Abstract
Very high-frequency oscillations (VHFOs, > 500 Hz) are more specific in localizing the epileptogenic zone (EZ) than high-frequency oscillations (HFOs, < 500 Hz). Unfortunately, VHFOs are not visible in standard clinical stereo-EEG (SEEG) recordings with sampling rates of 1 kHz or lower. Here we show that “shadows” of VHFOs can be found in frequencies below 500 Hz and can help us to identify SEEG channels with a higher probability of increased VHFO rates. Subsequent analysis of Logistic regression models on 141 SEEG channels from thirteen patients shows that VHFO “shadows” provide additional information to gold standard HFO analysis and can potentially help in precise EZ delineation in standard clinical recordings.
Similar content being viewed by others
Introduction
Epilepsy is a severe chronic neurological disorder that is mostly well-controlled using anti-seizure medication (ASM). Long-term seizure freedom can be achieved in the majority of patients. Unfortunately, despite considerable recent developments in ASM and well-adjusted pharmacological treatment, 30–40% of all patients remain drug-resistant with ongoing seizures1. Consequently, epilepsy surgery (either resective or neurostimulation) should always be considered to eliminate seizures in drug-resistant subjects suffering from focal epilepsy2. Nowadays, the best practice represents performing a curative resective surgical procedure aiming to remove brain tissue responsible for seizure genesis—the epileptogenic zone (EZ). To localize and delineate EZ, intracranial EEG (iEEG) is performed in selected patients eligible for resective epilepsy surgery3. Although iEEG is a powerful tool for the identification of irritative zone (IZ—brain regions producing interictal epileptic spikes) and actual seizure onset zone (SOZ—brain region with the very first ictal change of the EEG pattern), it is still somewhat limited in precise delineation of EZ which remains to be a grossly theoretical concept4. Importantly, the removal of SOZ is mostly, but not always resulting in a long-term seizure-free outcome5.
With EEG advances, it is currently possible to monitor brain activity with frequencies over 100 Hz. The recordings with amplifiers using high sampling frequency allow us to investigate interictal high-frequency oscillations (HFOs) which are EEG activities in the frequency range 80–500 Hz6. In combination with interictal spikes, HFOs have high specificity in indicating the SOZ7,8, and removal of interictal HFO-generating areas correlated with good postsurgical seizure outcomes6,9,10,11,12. Thus it seems that HFOs contain information that can optimize the diagnosis and treatment of epilepsy. However, using HFOs in an individual prognostication of seizure outcome is correct in 67% of patients only7. Recent studies suggest that within mesiotemporal regions, interictal very high-frequency oscillations (VHFOs) with frequencies over 500 Hz are more specific biomarkers in localizing SOZ, and possibly even EZ, than interictal HFOs or spikes13,14.
In spite of technological advancement, recordings with extremely high sampling frequencies that are necessary for VHFO identification are still too demanding and expensive to be routinely used in standard clinical settings. The aim of the present study is to investigate whether “shadows” of the VHFOs can be captured in the EEG containing frequencies up to 450 Hz. We hypothesized that it is possible to distinguish iEEG signals/channels with or without the presence of VHFOs, using recordings with a reasonable sampling frequency of 1 kHz.
Results
Patients
The whole database of all SEEG patients consisted of 103 patients. Two patients were not adults at the time of the surgery, 14 had non-resective surgery or were not operated on at all and 20 patients did not have a 2-year post-surgical outcome. The initial cohort consisted of 67 patients. 45 patients had poor outcome > Engel IB and were excluded in order to analyze only channels which resection would be valuable, ensuring that the proposed methods would aid better postoperative results. Consequently, two patients did not have clearly localized SOZ, one patient had no overlap between SOZ and resection, four patients were not implanted in the hippocampus, and in two patients no VHFOs were detected. The selection flowchart is shown in Fig. 1. In the final 13 selected patients (8 females, age 36.63 ± 12.10; 5 males, age 40.40 ± 5.99), data from 141 contacts positioned in the hippocampus or uncus gyri parahippocampalis or amygdala was investigated, as described in Table 1.
SEEG channels manual classification
Raw SEEG recordings with a sampling rate of 5 kHz were manually checked for the presence of HFO and VHFO. Channels with HFOs and VHFOs were marked as VHFO channels. Channels with HFOs in ripple (R) or fast ripple (FRs) range and without the presence of VHFOs were marked as HFO channels. Channels without any HFO activity were removed from further processing. Consequently, 50 VHFO SEEG channels and 91 HFO SEEG channels were downsampled to 1 kHz and the following features were calculated: Amplitude Maximum, 75th Percentile of power spectral density, Shannon Entropy, and Teager–Kaiser Energy Operator (TKEO).
Differences between VHFO and HFO channels
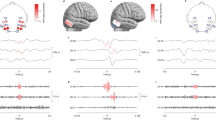
There were significant differences between VHFO and HFO channels in all of the features calculated from undersampled 1 kHz SEEG channels (Fig. 2). Calculated p values after Bonferroni correction and effect sizes are concluded in Table 2. When filtered in different traditional EEG frequency bands, the results show significant differences between VHFO and HFO channels in frequencies mainly above the Theta band. The p values after Bonferroni correction calculated in 7 different frequency bands are summarized in Table 3.
HFO rates
Furthermore, statistically significant differences were proven between the ripple rates of VHFO and HFO channels (p = 0.0007, large Cliff’s delta = 0.4798) and between the fast ripple rates of VHFO and HFO channels (p = 0.0001, large Cliff’s delta = 0.5176) calculated from data with sampling frequency 5 kHz (Fig. 3).
Logistic regression
The accuracies and F1 scores of logistic regression models trained on ripple rates detected on 5 kHz data (Model 1), described features (Model 2), and HFO rates detected on 5 kHz and features combined (Model 3) are summarized in Table 4. The p-values of the Wilcoxon rank-sum test after Bonferroni correction between accuracies and F1 scores are significant for Model 1 and Model 2, and Model 1 and Model 3, while the p values are insignificant for Model 2 and Model 3.
Discussion
Recently two independent research groups published influential studies suggesting an extraordinary role of VHFOs (500–2000 Hz) in human SEEG recordings for more precise delineation of the epileptogenic zone. These oscillations seem to be preferentially observed within mesiotemporal regions and were promisingly described as more specific biomarkers than ripples and FRs13,14. Similarly to red and green spikes, where the red spike zones are recognized as zones generating seizures and the green spike zones are recognized as zones generating only sporadic, isolated, spatially confined discharges15, the examined channels could be divided into two groups of VHFO and HFO channels based on the VHFO information. The division could possibly resolve which HFOs are physiological, seemingly the HFO channels, and which HFOs are not physiological—the VHFO channels. However, high-quality, high-resolution, and low-noise recordings are critical for the identification of VHFOs. These requirements usually cannot be fulfilled in traditional clinical settings and, therefore, practical use of VHFO detection in clinical practice can be hardly expected in the near future. Thus, a question arose if the presence of VHFO could be estimated from standard SEEG recordings (sampled at 1 kHz) utilizing any other specific feature/s.
Our study investigated 1 kHz SEEG recordings, manually assessed in section Methods—SEEG channel selection and manual classification as with/without VHFOs, for shadows of VHFOs in lower frequencies, focusing on the possibility of determining their presence or absence.
This study included 141 SEEG recordings, from a cohort of 13 patients, undersampled from 5 to 1 kHz with a focus on VHFOs. 126 out of the total 141 (89%) of the contacts with present VFRs and/or UFRs were contacts that were also resected in the surgery. Contacts that were not resected were found in four patients: patient 15 contact C′2, patient 26 contacts B1–3, C1–2, patient 71 contacts B1–5 and C′1–2, and patient 82 contacts C1–2 (Table 1). Even if the contacts were not resected they were still used in the analysis and are included in the results.
Our results suggest that based on the calculated features it is feasible to differentiate between HFO and VHFO channels even in lower sampling frequencies. The calculated features, Maximum amplitude, Shannon entropy, and TKEO were calculated in the time domain of a signal while the 75th percentile was calculated from power spectral density estimates proposing that VHFOs to some extent directly affect the power spectrum below 450 Hz of the recorded signal. When the recordings were filtered into seven distinctive frequency bands, bands above theta also show significant differences in Amplitude Maximum, 75th percentile, and TKEO features, suggesting that VHFO activity affects the signal recording in certain frequency bands. On the contrary, Shannon Entropy is non-significant in most of the seven respective ranges, providing significant values in theta, alpha, and ripple bands. Shannon Entropy is expressing a value unrelated to the remaining features, describing the information and variability of the signal. Filtered EEG signal becomes more deterministic and causes the entropy to lower even in HFO channels, which have significantly higher Shannon Entropy than VHFO channels, causing the medians of these two groups to overlap after the filtration into narrow frequency ranges. (Table 3, Supplement Fig. 1).
Alternatively, the significant differences could be justified by the presence of HFO—ripples and fast ripples. The analysis of HFO rates and the non-significant p values validates that the HFO rates detected on the original 5 kHz are sufficient to differentiate between HFO and VHFO channels. Accuracies of the three trained models confirm that the proposed features bring new information compared to rates of HFOs only as they improve with the inclusion of the proposed features. The model trained on the calculated features and HFO rates has the best accuracy and F1 score, while the model trained in HFO rates has lower accuracy and F1 score than the model trained on feature values. The p-value of the Wilcoxon rank-sum test after Bonferroni correction between accuracies of proposed models describes significant differences between accuracies of Model 1 and 2, and Model 1 and 3, and no significant differences were confirmed between accuracies of Model 2 and 3. To conclude, shadows of VHFOs are present in lower frequencies of the SEEG recordings. The results suggest that it is possible to distinguish SEEG signals with or without the presence of VHFOs, using recordings with a sampling frequency of 1 kHz. The differences between the accuracies and F1 scores also suggest that our features provide additional information to traditional ripple and fast ripple rates. Introducing a computation of Amplitude Maximum, 25th Percentile, Shannon Entropy, and Teager–Kaiser energy operator into the analyses of SEEG recordings with standard sampling frequency improves our ability to differentiate HFO and VHFO channels. The determined VHFO channels could facilitate the resection and potentially improve epilepsy surgery outcomes14.
Methods
Patients
The selected patients were all adults with drug‐resistant focal epilepsy who underwent stereoelectroencephalography (SEEG) and subsequent resective surgery with ≥ 2 years follow-up at the St. Anne's University Hospital since 2012 when we started with SEEG recordings at a 25 kHz sampling frequency. Inclusion criteria for our study were: (a) good post-surgical outcome defined as Engel IA or IB16; (b) identified SOZ contacts; (c) overlap between SOZ and resection; (d) hippocampus, parahippocampal gyrus, or amygdala implanted; (e) detected VHFOs in selected SEEG electrodes. The study was approved by the St. Anne’s University Hospital Research Ethics Committee and the ethics committee of Masaryk University. The study was carried out in accordance with relevant guidelines and regulations. All patients signed an informed consent form.
SEEG recordings
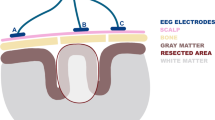
For depth SEEG recordings, standard intracerebral multi-contact platinum electrodes (5, 8, 10, 12, 15, and 18 contacts) with a diameter of 0.8 mm, a contact length of 2 mm, an intercontact distance of 1.5 mm, and a contact surface area of 5 mm2 were used in all patients. Each patient received 5–13 orthogonal SEEG electrodes in the temporal and/or frontal, parietal, and occipital lobes using the stereotaxic coordinate system of Talairach17. Their position within the brain was verified using MRI with electrodes in situ. A 192-channel research EEG acquisition system (M&I; BrainScope, Prague, Czech Republic) was used for recording 30 min of awake resting interictal EEG recordings with a sampling rate of 25 kHz and dynamic range of 625 mV with 10 nV (24 bits) and downsampled to 5 kHz with a 2 kHz frequency band. The EEG acquisition unit was battery-powered to reduce line noise. No special shielded environment was used. Thirty minutes of artifact-free continuous interictal SEEG data (recorded during wakefulness) was analyzed for each subject. All recordings were acquired using a referential earlobe reference. For analysis, the signals were subtracted from an averaged signal from all SEEG signals. All data processing and statistics were performed using open platform software SignalPlant18 and a Python environment.
Preprocessing
Artifact elimination
To detect artificial segments in the individual recordings, SignalPlant visualization of raw signals simultaneously with Power Distribution Matrices (PDMs) was used18.
Artifact detection is crucial for the computation of numerical parameters and statistical evaluation. PDMs provide a complete overview, including artificial signals. SignalPlant visualization of the raw signal simultaneously with PDMs was used to manually identify noisy contacts or time periods with artifacts. Artifacts were detected in each frequency band in PDM format. The number of detected artifacts was the highest in the VFR and UFR bands. Artificial segments and contacts were removed from further processing. The same set of all deselected contacts was used for the R, FR, VFR, and UFR bands14.
SEEG channel selection and manual classification
Firstly, channels that were located in anatomical structures outside the hippocampus, parahippocampal gyrus, or amygdala, were eliminated from the analysis and were not further considered for visual identification.
The remaining channels were examined for the presence of HFOs and VHFOs (VFRs/UFRs) using visual inspection of graphical PDMs filtered in four different frequency bands (ripples, 80–200 Hz; fast ripples, 200–500 Hz; very fast ripples VFR, 500–1000 Hz; and ultrafast ripples UFR, 1000–2000 Hz). Referred 2-dimensional PDMs consist of rows corresponding to smoothed power estimates (PEs) of respective recorded contacts and of columns corresponding to time intervals. The PDMs were calculated according to the algorithm described in14. Based on the reviewed PDMs, 141 channels were selected as channels that showed elevated PEs in ripple and fast ripple frequency bands. These channels’ PEs were subsequently analyzed in VFR and UFR frequency bands. If the channel’s PE was elevated above other channels’ PEs, the channel was subsequently inspected in 5 kHz raw data format for the occurrence of visible oscillations. Identification of contacts with HFOs and VHFOs was blind, without any a priori knowledge of the resected areas and postsurgical outcome (Z.V., P.K., P.J.).
HFO rates
An automated HFO detection based on a line-length algorithm19 with the threshold set to 6 was used to estimate the HFO rates in all selected channels. The patient HFO rates were min–max normalized and then compared in HFO and VHFO channels in order to test whether it is possible to differentiate between these two groups of channels solely on the information on the HFO rates. The HFO rates were detected in the original signals with a sampling frequency of 5 kHz. HFO rates were not detected on 1 kHz data as it is not feasible to reliably detect FR rates due to sampling frequency constraints.
Signal processing
For further processing, all recordings were filtered in the 0–450 Hz range and downsampled from 5 to 1 kHz. Subsequently, multiple features were calculated in a 10-s sliding window moving through the 30 min long recording with an overlap of 50%. The single feature value representing the 30 min long patient channel recording is a median of values of all windows of the recordings. All of the features were calculated on downsampled signals with 0.5–450 Hz frequency band as well as in traditional frequency bands: delta (0.5–4 Hz); theta (4–7 Hz); alpha (8–12 Hz); beta (14–30 Hz); lower gamma (30–45 Hz); upper gamma (55–80 Hz); ripples (80–200 Hz). The frequency range 45–55 Hz was omitted on purpose to avoid the negative effects of the power line hum.
Calculated features
Amplitude maximum
Absolute instantaneous maximum of the signal’s amplitude was estimated using an envelope determined by Hilbert’s transform as envelope = abs(H(x)), where abs is the absolute value, and the final maximum values were calculated as max(abs(envelope))20,21.
Shannon entropy
Shannon (information) entropy was developed as a unified definition of information-theoretic divergence. It is one of the applicable entropy measures that measure the spread of the data and describe the variability in the EEG signal. High values correspond with a flat probability distribution (typical for noise or random signal) and on the other hand, low values correspond with a narrow probability distribution (typical for deterministic signal). Shannon entropy is defined as H = − sum(p(i)log2(p(i))), where the p are probabilities of a raw signal datum being in a histogram bin22,23.
Teager–Kaiser energy operator
Teager–Kaiser energy operator (TKEO) is a nonlinear operator determining the instantaneous energy of a non-stationary signal. TKEO is for discrete signal defined as Ψ(x(n)) = x2(n) − x(n + 1)x(n − 1), where x is the signal value and n is the sample number24,25.
75th Percentile
The 75th percentile is a statistical value of power spectral density estimate. In order to estimate the power spectral density, a nonparametric method of Welch periodogram was used. Welch’s method consisted of dividing the 5-min time series window into segments of length L = 256 with 50% overlap covering the entire window. Afterward, a modified periodogram for each of these segments is calculated, and periodograms for each segment are obtained using the finite Fourier transform. The final spectral estimate of the 5-min window is the average of these periodograms26. The final feature value is the 75th percentile of this power spectral density estimate.
Statistical analyses
Analysis of the relationship between HFO and VHFO channels was carried out by Wilcoxon rank-sum test for two samples of min–max patient-wise normalized data. As an additional statistic, Cliff’s delta calculated the effect size. Wilcoxon rank-sum test for two samples and Cliff’s delta statistics were also used to describe the relationship between HFO ripple and FR rates. Results with p ≤ 0.01 and large Cliff’s delta were considered significant.
Logistic regression
To test whether our proposed features can differentiate between HFO and VHFO channels and whether they provide additional information to traditional HFO rates, three different logistic regression models were created using all available channels in a cross-validation procedure with 3 splits and 15 repeats. Model 1 used two features: normalized ripple and fast ripple rates detected from 5 kHz recordings. Model 2 used 4 features: Amplitude Maximum, 75th Percentile, Shannon Entropy, and TKEO, calculated from undersampled 1 kHz recordings.
Finally, Model 3 used 6 features: combining ripple and fast ripple rates from 5 kHz data with the calculated features from 1 kHz data. Mean accuracy was calculated for each model separately.
Additionally, the Wilcoxon Rank-sum test of the differences between the accuracies of the proposed models was applied to each possible model pair in the two sets of models in order to test if the difference in accuracies between models was significant.
Data availability
Data is available upon reasonable request to the corresponding author.
References
Schuele, S. U. & Lüders, H. O. Intractable epilepsy: Management and therapeutic alternatives. Lancet Neurol. 7, 514–524 (2008).
Jacobs, J. et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog. Neurobiol. 98, 302–315 (2012).
Ryvlin, P., Cross, J. H. & Rheims, S. Epilepsy surgery in children and adults. Lancet Neurol. 13, 1114–1126 (2014).
Jehi, L. The epileptogenic zone: Concept and definition. Epilepsy Curr. 18, 12–16 (2018).
Boling, W., Aghakhani, Y., Andermann, F., Sziklas, V. & Olivier, A. Surgical treatment of independent bitemporal lobe epilepsy defined by invasive recordings. J. Neurol. Neurosurg. Psychiatry 80, 533–538 (2009).
Zijlmans, M. et al. How to record high-frequency oscillations in epilepsy: A practical guideline. Epilepsia 58, 1305–1315 (2017).
Jacobs, J. et al. Removing high-frequency oscillations: A prospective multicenter study on seizure outcome. Neurology 91, e1040–e1052 (2018).
Roehri, N. et al. High-frequency oscillations are not better biomarkers of epileptogenic tissues than spikes. Ann. Neurol. 83, 84–97 (2018).
Jacobs, J. et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann. Neurol. 67, 209–220 (2010).
Wu, J. Y. et al. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology 75, 1686–1694 (2010).
Akiyama, T. et al. Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia 52, 1802–1811 (2011).
Gloss, D., Nevitt, S. J. & Staba, R. The role of high-frequency oscillations in epilepsy surgery planning. Cochrane Database Syst. Rev. 10, CD010235 (2017).
Usui, N. et al. Significance of very-high-frequency oscillations (over 1,000 Hz) in epilepsy. Ann. Neurol. 78, 295–302 (2015).
Brázdil, M. et al. Very high-frequency oscillations: Novel biomarkers of the epileptogenic zone. Ann. Neurol. 82, 299–310 (2017).
Serafini, R. Similarities and differences between the interictal epileptiform discharges of green-spikes and red-spikes zones of human neocortex. Clin. Neurophysiol. 130, 396–405 (2019).
Engel, J. Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992). Neurology 43, 1612–1617 (1993).
Talairach, J. et al. Atlas d’anatomie stéréotaxique du télencéphale; études anatomo-radiologiques (Masson, 1967).
Plesinger, F., Jurco, J., Halamek, J. & Jurak, P. SignalPlant: An open signal processing software platform. Physiol. Meas. 37, N38-48 (2016).
Gardner, A. B., Worrell, G. A., Marsh, E., Dlugos, D. & Litt, B. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin. Neurophysiol. 118, 1134–1143 (2007).
Hahn, S. L. Comments on “A tabulation of Hilbert transforms for electrical engineers”. IEEE Trans. Commun. 44, 768 (1996).
Polat, H. & Ozerdem, M. S. Epileptic seizure detection from EEG signals by using wavelet and Hilbert transform. In 2016 XII International Conference on Perspective Technologies and Methods in MEMS Design (MEMSTECH) 66–69 (IEEE, 2016). https://doi.org/10.1109/MEMSTECH.2016.7507522.
Lin, J. Divergence measures based on the Shannon entropy. IEEE Trans. Inform. Theory 37, 145–151 (1991).
Kannathal, N., Choo, M. L., Acharya, U. R. & Sadasivan, P. K. Entropies for detection of epilepsy in EEG. Comput. Methods Programs Biomed. 80, 187–194 (2005).
Kaiser, J. F. On a simple algorithm to calculate the “energy” of a signal. In International Conference on Acoustics, Speech, and Signal Processing 381–384 (IEEE, 1990). https://doi.org/10.1109/ICASSP.1990.115702.
Badani, S., Saha, S., Kumar, A., Chatterjee, S. & Bose, R. Detection of epilepsy based on discrete wavelet transform and Teager–Kaiser energy operator. In 2017 IEEE Calcutta Conference (CALCON) 164–167 (IEEE, 2017). https://doi.org/10.1109/CALCON.2017.8280717.
Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 15, 70–73 (1967).
Acknowledgements
The study was supported by the GACR project 22-28784S, the European Regional Development Fund—Project ENOCH No.CZ.02.1.01/0.0/0.0/16_019/0000868, and the CAS project RVO:68081731.
Author information
Authors and Affiliations
Contributions
All co-authors have been substantially involved in the study and/or the preparation of the manuscript. No undisclosed persons have had a primary role in the study. All co-authors saw and approved the submitted version of the paper and accepted responsibility for its content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasickova, Z., Klimes, P., Cimbalnik, J. et al. Shadows of very high-frequency oscillations can be detected in lower frequency bands of routine stereoelectroencephalography. Sci Rep 13, 1065 (2023). https://doi.org/10.1038/s41598-023-27797-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27797-9
This article is cited by
-
The high frequency oscillations in the amygdala, hippocampus, and temporal cortex during mesial temporal lobe epilepsy
Cognitive Neurodynamics (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.