Abstract
To compare the safety and effectiveness of surgical treatment of jugular paragangliomas (JPs) following the application of our modified surgical techniques. Fifty-six patients with JPs were analyzed for tumor classification, surgical outcomes, and intratumor blood vessels. The gross total resection in C1–2 (100%) was significantly greater than that in C3 and D (66.7%). Good postoperative facial nerve (FN) function (House–Brackmann I–II) was achieved in 89.5% C1–2 cases, which was not significantly different from C3 and D (93.3%) (P = 0.694). Preoperative and postoperative lower cranial nerve (LCN) deficits correlated with the Fisch’s classification of tumors (P < 0.05), and intraoperative blood loss was greater in advanced tumors (P = 0.050). Further study showed that the cross-sectional area of intratumor blood vessels was positively associated with intraoperative blood loss (P < 0.001). Surgical excision of JPs is a safe and effective strategy, and early surgical treatment is a good option for patients with C1–2 tumors without surgical contraindications.
Similar content being viewed by others
Introduction
Head and neck paragangliomas (HNPs) are rare slow-growing, benign, highly vascularized tumors, but are locally destructive lesions, accounting for only 0.6% of all head and neck tumors1. Jugular paragangliomas (JPs) are the most common primary neoplasms in the jugular foramen, arising from paraganglia cells located within the adventitia of the dome of the jugular bulb. Although histologically considered benign, JPs represent locally aggressive, destructive neoplastic lesions and close proximity to the facial nerve (FN), lower cranial nerves (LCNs), internal carotid artery (ICA), jugular bulb, posterior fossa, cochlea and labyrinthine, and surgical management of JPs is challenging2,3. In rare cases, JPs can secrete catecholamines and metastasize to lymph nodes and distant organs, which significantly worsens the disease prognosis1,4.
There has been intensive debate over the various treatment options for JPs, including surgical excision, radiotherapy and wait-and-scan3,5,6. Currently, due to a thorough understanding of the anatomic and surgical approaches of the skull base and various modified surgical approaches, technological improvements in microsurgery, neuromonitoring, and neuroradiology have made surgical removal of paragangliomas in the jugular foramen easier and safer7,8,9,10,11,12. According to the Fisch classification of temporal bone paragangliomas (TBPs)13,14, TBPs are traditionally classified into classes A, B, C, and D based on the location and extension assessed by high-resolution computed tomography (HRCT) and magnetic resonance imaging (MRI) of the temporal bone. The Fisch class A and B tumors can be treated surgically by skillful otologists with standard approaches with almost no complications, preserving the FN and inner ear function and offering the patient a complete cure15. Fisch class C or D JPs are still a great challenge to skull base surgeons, and cranial nerve injury is the main source of postoperative morbidity. In recent years, our group has developed two improved surgical techniques, tunnel packing or push packing of the inferior petrous sinus and tension-free FN anterior rerouting, that have resulted in significant control of bleeding from the inferior petrous sinus and improved postoperative FN function11,12. The purpose of this study was to compare the safety and effectiveness of surgical treatment of JPs between early-stage and advanced-stage tumors following the application of our modified surgical techniques and to propose a reasonable strategy to manage JPs.
Materials and methods
This was a retrospective review of 56 patients with JPs diagnosed and treated at the Eye Ear Nose and Throat Hospital of Fudan University (Shanghai, China) between October 2010 and January 2021. Patients who were managed with radiotherapy or wait-and-scan protocols were not enrolled in this study. This study was approved by the Ethics Committee of the Eye Ear Nose and Throat Hospital of Fudan University (No. 2021048). Our study involving human research participants had been performed in accordance with the Declaration of Helsinki. At our center, all patients underwent temporal-bone HRCT and gadolinium-enhanced MRI, and 24 of 56 patients underwent magnetic resonance arteriography (MRA) and magnetic resonance venography. Digital subtraction angiography was performed to identify the feeding arteries, as well as to assess ICA involvement and contralateral venous drainage, and then preoperative endovascular embolization was carried out on all patients to minimize intraoperative bleeding. Blood catecholamine levels were routinely evaluated preoperatively. The diagnostic work-ups for the postoperative evaluation of clinical signs associated with LCN deficits include laryngoscopy, shoulder and tongue movement. The Fisch classification was used for staging tumors13,14. Preoperative and postoperative FN function were graded according to the House-Brackmann grading system16.
The infratemporal fossa approach type A (IFTA-A) was employed in all the patients, and the surgical procedures have been described elsewhere14,17,18. The IFTA-A with either anterior transposition or nerve grafting of the FN was applied in the present study. If the epineurium was intact, we performed FN transposition. If the tumor invades the FN and the perineurium was infiltrated by the tumor, the FN was sacrificed and a simultaneous FN grafting is performed with the great auricular nerve. Several modifications were made to reduce FN tension during FN anterior transposition, which we defined as tension-free FN anterior rerouting12. (1) The FN, digastric muscle and parotid gland were anteriorly displaced without dissecting the FN within the parotid gland. The upper parotid gland was then sutured tightly to the lower margin of the temporal muscle to shorten the distance from the geniculate ganglion to the FN in the stylomastoid foramen. (2) A long articulated retractor was placed at a 45° angle to push the posterior belly of the digastric muscle and parotid gland anteriorly and superiorly to further shorten the distance of the facial nerve from the genicular ganglion to the stylomastoid foramen. To control bleeding from the inferior petrous sinus and to better preserve LCN function, the sigmoid sinus tunnel packing or push packing technique with surgical and intrabulbar dissection was applied intraoperatively 11,19.
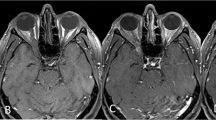
To identify whether the intratumor blood vessels were correlated with intraoperative blood loss and the Fisch classification of the tumor, preoperative MRA images were evaluated by a surgeon and a senior radiologist. In the axial plane, we selected the maximum cross-sectional area with intratumor blood vessels (Figs. 1, 2) and applied ImageJ software to measure the maximum cross-sectional area of intratumor blood vessels. We measured and summed the cross-sectional area of all intratumor blood vessels if there were multibranch arteries in the tumor.
The preoperative axial MRA image of patient with C3 JPs on the right. There are multiple intratumor blood vessels and intraoperative blood loss was 600 ml. (A) The black star indicates the internal carotid artery. (B) The red areas represent the maximum cross-sectional area of intratumor blood vessels.
The preoperative axial MRA image of patient with C2 JPs on the right. There is only one intratumor blood vessel and intraoperative blood loss was 100 ml. The black star indicates the internal carotid artery. (A) The white arrow indicates intratumor blood vessel. (B) The red area represents the maximum cross-sectional area of intratumor blood vessel.
Intraoperative management of the FN, LCNs and inferior petrosal sinus was noted, and blood loss and surgical complications were reviewed. Gross total resection (GTR) and recurrence were assessed by gadolinium-enhanced MRI. Follow-up duration was defined as the period from surgery to the most recent office visit or patient contact. Follow-up included physical examination and temporal-bone MRI with enhancement. MRI with enhancement was usually performed at a minimum of 3 months postoperatively and annually thereafter in our hospital.
In addition, to analyze the efficiency of surgical treatment of classes C1, C2, C3 and D, we pooled C1-4 De1-2/Di1-2 tumors as class D tumors. We defined C1 and C2 tumors as the early class and C3 and D tumors as the advanced class. The Chi-square test was applied to compare surgical outcomes among different groups using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Spearman’s correlation was used to analyze the correlation between the Fisch classification of the tumor, intraoperative blood loss and the maximum cross-sectional area of the feeding artery. P-values < 0.05 were considered to be statistically significant.
Ethics declarations
Our study involving human research participants had been performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all subjects and/or their legal guardian(s).
Results
Demography
A total of 56 patients with JPs underwent surgery at our center between October 2010 and January 2021. Of the 56 patients, the mean age of the cohort was 45.5 years, and 18 (32.1%) patients were male and 38 (67.9%) were female. Eight patients presented with bilateral or multiple HNPs, including two with ipsilateral carotid body tumor (CBT), four with ipsilateral vagal paragangliomas (VP), and one with contralateral CBT. In one patient, JPs coexisted with bilateral CBT, while in another patient, JPs coexisted with contralateral CBT and adrenal pheochromocytoma. No patient was diagnosed with malignant JP in this cohort. The mean follow-up duration was 29.5 months (range, 6–94 months).
Clinical signs and symptoms
The clinical features of patients with JPs are summarized in Table 1. The most common symptoms were hearing loss (43 patients, 76.8%) and pulsatile tinnitus (42 patients, 75%). Fifteen (26.8%) patients presented with FN palsy and 20 patients (35.7%) presented with LCN palsy. Fifty patients (89.3%) had a red tumor behind the eardrum or in the external auditory canal. All patients had one or more symptoms at the initial diagnosis.
Classification of tumors
In the present study, 4 (7.1%) patients were classified as C1 according to the Fisch classification, 25 (44.6%) as C2, 14 (25%) as C3, and 13 (23.2%) as D. Of them, 7 (12.5%) cases were of extradural extension and 6 (10.7%) were of intradural extension.
FN management and FN function
All 56 tumors were removed via IFTA-A. The preoperative, postoperative FN function and intraoperative management of FN were shown in Table 2. There was no significant difference of FN between early-stage and advanced-stage tumors either preoperatively or postoperatively. Thirty-four patients with HB-I FN function preoperatively were confirmed with intact epineurium intraoperatively. Nineteen out of 34 patients were of C1-2, 15 patients were of C3-D. Of them, 17 patients (89.5%) had preserved good FN function (HB I–II) in classes C1 and C2 tumors postoperatively, while 14 patients (93.3%) had good FN function (HB I–II) in classes C3 and D tumors. There was no significant difference in FN function between the two groups (χ2 = 0.155, P = 0.694) (Table 2).
Preoperative and postoperative LCN dysfunction
Fourteen patients (25%) suffered from at least one LCN deficit in the preoperative evaluation. The presence of preoperative LCN deficits was correlated with the Fisch classification of tumors (Table 3), which showed a lower incidence of LCN dysfunction in classes C1 and C2 (3 cases, 10.3%) and poorer outcomes of LCN dysfunction in classes C3 and D (11 cases, 40.7%) (χ2 = 5.364, P = 0.021). The presence of postoperative LCN deficits in classes C3 and D (15 cases, 55.6%) was obviously higher than that in classes C1 and C2 (7 cases, 24.1%) (χ2 = 5.364, P = 0.033). Furthermore, CN X was the most commonly affected LCN, followed by CN XII.
Correlation among Fisch classification of tumors, intraoperative blood loss and the maximum cross-sectional area of intratumor blood vessels
The estimated blood loss averaged 502 ml (100 to 2500 ml) in 56 patients. The mean intraoperative blood loss was 358.6 ml in class C1–2 tumors and 657.4 ml in class C3 and D tumors respectively. More intraoperative blood loss was not statistically correlated with advanced Fisch classification (r = 0.404, P = 0.050). Further study showed that the cross-sectional area of intratumor blood vessels was positively associated with intraoperative blood loss (r = 0.791, P < 0.001); the greater the cross-sectional area of intratumor blood vessels was, the more the intraoperative blood loss would be. However, a more advanced Fisch classification was not correlated with the maximum cross-sectional area of intratumor blood vessels (r = 0.285, P = 0.177) (Table 4).
Tumor removal, residuals and recurrences
Gross total tumor removal was achieved in 47 cases (83.9%), and subtotal removal was achieved in 9 cases (16.1%). The GTR rate in classes C1 and C2 (100%) was significantly greater than that in classes C3 and D (66.7%). Three patients complained of recurrence, including two patients with Class D tumors, and one with C3 tumors. One patient underwent surgery again. The other 2 patients treated with radiotherapy reported no tumor progression during follow-up.
Complications
One patient suffered from postoperative cerebrospinal fluid (CSF) leakage and underwent an additional procedure to repair the CSF leakage. Two patients had wound infections, which were successfully treated after surgical debridement. Two patients developed mild cerebral infarction postoperatively and recovered after conservative medical treatment without sequelae. No patients underwent tracheotomy or nasal feeding postoperatively. There were no perioperative or postoperative vascular injuries or deaths in the present series.
Discussion
Although considered histologically benign, surgical management of JPs is challenging due to their infiltrative nature and proximity to important neurovascular structures. The Fisch class A and B tumors can be treated surgically by skillful otologists with standard approaches with rare complications, preserving the FN and inner ear function and offering the patient complete cure15,20. The management of the Fisch class C and D tumors remains controversial, and there are still various opinions, such as surgery, radiotherapy or wait-and-scan3,6,21,22,23,24,25,26. This study retrospectively analyzed the clinical efficacy of surgical treatment of Fisch class C and D tumors in our institution during the past 10 years following the application of our modified surgical techniques and concluded that surgical treatment of JPs is a safe and effective strategy, especially for C1 and C2 tumors.
In the case of surgical management of JPs, IFTA-A, with the permanent anterior rerouting of the FN and exposure of the infratemporal course of the ICA as described by Fisch in 1977, is considered as the standard procedure. Due to permanent anterior FN transposition, the FN might lose most of its extrinsic vascularity, resulting in a certain degree of facial paralysis after surgery. Advances in neuroimaging, skull base techniques and modified surgical approaches have markedly reduced the incidence of FN dysfunction during the last two decades7,8,9,11,12,27; however, a majority of surgeons are still concerned about postoperative FN function. In this study, preoperatively, six patients (20.7%) of classes C1 and C2 had HB grade III–VI, while nine (33.3%) had HB grade III–VI in classes C3 or D. Although there was no significant difference in FN function between the two groups, the incidence of facial paralysis in classes C3 and D were greater than that in classes C1 and C2 preoperatively. Thirty-four patients with normal FN function preoperatively and the epineurium were identified as intact intraoperatively in our study. They underwent tension-free FN anterior rerouting, and 31 cases (91.2%) achieved good facial nerve function (House-Brackmann grade I–II), which indicated that good FN function was achieved in more than 90% of patients who underwent tension-free FN anterior rerouting. Wang et al. reported a postoperative rate of new FN deficits of 51.7% in eighty-nine patients with Fisch class C or D JPs28. In a recent study, 185 patients with Fisch class C or D JPs were treated surgically by Prasad and colleagues, and forty-three (23.2%) new FN deficits (House-Brackmann grade III–VI) were observed as a consequence of FN mobilization in IFTA-A3. In the present study, 31 patients (91.2%) achieved good facial nerve function following the application of tension-free FN anterior rerouting. The tension-free FN anterior rerouting technique significantly reduced the incidence of new FN deficits.
In our clinical practice, achieving optimal exposure of the jugular foramen while minimizing the damage to neurovascular structures and obtaining proximal and distal exposures of the ICA are the key points. The management of FN plays an important role in exposing of the jugular foramen. FN management strategies are included in the fallopian bridge technique and total or partial tension-free FN anterior rerouting technique, which are determined according to the extent of the lesion shown on temporal bone CT and MRI preoperatively. In the present study, most of the patients underwent tension-free FN anterior rerouting in cases of normal facial nerve function preoperatively, thereby reducing the incidence of FN dysfunction, and 31 patients (91.2%) achieved good facial nerve function postoperatively.
Given highly vascularized tumors, heavy intraoperative bleeding frequently occurs during the removal of JPs. To reduce intraoperative bleeding, preoperative super-selected embolization of the main feeding arteries of the tumor is routinely performed on patients with JPs. In addition, effectively controlling intraoperative bleeding from the inferior petrous sinus can provide a clearer surgical field and decrease the incidence of neurovascular structure damage. In the present study, we applied our modified technique, the sigmoid sinus tunnel packing and push packing technique, to control bleeding from the inferior petrous sinus11, which led to a mean blood loss of 502 ml.
Function preservation of LCNs is another key point that draws attention to the discussion of JP management options. The intrabulbar dissection technique was proposed in 2002 and was routinely applied to dissect tumors in our clinical practice as long as the tumor itself had not penetrated the medial wall of the jugular bulb or infiltrated the LCNs19. Prasad et al. noticed that the presence of intradural extension is usually associated with infiltration of the LCNs3. Grinblat et al. reported that early and limited C1 and C2 tumors without medial wall invasion reduces the risk of dysfunctionality of LCNs29. Thus resection of early JPs (classes C1-C2), where the lateral aspect of the jugular bulb is involved and the medial wall is not infiltrated, makes preservation of the LCNs possible. Our investigation also revealed a lower incidence (3 cases, 10.3%) of LCN dysfunction in classes C1 and C2 and a greater incidence (11 cases, 40.7%) in classes C3 and D preoperatively. Furthermore, surgery resulted in newly developed LCN deficits in 14.3% of patients. In addition, conservative surgical management of JPs with targeted subtotal resection based on the extent and location of tumors yields lower new-onset LCNs21,23,30. That is, the more tumor that is removed, the less likely the residual tumor is to grow31. Bacciu et al. demonstrated that class C1 and C2 tumors can be removed completely using IFTA-A with fewer LCN deficits32. Our results were consistent with a previous study, and GTR (100%) was able to achieve fewer postoperative new LCN deficits (4 cases, 13.8%) in classes C1 and C2. Therefore, radical excision is recommended for early-stage JPs to reduce the incidence rate of LCN deficits and prevent tumor recurrence instead of subtotal resection.
It has also been demonstrated that MR arteriography is not only useful to visualize the major feeding arteries of paragangliomas but is also helpful in the detection and characterization of paragangliomas33. Our study retrospectively reviewed the patient's preoperative MRA data and analyzed the correlation between the Fisch classification of tumors, intraoperative blood loss and the maximum cross-sectional area of intratumor blood vessels. We concluded that the advanced the more tumor class, the more intraoperative blood loss and the greater the cross-sectional area of intratumor blood vessels. However, there was no significant correlation between the Fisch classification of tumors and the maximum cross-sectional area of intratumor blood vessels. It is well known that the Fisch classification is based on the relationship of the tumor location with the ICA and intracranial extension, rather than the size of the tumor. The Fisch classification of tumors was positively correlated with intraoperative blood loss, indicating more bleeding in advanced tumors, resulting in unclear surgical fields, less GTR and increased injury of neurovascular structures. Therefore, we recommend early surgical intervention to reduce the incidence of perioperative complications and improve the GTR rate.
In recent years, radiotherapy has become popular as the first-line treatment for JPs due to its high tumor control and low morbidity compared with the surgical management of JPs5,22,34. Recently, Patel and colleagues reported a series of 40 cases with JPs treated by linear accelerator stereotactic radiosurgery22. Similar to the results of previous radiotherapy studies, the authors did not classify tumors according to the Fisch classification but preferred to report them based on tumor size. Considering that the mean pretreatment volume size was 8.9 cm3 (range 3.8–13.1 cm3), most of them were probably small tumors (possibly even class B tympanomastoid paragangliomas according to the Fisch classification), which can be treated surgically by skillful otologists with standard approaches with almost no complications and achieve gross total tumor removal. With improvements in surgical techniques and perioperative management, the incidence of cranial nerve injury and serious complications caused by surgical treatment has gradually decreased. However, the unique complications of radiotherapy cannot be avoided, such as temporal bone necrosis, brain necrosis, secondary malignancy and pituitary/hypothalamic insufficiency34. The most significant effect of radiotherapy is related to radiation‑induced fibrosis with obliteration of the vascular supply and not to direct destruction of tumor cells. Thus, it is important to re‑emphasize that radiotherapy only achieves tumor control and not a cure. Jansen et al. noted that the age of presentation was a risk factor for tumor growth, and 59 patients with JPs who underwent wait-and-scan with a median follow-up of 63.6 months showed that the age of presentation was an independent predictor of tumor growth and had a significant inverse correlation with growth rates: the younger the age of presentation, the greater the growth rate6. It is essential to achieve cure rather than tumor control in those who have more than 30 years of life expectancy. If radiotherapy is considered in young patients, it also means life-long surveillance scans and the psychological burden caused by living with the knowledge of having a residual tumor. Long-term treatment (surgery, radiotherapy or a combined modality) outcomes of 93 Fisch class C and D tumors were evaluated by Jansen’s group in 2018. One independent predictor of treatment outcome was reported: if treatment was delayed until tumor growth occurred, the chance of functional recovery was lower21. Taken together, we consider it important to make an early diagnosis and perform surgery as early as possible. As outlined above, although the management of JPs is challenging, we believe that surgical gross total resection of the tumor remains the mainstay of treatment of JPs.
Conclusion
The low incidence of tumor residuals, tumor recurrences, facial paralysis, LCN deficits and other surgical complications in this study demonstrated the safety and efficacy of surgical excision of Fisch class C and D tumors, especially tumors in the early stage following the application of our modified surgical techniques. Consequently, we advocate that early surgical treatment is a good option for Fisch class C1 or C2 patients with no contraindications.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Offergeld, C. et al. Head and neck paragangliomas: Clinical and molecular genetic classification. Clinics (Sao Paulo) 67(Suppl 1), 19–28 (2012).
Lope, A. R., Sivalingam, S., Konishi, M., De Donato, G. & Sanna, M. Oncologic outcome in surgical management of jugular paraganglioma and factors influencing outcomes. Head Neck 35, 527–534 (2013).
Prasad, S. C. et al. Strategies and long-term outcomes in the surgical management of tympanojugular paragangliomas. Head Neck 38, 871–875 (2016).
Forbes, J. A. et al. Jugulotympanic paragangliomas: 75 years of evolution in understanding. Neurosurg. Focus 33, E13 (2012).
Lloyd, S., Obholzer, R. & Tysome, J. British skull base society clinical consensus document on management of head and neck paragangliomas. Otolaryngol.-Head Neck Surg. 163, 400–409 (2020).
Jansen, T., Timmers, H., Marres, H. & Kunst, H. Feasibility of a wait-and-scan period as initial management strategy for head and neck paraganglioma. Head Neck 39, 2088–2094 (2017).
Bacciu, A. et al. Management of the cervico-petrous internal carotid artery in class C tympanojugular paragangliomas. Head Neck 38, 899–905 (2016).
Sanna, M. et al. Infratemporal fossa approach type a with transcondylar-transtubercular extension for Fisch type C2 to C4 tympanojugular paragangliomas. Head Neck 36, 1581–1588 (2014).
Bacciu, A. et al. Management of facial nerve in surgical treatment of previously untreated Fisch class C tympanojugular paragangliomas: Long-term results. J. Neurol. Surg. Part B Skull Base 75, 1–7 (2014).
Piazza, P. et al. Preoperative protective stenting of the internal carotid artery in the management of complex head and neck paragangliomas: Long-term results. Audiol. Neurotol. 18, 345–352 (2013).
Jang, M., Liu, H. & Dai, C. The application of sigmoid sinus tunnel-packing or push-packing of the inferior petrous sinus in the microsurgical management of jugular paragangliomas. Otol. Neurotol. 39, e166–e172 (2018).
Kong, D., Zhang, Y., Li, F., Li, W. & Dai, C. Tension-free anterior rerouting of the facial nerve in management of jugular foramen paragangliomas. Laryngoscope 131, 2684–2687 (2021).
Fisch, U. Microsurgery of the Skull Base. 149–152 (Thieme Company, 1988).
Fisch, U. Infratemporal fossa approach for glomus tumors of the temporal bone. Ann. Otol. Rhinol. Laryngol. 91, 474–479 (1982).
Medina, M. et al. The effects of tympanomastoid paragangliomas on hearing and the audiological outcomes after surgery over a long-term follow-up. Audiol. Neurootol. 19, 342–350 (2014).
House, J. W. & Brackmann, D. E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 93, 146–147 (1985).
Sanna, M., Piazza, P. & Shin, S.H. Microsurgery of Skull Base Paraganglioma. 226–343 (Thieme, 2013).
Fisch, U. Microsurgery of the Skull Base. 149–52 (Thieme Company, 1988).
Al-Mefty, O. & Teixeira, A. Complex tumors of the glomus jugulare: Criteria, treatment, and outcome. J. Neurosurg. 97, 1356–1366 (2002).
Patnaik, U. et al. Long term surgical and hearing outcomes in the management of tympanomastoid paragangliomas. Am. J. Otolaryngol. 36, 382–389 (2015).
Jansen, T. T. G. et al. Surgery, radiotherapy or a combined modality for jugulotympanic paraganglioma of Fisch class C and D. Clin. Otolaryngol. 43, 1566–1572 (2018).
Patel, A. K. et al. Long term outcomes with linear accelerator stereotactic radiosurgery for treatment of jugulotympanic paragangliomas. Head Neck 43, 449–455 (2021).
Nicoli, T. K., Sinkkonen, S. T., Anttila, T., Mäkitie, A. & Jero, J. Jugulotympanic paragangliomas in southern Finland: A 40-year experience suggests individualized surgical management. Eur. Arch. Oto-Rhino-L 274, 389–397 (2017).
Ivan, M. E. et al. A meta-analysis of tumor control rates and treatment-related morbidity for patients with glomus jugulare tumors. J. Neurosurg. 114, 1299 (2011).
Jansen, J. C. et al. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer-Am. Cancer Soc. 88, 2811–2816 (2000).
Jansen, T. T. G., Timmers, H. J. L. M., Marres, H. A. M., Kaanders, J. H. A. M. & Kunst, H. P. M. Results of a systematic literature review of treatment modalities for jugulotympanic paraganglioma, stratified per Fisch class. Clin. Otolaryngol. 43, 652–661 (2018).
Harati, A. et al. Microsurgical treatment of large and giant tympanojugular paragangliomas. Surg. Neurol. Int. 5, 179 (2014).
Wang, Z., Zhang, Z., Huang, Q., Yang, J. & Wu, H. Surgical management of extensive jugular paragangliomas: 10-year-experience with a large cohort of patients in China. Int. J. Surg. 11, 853–857 (2013).
Grinblat, G. et al. Comparison of lower cranial nerve function between tympanojugular paraganglioma class C1/C2 with and without intracranial extension: A four-decade experience. Otol. Neurotol. 43, e122–e130 (2022).
Manzoor, N. F. et al. Contemporary management of jugular paragangliomas with neural preservation. Otolaryngol.-Head Neck Surg. 164, 391–398 (2021).
Wanna, G. B., Sweeney, A. D., Haynes, D. S. & Carlson, M. L. Contemporary management of jugular paragangliomas. Otolaryngol. Clin. N. Am. 48, 331–341 (2015).
Bacciu, A. et al. Lower cranial nerves function after surgical treatment of Fisch class C and D tympanojugular paragangliomas. Eur. Arch. Oto-Rhino-L 272, 311–319 (2015).
van den Berg, R., Verbist, B. M., Mertens, B. J., van der Mey, A. G. & van Buchem, M. A. Head and neck paragangliomas: Improved tumor detection using contrast-enhanced 3D time-of-flight MR angiography as compared with fat-suppressed MR imaging techniques. AJNR Am. J. Neuroradiol. 25, 863–870 (2004).
Gottfried, O. N., Liu, J. K. & Couldwell, W. T. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg. Focus 17, E4–E30 (2004).
Funding
This study was funded by Shanghai Municipal Key Clinical Specialty (No. shslczdzk00801).
Author information
Authors and Affiliations
Contributions
P.Z., C.D., Y.Z. and F.L. wrote the main manuscript text. C.D. and Y.Z. contributed to the conception of the study; D.D.K and Y.S.F prepared figures and tables. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, P., Zhang, Y., Lin, F. et al. Comparison of surgical outcomes between early and advanced class of jugular paragangliomas following application of our modified surgical techniques. Sci Rep 13, 885 (2023). https://doi.org/10.1038/s41598-023-27821-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27821-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.