1 and day 7 while in the ICU. The mean (95% CI) SOFA score on day 7 was 6.9 (5.8–7.9) in patients who exhibited a < 10% decrease in RFcsa and 7.6 (6.6–8.6) in patients who exhibited ≥ 10% decreases in RFcsa. The mean duration of mechanical ventilation was 12.3 (9.9–14.7) and 11.4 (9.4–13.4) days for patients with decreases in RFcsa of < 10% and ≥ 10%, respectively. The mean length of stay in the ICU was 18.8 (16.6–21.1) and 17.0 (15.1–18.9) days for these two groups, respectively. The mean 28-day mortality was 16.7% in patients that exhibited a < 10% decrease in RFcsa and 30.6% in patients that exhibited a ≥ 10% decrease in RFcsa. Although mortality nearly doubled in the group of patients that exhibited a ≥ 10% decrease in RFcsa compared with those that exhibited a < 10% decrease in RFcsa. None of these differences achieved statistical significance (see p-values in Table 3). The outcome data are summarized in Table 3.
Predictors for a ≥ 10% decrease of RFcsa
All characteristics listed in Table 1 were explored as potential predictors for their relationship with RFcsa reduction graphically and by means of univariate logistic regression. Further forward stepwise selection in a multivariate logistic regression model was used to identify significant predictors. Patient age was the only parameter identified as a significant predictor, however on borderline statistical signifikance, for a ≥ 10% decrease in RFcsa. The results of this logistic regression analysis are shown in Table 4. The odds ratio suggests that, for each one-year increase in age, the odds that an ICU patient will experience a ≥ 10% decrease in RFcsa increase by 2%.
Discussion
Muscle wasting contributes significantly to weakness among patients who have recovered from a critical illness and can have a negative impact on a patient’s quality of life7. Recent research has focused on identifying adequate techniques of assessment of muscle wasting as well as factors contributing to muscle wasting in critical care settings. Ultrasound measurements of muscle mass and other parameters of different muscles were investigated and reported in critically ill patients recently18. Ultrasound is thus a well-established method for achieving this goal. For example, Mourtzakis et al.19 reported the outcomes of 11 observational and three interventional studies that used ultrasound measurements to assess muscle wasting in critically ill patients. Ultrasound has specifically been used to assess muscle mass by measuring the RFcsa, which could replace methods that measure muscle thickness14. Ultrasound measurements of RFcsa are feasible at the bedside, require no patient cooperation, and show good intra- and interobserver agreement20,21.
Previous studies that assessed muscle wasting using RFcsa concluded that a > 9.24% decrease in RFcsa was significant16,17. Observational and interventional studies aimed at the preservation of muscle mass have used a cut-off of a ≥ 10% reduction in RFcsa14,22. To our knowledge, no studies in critical care settings that have used a muscle-wasting criterion of a > 10% reduction in RFcsa as a cut-off for significant muscle wasting have reported its incidence. In this study, the incidence of significant muscle mass wasting was 59.6%, which is similar to the incidence reported in cardiac surgery16,17.
While loss of muscle mass has frequently been observed in association with critical illness, the relationship of muscle wasting degree to clinical outcomes remains unclear.
Only a few studies reported the effect of loss of muscle mass on clinical outcomes and none of these studies focused on clinical outcome measures as primary endpoints16,22. Bloch et al. conducted an observational study in high-risk cardiovascular surgery patients and found no statistical differences in the length of ICU or hospital stay between patients with or without muscle wasting, which was defined as reduction of RFcsa > 9.24%16,18. We obtained similar results in this study. The relationship between significant muscle wasting and mortality in adult patients in critical care settings also remains unknown. Our results revealed a nearly two-fold increase in 28-day mortality in patients with significant muscle wasting (30.6% versus 16.7% in the groups with ≥ 10% and < 10% decreases in RFcsa, respectively). Although this difference was not statistically significant, the study sample size was not pre-set to evaluate this question.
Patients frequently lose muscle mass in critical care settings; for example, one study showed that patients with multiple organ failure experienced a 15.7% reduction in RFcsa during their first 7 days in the ICU2. In study by Mayer et al. found decrease of 18.9% of the RFcsa23. In our study, the mean decrease in RFcsa over 7 days in all enrolled ICU patients was 14.9%. The higher reduction of RFcsa in study by Meyer may be related do differnet study population, as the majority of patients in that study were admitted to medical ICU.
Predictors for muscle wasting remain poorly understood. Puthucheary et al.2 found that age, bicarbonate level at hospital admission, and the ratio of PaO2 to FiO2 were associated with a > 10% decrease in RFcsa measured on day 10 of an ICU stay. Mayer et al.23 recently reported age among risk factors of muscle sit-to-stand performance at hospital discharge in patients with critical illness. In our study, age was found of borderline signifikance as a predictor for a > 10% decrease in RFcsa measured on day 7 of an ICU stay. Larger study is needed to prove this finding.
There were several limitations associated with this study. First, the small sample size precludes a full evaluation of the relationship between the loss of muscle mass and 28-day mortality. Second, the patient cohort surveyed in this study is highly heterogeneous. For example, we enrolled significantly more male than female patients, and our results may therefore not be valid for both sexes. The reasons patients were admitted to the ICU were also not evenly represented, as most patients were admitted for internal complications or trauma, whereas few were admitted for surgical complications. Third, the site of ultrasound measurement did not follow any generally accepted protocol as none such exists. On the other hand, as shown in a review by Nascimento18, the most frequently used site for assessing quadriceps muscle is 2/3 of the anterior iliac spine—patella distance. However, we found in the preparation phase of the study that with this protocol we were not able to obtain a cross-section of rectus femoris muscle in total. This was due to the type of ultrasound probe available. Fourth, the side where the RFcsa measurement was performed was not standardized across all patients and was instead subjectively chosen by the investigator. Fifth, we did not document the hand dominance of each patient in this cohort. We therefore may have measured RFcsa on the patient’s non-dominant side, leading to potential overestimation of muscle wasting. We finally recognize the potential for selection bias, as primary patient selection was based on the investigator’s subjective assessment of which individuals were likely to need mechanical ventilation for more than 48 h after ICU admission.
Our results suggest that many adult patients in critical care settings experience a significant loss of muscle mass, defined as a ≥ 10% decrease in RFcsa over seven days of ICU care. Our results could be used to determine the required sample sizes for randomized controlled trials that further examine muscle wasting in critical care patients and identify strategies for mitigating muscle loss.
The generalizability of this study is somewhat limited due to the observational design and the fact that it was carried out at a single medical center.
Significant loss of muscle mass was assessed by ultrasound measurements of RFcsa in 104 critically ill adult patients. Patient age at admission was associated with a risk of developing clinically significant muscle wasting. Mortality rates were two-fold higher in patients with significant muscle wasting (≥ 10% over the first seven days in the ICU) than among those without significant wasting, though this difference in mortality was not statistically significant.
Muscle wasting occurs rapidly2 and has an important role in development of ICU acquired weakness7. It also has a negative impact on clinical outcomes as functional disability at ICU discharge9. Earlier and greater loss of muscle mass is associated with prolonged mechanical ventilation, ICU acquired weakness and in-hospital mortality13. Our result showed that significant muscle wasting is frequent among critically ill patients. The results of this study could be used to plan future studies evaluating strategies to prevent muscle wasting and thus positively affect the outcomes of critically ill patients, e.g. nutrition regimens or physiotherapy.
Materials and methods
Study design, setting, and participants
This single-center prospective observational cohort study is registered at ClinicalTrials.gov (NCT03865095). Ethical approval for this study (Ethical Committee No. 05-130219/EK) was provided by the Ethical Committee of University Hospital Brno, Brno, Czech Republic (Chairperson PharmDr.S.Kozakova, MBA) on 13 February 2019. This work was performed in accordance with relevant guidelines and regulations and in accordance with the Declaration of Helsinki. Inform consent was obtained from all enrolled patients. If a patient has impaired consent capacity, the informed consent was obtained from a legal representative. If a legal representative was not established or known at the time of enrolment, the informed consent was obtained from a physician who was independent on study conduct and familiar with study protocol. All patients admitted to one of the four ICUs of the Department of Anesthesiology and Intensive Care Medicine of University Hospital Brno from March 2019 to September 2020 were screened for eligibility for enrolment in this study. Inclusion criteria at ICU admission included ≥ 18 years of age and the physician’s subjective evaluation that the patient would require mechanical ventilation for at least 48 h. Exclusion criteria included age < 18 years, a Clinical Frailty Score > 7 prior to admission, a past medical history of neuromuscular disease, amputated lower extremities, prior trauma to the lower extremities involving thighs and inability to cooperate with ultrasound examinations. Patients whose ultrasound measurement was not feasible on day 7 were excluded from the final analyses, reasons for exclusion were provided (Fig. 1).
Variables, data sources, and measurements
The primary outcome of the study was the quantitative assessment of decreased RFcsa, defined as the percentage reduction in RFcsa based on the results of ultrasound evaluation on day 1 and day 7 of the patient’s ICU stay. We considered a decrease in RFcsa ≥ 10% to be clinically significant muscle wasting. The secondary outcome of the study was 28-day mortality. We also examined predictors for a clinically significant decrease in RFcsa.
Ultrasound measurements (Vivid S6, GE Healthcare) of RFcsa were performed by the same investigator within the first 24 h after ICU admission (day 1) and again on day 7 of the patient’s ICU stay. The side of measurement was left at the discretion of the ultrasonographer. The physical point at which the ultrasound measurement was performed was drawn on the patient’s skin as a line perpendicular to the long axis of the thigh, three-quarters of the distance between the anterior superior iliac spine and the middle of the upper part of the patella. A linear ultrasound probe with a frequency of 3–9 MHz was placed on the marked line perpendicular to the skin using an excess of gel and minimal pressure. Six images of the rectus femoris muscle cross were collected and stored. An ultrasound machine tool for area measurement was used for RFcsa measurements. The inner side of the fascia of the rectus femoris muscle was manually traced in each image. For the calculation of average value of ultrasound measurements we adopted the method described by Turton et al.24, we omitted the smallest and largest values and calculated the average of the remaining four values. The member of the study team who performed the examination entered the ultrasound measurements into the case report form. To assess the reproducibility of our ultrasound measurements we performed separate analysis of intra- and interobserver agreement in 25 consecutive patients. The measurements were performed by two observers. Observer A repeated the measurement 30 min after the first one, and observer B performed the measurement independently of observer A and was blinded to the results of observer A. The inter-class correlation coefficients for intra- and interobserver agreement showed excellent correlation21.
All study-related patient data (age, sex, APACHE II score, SOFA score, Frailty scale value, and ICU Mobility score on admission and day 7) were obtained from medical records and entered into the case report form by a study team member.
Bias and sample size
We cannot rule out selection bias in this study, as one of the primary inclusion criteria (i.e., the physician’s personal assessment of the need for 48 h of mechanical ventilation) was subjective in nature. The likelihood that a patient would need mechanical ventilation for > 48 h was assessed after discussion between the investigator and the senior ICU physician. This discussion took into account findings from initial diagnostic work-up and the reaction to therapeutic interventions. Because we were unable to locate any previous studies that documented the incidence of significant muscle wasting in critically ill adult patients, we were unable to use previous data to determine the relevant sample size. However, we did note that Bloch et al.17 reported significant muscle wasting (defined as a decrease in RFcsa of more than 10% at day 7 of an ICU stay) in 55% of patients who had undergone cardiothoracic surgery. Based on these findings, we estimated that 100 patients would provide the appropriate sample size for determining the incidence of significant muscle wasting. Given the possibility that many of the enrolled patients might not fully complete the study protocol, we increased the number of participants to 175. To cover potential loss of follow-up, the number of participants was increased to 186.
Statistical analysis
Continuous demographic data and baseline patient characteristics are presented as means with standard deviations (SDs). Continuous outcome characteristics and measurements of RFcsa are presented as means and 95% confidence intervals (CIs) with SDs. Categorical characteristics are summarized using absolute counts and percentages. There were no missing RFcsa measurements, outcome characteristics, demographic, or baseline data for any of the 104 patients we evaluated.
All potential predictors were examined using both descriptive statistics and forward stepwise selection in a logistic regression model. Predictors were retained in the model if they had p-value < 0.1. A logistic regression model that included change in RFcsa as a dependent binary variable and predictors that were retained after forward selection as predictors were constructed to estimate odds ratios and 95% Wald CIs.
The relationships between patient characteristics and decreases in RFcsa were examined using Wilcoxon–Mann–Whitney U-tests. The relationship between a decrease in RFcsa and 28-day mortality was evaluated using Fisher’s exact test with an alpha level of 0.05. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary NC).
References
Shefold, J. C. et al. Muscular weakness and muscle wasting in the critically ill. J. Cachexia Sarcopenia Muscle 11, 1399–1412 (2020).
Puthucheary, Z. A. et al. Acute skeletal muscle wasting in critical illness. JAMA 310, 1591–1600 (2013).
Biolo, G. et al. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J. Clin. Endocrinol. Metab. 87, 3378–3384 (2002).
Paddon-Jones, D. et al. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J. Clin. Endocrinol. Metab. 91, 4836–4841 (2006).
Mayer, K. P. et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit. Care 24, 1–12 (2020).
Lee, Z. Y. et al. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: A single-center prospective observational study. Clin. Nutr. 40, 1338–1347 (2021).
Herridge, M. S. et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 348, 683–693 (2003).
Dinglas, V. D. et al. Muscle weakness and 5-year survival in acute respiratory distress syndrome survivors. Crit. Care Med. 45, 446–453 (2017).
Parry, S. M. et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J. Crit. Care 30, e9-14 (2015).
Ten Haaf, D. et al. The magnitude and time course of muscle cross-section decrease in intensive care unit patients. Am. J. Phys. Med. Rehabil. 96, 634–638 (2017).
Tillquist, M. et al. Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN 38, 886–890 (2014).
Sabatino, A. et al. Reliability of bedside ultrasound for measurement of quadriceps muscle thickness in critically ill patients with acute kidney injury. Clin. Nutr. 36, 1710–1715 (2017).
Hadda, V. et al. Reliability of ultrasonographic arm muscle thickness measurement by various levels of health care providers in ICU. Clin. Nutr. ESPEN 24, 78–81 (2018).
Puthucheary, Z. A. et al. Rectus femoris cross-sectional area and muscle layer thickness: Comparative markers of muscle wasting and weakness. Am. J. Respir. Crit. Care Med. 195, 136–138 (2017).
Palakshappa, J. A. et al. Quantitative peripheral muscle ultrasound in sepsis: Muscle area superior to thickness. J. Crit. Care 47, 324–330 (2018).
Bloch, S. A. et al. Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit. Care Med. 41, 982–989 (2013).
Bloch, S. A. et al. MiR-181a: A potential biomarker of acute muscle wasting following elective high-risk cardiothoracic surgery. Crit. Care 19, 147 (2015).
Nascimento, T. S. et al. Ultrasound protocols to assess skeletal and diaphragmatic muscle in people who are critically ill: A systematic review. Ultrasound Med. Biol. 47, 3041–3067 (2021).
Mourtzakis, M., Parry, S., Connolly, B. & Puthucheary, Z. A. Skeletal muscle ultrasound in critical care: A tool in need of translation. Ann. Am. Thorac. Soc. 14, 1495–1503 (2017).
Sarwal, A. et al. Interobserver reliability of quantitative muscle sonographic analysis in the critically ill population. J. Ultrasound Med. 34, 1191–1200 (2015).
Hrdy, O., Vrbica, K., Kovar, M., Korbicka, T. & Gal, R. Intra- and interobserver agreement of rectus femoris cross-sectional area in critically ill patients. Minerva Anestesiol. 87, 494–495 (2021).
McNelly, A. S. et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: A phase 2 clinical trial. Chest 158, 183–194 (2020).
Mayer, K. P. et al. Acute skeletal muscle wasting and dysfunction predict physical disability at hospital discharge in patients with critical illness. Crit. Care 24, 637 (2020).
Turton, P., Hay, R., Taylor, J., McPhee, J. & Welters, I. Human limb skeletal muscle wasting and architectural remodeling during five to ten days intubation and ventilation in critical care—An observational study using ultrasound. BMC Anesthesiol. 16, 119 (2016).
Acknowledgements
We would like to thank Dr. Eva Strazevska for her assistance with the study.
Funding
This work was supported by a Specific University Research grant provided by MŠMT (MUNI/A/1058/2019 and MUNI/A/1091/2020). The funding sources were not involved in study design, the collection, analysis, and interpretation of data, in the writing of the report, or the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by O.H., K.V., T.K., R.S. and M.K. The manuscript was written by O.H. and R.G. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Gal has nothing to disclose. Dr. Hrdy reports grants and personal fees from MŠMT, during the conduct of the study. Dr. Vrbica reports grants and personal fees from MŠMT, during the conduct of the study. Dr. Kovar has nothing to disclose. Dr. Korbicka has nothing to disclose. Mrs. Radka Stepanova has nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hrdy, O., Vrbica, K., Kovar, M. et al. Incidence of muscle wasting in the critically ill: a prospective observational cohort study. Sci Rep 13, 742 (2023). https://doi.org/10.1038/s41598-023-28071-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28071-8
This article is cited by
-
Assessment of quadriceps muscle mass by ultrasound in the postoperative period of cardiac surgery
The Ultrasound Journal (2024)
-
Ultrasound assessment of muscle mass and correlation with clinical outcomes in critically ill patients: a prospective observational study
Journal of Ultrasound (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.