Abstract
Salinity is one of the most important abiotic stresses that reduce plant growth and performance by changing physiological and biochemical processes. In addition to improving the crop, using nanomaterials in agriculture can reduce the harmful effects of environmental stresses, particularly salinity. A factorial experiment was conducted in the form of a completely randomized design with two factors including salt stress at three levels (0, 50, and 100 mM NaCl) and chitosan-salicylic acid nanocomposite at three levels (0, 0.1, and 0.5 mM). The results showed reductions in chlorophylls (a, b, and total), carotenoids, and nutrient elements (excluding sodium) while proline, hydrogen peroxide, malondialdehyde, total soluble protein, soluble carbohydrate, total antioxidant, and antioxidant enzymes activity increased with treatment chitosan-salicylic acid nanocomposite (CS-SA NCs) under different level NaCl. Salinity stress reduced Fm', Fm, and Fv/Fm by damage to photosynthetic systems, but treatment with CS-SA NCs improved these indices during salinity stress. In stress-free conditions, applying the CS-SA NCs improved the grapes' physiological, biochemical, and nutrient elemental balance traits. CS-SA NCs at 0.5 mM had a better effect on the studied traits of grapes under salinity stress. The CS-SA nanoparticle is a biostimulant that can be effectively used to improve the grape plant yield under salinity stress.
Similar content being viewed by others
Introduction
Salinity stress is one of the most important environmental stresses that threaten agricultural production worldwide1. Salinity hinders growth, photosynthesis, transpiration, and stomata conductance. It increases the reactive oxygen species (ROS) content in plant cells, resulting in ion poisoning and impaired ion homeostasis. Therefore, it causes an imbalance in nutrient uptake and destruction of various membranes leading to osmotic and ionic stress2. The absorption of toxic sodium and chlorine ions restricts nutrient uptake, transport, and distribution, resulting in a nutritional imbalance in the plant3,4,5. Salinity stress causes an ionic imbalance in the cell due to the overaccumulation of Na+ and Cl−, which reduces the uptake of nutrients such as nitrogen, potassium, calcium, magnesium, and manganese, thereby inhibiting plant growth6. The accumulation of Na+ and Cl− in leaves reduces the leaf photosynthetic area in plants, ultimately affecting plant performance7. ROS accumulation under salinity stress causes damage to photosynthetic pigments and chlorophyll degradation8,9. Many plants commonly react to salinity through the overproduction of a set of organic compounds to ameliorate its harmful effects. These compounds are carbohydrates, amino acids, and proteins that act as osmolytes to counteract stress10. In addition, plants containing dynamic antioxidant enzymes can reduce damage from environmental stressors. An efficient antioxidant system shows good tolerance to environmental stress and salinity11,12.
The effectiveness of salicylic acid (SA) in resistance to environmental stresses in plants has been documented in numerous studies. The effective application of SA in salinity stress of grapes cv. ‘Sultana’ led to significantly increased salinity tolerance by reducing the Na+/K+ ratio, leaf electrolyte leakage, MDA, and H2O2 and increasing proline and enzymatic activities (POD, APX, CAT, and SOD)13,14.
Chitosan acts as a biostimulant and a potential stimulant in agriculture. This non-toxic, biodegradable, and biocompatible substance reduces the adverse effects of abiotic stresses through the stress transfer pathway by secondary signaling15. In addition, chitosan upgrades several defensive genes in plants such as pathogenesis-related genes (glucanase and chitinase)16. Chitosan reduces the effect of salinity stress on plants and enhances plant growth by regulating cellular osmotic pressure by increasing the availability and uptake of water and essential nutrients17. Chitosan biopolymer has been used in plant growth and protection, particularly as a nano-coating for chemicals18,19. Treatment with chitosan improved the antioxidant potential in different tissues of V. vinifera. Antioxidant activity and antimicrobial compounds increased in different components of grapes treated with chitosan solution20. SA-functionalized chitosan nanoparticles have greater potential in improving plant immunity as they are involved in the transfer of plant signals19. CS-SA NCs were studied as a biostimulant to enhance plant defense and growth. The results showed that CS-SA NCs expressed considerable physiological-biochemical reactions in vitro and in vivo. These responses occur as high activities of antioxidant defense enzyme, modulation of ROS, reinforcement of cell wall by lignin deposition, disease control, and plant growth21. Considering that SA and CS have shown effective effects on plant growth, physiological, and biochemical parameters, especially anthocyanins and enzymes, the combination of nanoparticles (CS-SA NCs) may have a synergistic effect due to the effect of chitosan22 CS-SA treatments with the lowest encapsulated ratio reduced the toxicity of free SA on Arabidopsis thaliana. In this system, plants treated with capsule, CS-SA had more root and rosette growth than plants treated with free SA23.
Grape (Vitis vinifera L.) is a perennial plant belonging to the Vitaceae family that is widely cultivated around the world and is one of the most economically important fruit crops24. Grape growth and development are affected by abiotic stresses such as drought, salinity, extreme temperatures, chemical toxicity, and oxidative stress25. Salinity reduces grape biomass production and, ultimately, the death of the whole plant. Leaf, root, and shoot biomasses decreased in the grape plant under salinity stress26. A positive correlation between osmolytes accumulation and stress tolerance has been reported in several studies27. Fozouni et al.28 showed that in the response of four cultivars of rooted table grapes (Vitis vinifera L.) to different concentrations of salt, proline accumulation increased significantly with increasing salinity.
Based on previous research on the beneficial effects of SA and chitosan on abiotic stresses and the other hand the positive effect of nanocomposites, the response of grapes cv. ‘Sultana’ to salinity stress and the role of a CS-SA nanocomposite in reducing the effects of salt were studied through the antioxidant defense system, fluorescence chlorophyll, and ionic homeostasis.
Results
Chlorophyll fluorescence parameters
According to the results, chlorophyll fluorescence parameters were significantly affected by salinity stress and foliar application of CS-SA NCs at 1% and 5% probability (Table 1). The salinity stress and foliar application along with the CS-SA NCs on the photosynthetically active radiation (PAR) value revealed that the foliar application in 0 and 50 mM salinity treatments did not significantly affect the PAR value. The highest PAR values were observed in 0.5 mM of CS-SA NCs under 100 mM salinity. Our findings indicated that salinity stress and the foliar application of the nanocomposite did not significantly influence the minimum fluorescence value (F0). But, maximum fluorescence (Fm) decreased with increasing salinity stress and the mentioned treatments, so the highest Fm value was observed in the treatment with 0.5 mM of CS-SA NCs without salinity stress. Also, the application of CS-SA NCs (0.5 mM) at different salinity levels increased the electron transfer rate (ETR). Fm' decreased with enhancing salinity stress, but this reduction was significantly higher in unsprayed samples than in the plants treated by CS-SA NCs. Based on the obtained results, the highest maximum quantum efficiency of photosystem II (Fv/Fm) was observed in 0.5 mM of CS-SA NCs without salinity, and the lowest belonged to 100 mM salinity treatment and no foliar application (Table 1).
Chlorophyll and carotenoid content
The results showed that chlorophyll a, b, and total, and carotenoids content were significantly influenced by salinity stress and foliar application of CS-SA NCs at 1% and 5% probability (Table 2). Based on the results of the comparison of means, the highest chlorophyll a content (22.8 mg/g FW) was measured in without salinity along with 0.5 mM of CS-SA NCs, and the lowest was recorded in grape plants under 100 mM salinity and without any foliar application. also chlorophyll b content reduced using salinity stress that the maximum obtainedat 0.5 mM of CS-SA NCs in stress-free conditions, and the lowest content was observed in 100 mM salinity treatment and no CS-SA NCs foliar application. The application of CS-SA NCs (0.5 mM) at different salinity levels significantly increased the total chlorophyll compared to the other treatments. The foliar application significantly increased leaf carotenoid content and the highest was obtained from the plants sprayed with 0.5 mM of CS-SA NCs and stress-free conditions (Fig. 1).
Effect of CS-SA NCs on Chlorophyll a (A), b (B), total chlorophyll (C) and carotenoid (D) of grapevine cv. ‘Sultana’ under salinity stress. Means followed by the same letter on columns are not significantly different at 0.05 level, according to Duncan's multiple range test. Data are mean ± SD (n = 3 replicates).
Osmolytes and membrane stability
Based to the ANOVA, salinity stress and foliar application of CS-SA NCs had a significant impact on proline, MDA, carbohydrate, and electrolytes at 1% probability, while the effects were not significant on protein content (Table 3). The results showed that leaf proline content enhanced with increasing salinity stress. The 100 mM salinity and 0.5 mM of CS-SA NCs foliar application contained the highest proline content, and the lowest was observed in the control plants. Lipid peroxidation of the membrane boosted under salinity stress. The highest content of MDA belonged to the grape plants supplemented with 100 mM salinity and no foliar application treatments, and the lowest amount was found in the control. Increasing the concentration of CS-SA NCs foliar treatment improved the total soluble protein content. Electrolyte leakage increased with increasing salinity stress. Application of 0.5 mM CS-SA NCs caused a significant reduction in electrolyte leakage at 100 mM NaCl salt stress. Application of CS-SA NCs under salt stress increased the soluble carbohydrates in grape plants (Fig. 2).
Effect of CS-SA NCs on Proline (A), MDA (B), Total soluble protein (C, D), EL (E) and Soluble carbohydrate (F) of grapevine cv. ‘Sultana’. Means followed by the same letter on columns are not significantly different at 0.05 level, according to Duncan's multiple range test. Data are mean ± SD (n = 3 replicates).
Biochemical parameters
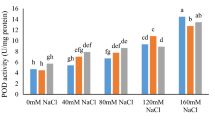
According to the findings, H2O2, enzyme antioxidant activity, and total antioxidant activity were significantly affected by salinity stress and foliar application of CS-SA NCs at 1% and 5% probability (Table 4). H2O2 levels were maximized at 100 mM salinity level without foliar application, and the lowest belonged to the control treatment. The GPX activity increased in the plants subjected to salinity stress, and the uppermost was observed in 100 mM salinity stress along with 0.5 mM of CS-SA NCs. The increasing salinity level led to a significant enhancement in SOD activity, and it increased at 0.5 and 0.1 mM of CS-SA NCs compared to the control treatment. Also, the APX activity showed a significant increase in plants treated with salinity stress, so that the utmost activity was showed in 0.5 mM of CS-SA NCs (Fig. 3).
Nutrients content
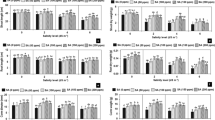
Based to the results, N, P, K, Mg, Zn, Fe content, and Na+/K+ were significantly modified by salinity stress and foliar application of CS-SA NCs at 1% and 5% probability (Table 5). According to the findings, the N content declined significantly with increasing salinity levels, while CS-SA NCs foliar application ameliorated it at the different salinity levels. The P content lessedd significantly with increasing salinity stress, and the maximum content belonged to 0.5 mM of the CS-SA NCs treatment without salinity; the 100 mM NaCl without foliar application contained the lowest P level. The results revealed that the K content was minimal in 100 mM salinity treatment without CS-SA NCs foliar application, and the highest K level was obtained at 0.5 mM of CS-SA NCs without NaCl treatment. The lowermost of Mg content was recordedin the 100 mM salinity treatment without foliar application, which reduced with enhancing the salinity concentration. With increasing salinity stress, the Fe content showed a significant reduction in the lowest level in 100 mM of NaCl stress. The Zn content decreased significantly in the grape plants exposed to salinity stress. The application of CS-SA NCs at 0.5 mM could effectively improve the Zn content of the grape leaves under salinity stress. At different levels of salinity stress, the Na+/K+ ratio declined significantly in the plants treated with the CS-SA NCs foliar application concentrations (Table 5). The salinity- CS-SA NCs interaction was not statistically significant on the Na+ content. The data showed that the Na+ content increased significantly with increasing salinity levels compared to the control treatment. Na+ content showed a significant decrease in 0.5 mM of CS-SA NCs compared to the control treatment (Fig. 4).
Multivariate analysis of Vitis vinifera cv. ‘Sultana’ under normal and salinity stress and CS-SA NCs treatments
Pearson’s correlations of chlorophyll fluorescence, photosynthetic pigments, antioxidant enzymes, and some biochemical traits are exhibited in Fig. 5. The findings showed that photosynthetic pigments, SOD, APX, N, P, K, Mg, Fm, Fm`, Fv/Fm positively correlated with each other, while significant negatively correlated EL, MDA, Na, Na+/K+, and H2O2. A negative significant correlation was recorded among EL, MDA, H2O2, proline, total soluble protein, and total soluble carbohydrate with N, P, K, and Mg content, but these traits positively correlated with Na, Na/k, APX, SOD, CAT, GPX, DPPH, Fm', ETR and PAR. A negative correlation was observed between Na and Na/K and other evaluated nutrients.
Heat map of Pearson’s correlation analysis for the response of Vitis Vinifera cv. ‘Sultana’ under salinity stress with application CS-SA NCs. Heat map representing of Chlorophyll a (Chl a), Chlorophyll b (Chl b), Total chlorophyll (Total Chl), Carotenoids (CARs), Electrolyte leakage (EL), Malondialdehyde (MDA), H2O2 content, Proline content, Carbohydrate (Carb), Total soluble protein content, Superoxide dismutase (SOD) activity, Ascorbate peroxidase (APX) activity, Guaiacol peroxidase (GPX) activity, catalase (CAT), total antioxidant (DPPH), nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), zinc (Zn), iron (Fe), sodium (Na), Na/K, (PAR), (Fm'), electron transport rate (ETR), maximal fluorescence (Fm), maximum photochemical quantum yield of photosystem II (Fv/Fm).
Heat map analysis based on the response of the plants to salinity and CS-SA NCs foliar application revealed that the traits including MDA, H2O2, EL, Na, Na/K, SOD, APX, PAR, ETR, DPPH, CAT, GPX, proline, total soluble protein, and total soluble carbohydrate had positive accordance to salinity stress, and on the other hand, the evaluated nutrient content, photosynthesis pigment, and fluorescence chlorophyll decreased with increasing salinity stress. The heat map analysis showed CS-SA NCs foliar application recovered the adverse effect of salinity stress by improving the physiological and nutritional traits (Fig. 6).
Heat map (a), loading biplot of the evaluated traits (b) and Principal component analysis heat map (C) of the enzymatic antioxidants pool, the biochemical changes, chlorophyll fluorescence and nutrient elements content in Vitis Vinifera cv. ‘Sultana’ under salinity stress with application CS-SA NCs. Heat map representing of Chlorophyll a (Chl a), Chlorophyll b (Chl b), Total chlorophyll (Total Chl), Carotenoids (CARs), Electrolyte leakage (EL), Malondialdehyde (MDA), H2O2 content, Proline content, Carbohydrate (Carb), Total soluble protein content, Superoxide dismutase (SOD) activity, Ascorbate peroxidase (APX) activity, Guaiacol peroxidase (GPX) activity, catalase (CAT), total antioxidant (DPPH), nitrogen (N), phosphorus (P), potassium (K), magnesium (Mg), zinc (Zn), iron (Fe), sodium (Na), Na/K, (PAR), (Fm'), electron transport rate (ETR), maximal fluorescence (Fm), maximum photochemical quantum yield of photosystem II (Fv/Fm).
Cluster analysis and dendrograms in the heat map (Fig. 6) showed three main clusters in the evaluated features of the plants under salinity stress and CS-SA NCs foliar application. Cluster I comprised MDA, H2O2, EL, Na, Na/K, SOD, APX, PAR, ETR, DPPH, CAT, GPX, proline, total soluble protein and total soluble carbohydrate; cluster II comprised nutrients content and Fm; cluster Ш included photosynthesis pigments, Fm' and Fv/Fm (Fig. 7). In general, cluster analysis of heat maps for salinity stress combined with CS-SA NCs indicated three classes. Class I contained the plants under 50 and 100 mM of NaCl with 0.5 mM foliar application of CS-SA NCs; Class II contained the plants treated with 50 and 100 mM of NaCl with 0.1 mM Cs-SA NCs foliar application, as well as the raised plants under 50 and 100 mM of NaCl with no-foliar application. Finally, class III included the grape plants under normal conditions which were sprayed by 0.1 and 0.5 mM CS-SA NCs and also the control plants (Fig. 6).
Discussion
The measurement of chlorophyll fluorescence is one of the important, simple, and non-destructive methods to evaluate photosynthetic efficiency. Plant response to salinity depends on the ability of PSII to respond to salinity stress29. Salinity reduces the quantum yield of the PSII electron transfer, the amount of light energy reaching the reaction center, and the complex involvement of oxygen. When plastoquinone (PQ) is oxidized under natural conditions, the electron transfer system has a minimum value of Fo. In salinity stress, however, Fo increases due to changes in the structure of the thylakoid membrane and damage to the PSII reaction centers30,31. The Fv/Fm index indicates the initial yield of photosystem II and acts as a stress indicator as it is sensitive to early plant responses to stress32. A decrease in the Fv/Fm index was reported in wheat under salinity stress33. Under stress conditions, reductions in Fm, Fv, and Fv/Fm can inhibit electron transfer from the PSII reaction center to electron transfer34. In sweet pepper, salinity stress influenced chlorophyll fluorescence parameters and caused a significant reduction in the maximum PSII yield (Fv/Fm). The useful role of chitosan was reported in increasing the production of protective metabolites, increasing the contents of N and K as well as the number of chloroplasts under stress, thereby improving the chlorophyll fluorescence parameter35. In a study of salinity stress on strawberry32 and sweet pepper35 induction of chlorophyll fluorescence of leaves increased with increasing salinity levels. Also, NPQ and F0 increased with increasing stress, but Fv/Fm and Fm decreased, which was consistent with our findings.
Based on the present results, a decrease in chlorophyll content was observed under salinity stress. Reductions of photosynthetic pigments under salinity stress may be caused by the deficiency in the leaf area responsible for light absorption and photosynthesis or may be due to chlorophyll degradation by increasing the activity of chlorophyll-degrading enzymes under salinity stress36,37. Other reasons for the reduction of photosynthetic pigments in salinity conditions include various types of ROS that cause chlorophyll degradation and damage to photosynthetic pigments8. According to our results, an increase in carotenoids with SA application was reported in tomato38, strawberry39, and myrtle (Catharanthus)40. Chitosan foliar application reduced the adverse effect of salinity stress by increasing the chlorophyll content. This increase was attributed to improvements in stomatal conductance, transpiration rate, and cell size and number41,42. Chitosan improved leaf chlorophyll content due to a higher nitrogen uptake, its transfer to leaves, and thus increasing chlorophyll pigments42. An increase in leaf chlorophyll content with the use of chitosan was reported in tomatoes43 and cucumber44. Our results in chlorophyll content under stress and chitosan treatment were similar to the results of other researchers43,44,45,46.
Proline protects cells by improving osmotic regulation, inhibiting ROS increase, and protecting the membrane structure47. Proline plays an essential role as an osmotic stabilizer as well as a stabilizer and protector of enzymes, proteins, and membranes48. An increase in proline content is an indicator of stress reduction47. In a study on salinity stress in tomatoes, proline content increased due to its role in the regulation and inhibition of ROS43. Similar to the results of our research, an increase in proline content was reported in chitosan-treated tomatoes43 and sunflower47 under salinity stress conditions. In this study, an increase in proline was observed in salicylic acid and chitosan treatments, which was consistent with the results of other researchers43,47,49. An increase in lipid degradation rate and MDA production was observed under stress conditions with the formation of ROS, leading to cell damage and destruction43, which corresponds to the present results. SA application in salinity stress reduced the amount of MDA in tomatoes50. Chitosan pretreatment under salinity stress increased the activity of antioxidant enzymes and reduced MDA levels and the negative effects of salinity stress43,51. SA application in stress conditions leads to the expression of genes in plants that produce proteins that activate signaling pathways and, ultimately, programmed cell death52. SA is reported to stimulate the synthesis of stress-related proteins by increasing nitrate reductase activity53 and increasing the content of abscisic acid54. Chitosan treatment also increased the content of total soluble proteins due to its role in increasing the expression of enzymes involved in glycolysis55,56. The increase in the production of malondialdehyde and decrease in degradation of lipids with the application of nano-chitosan-salicylic acid in the present results were consistent with the findings of other researchers43,50.
The activity of antioxidant enzymes increases in plants exposed to salinity stress because some antioxidant enzymes should be active to maintain lower levels of ROS43,57. SA activates the antioxidant enzymes SOD and CAT in stressed plants13. In myrtle, SA treatment in salinity stress increased the activity of SOD58. Chitosan can activate ROS-inhibitory systems in plants43. The use of chitosan as a bioelicitor with the potential to inhibit ROS has been shown in numerous studies. The activity of antioxidant enzymes increased significantly under the effect of chitosan treatment59. Chitosan could reportedly increase the activity of SOD and other antioxidant enzymes and cause tissue protection and delayed aging in stressed grapes60. Based on the results of the present study, the activity of superoxide dismutase and ascorbate peroxidase enzymes has increased significantly with increasing salinity and the application of salicylic acid-chitosan nanocomposite, which was consistent with the findings of other researchers in apple61, tomato43. The guaiacol peroxidase (GPX) enzyme is active in the cytosol, and glutathione is used as its cofactor55. This enzyme is present in the cellular and apoplasmic systems and is involved in many growth and development processes in the plant. Increasing salinity levels increased the activity of GPX13. The use of chitosan increased the activities of SOD, POX, and CAT in wheat and maize seedlings under salinity stress62. In the study of salt stress in spinach63, the activity of two enzymes, ascorbate peroxidase, and guaiacol peroxidase, increased significantly, which was consistent with our findings in grapes under salt stress.
NaCl salinity stress reduced the concentrations of Ca and Mg in all plant organs64. K uptake in salinity stress decreased significantly due to the effect of sodium on K transport in xylems and the inhibition of uptake processes4. Increased concentrations of Na+ and Cl− because of salinity stress reduced the uptake of K+, Ca2+, and NO3− and nutrient imbalance or deficiency65. In salinity conditions, a decrease in the Na+/K+ ratio in the plant indicates its resistance to salinity stress9,64. SA affects the intracellular ion balance of Na+ and K+ by increasing the regulation of H+-ATPase activity and thereby increases plant salinity resistance66, which is in agreement with our results. Decreased uptake of K and Ca has been reported under high salinity levels. Osmotic damage in plants occurs due to high levels of Na in leaf apoplasts7,57. SA significantly increased Fe uptake in strawberries39 and cucumber67. SA increases the amount of cytoplasmic K compared to Na by increasing the activity of the H+-ATPase pump in the cell membrane and providing a proton gradient, which contributes to reducing the toxic effects of Na and Cl and stimulates the activity of plant antioxidant systems and removal of ROS, thereby maintaining the integrity and protection of the cell membrane68. In the strawberry plant, leaf K content increased significantly by the effect of chitosan treatment69. Chitosan application in chickpea plants increased K content under salinity stress70. It seems that chitosan application caused the proper response of stressed grape plants to salinity stress by increasing and decreasing the concentrations of K and Na, respectively; in other words, chitosan could minimize ionic toxicity caused by salinity stress. In a study, the concentration of magnesium, calcium, potassium, iron and zinc elements decreased in the salinity stress of Selva strawberry71, which was consistent with our findings in grapes under salinity stress. At high levels of salinity, the ratio of sodium to potassium caused an ion imbalance, which is similar to the results of the present study, that salinity increased the ratio of sodium to potassium in cucumber plants72. In this study, salicylic acid treatment has reduced sodium ion concentration, which is in agreement with the findings of Jayakannan et al.73 in Arabidopsis.
Methods
Plant material and treatments
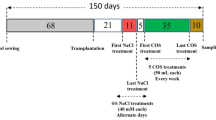
The current research was carried out in 2021 in the research greenhouse of the Department of Horticultural Science and Engineering, Faculty of Agriculture, located at Maragheh University, with a geographic location of 46,16° N latitude and 22,37° E longitude. The homogeneous one-year-old rooted cuttings of Vitis vinifera L. cv. ‘Sultana’ was provided by the nursery of the Horticultural Science Department, University of Maragheh, Iran by the relevant institutional and national guidelines and legislations. They were cultured in 5 L pots containing soil with a loamy sand texture (Table 6). For the initial growth and the adaptation of grapevines to greenhouse conditions (16 and 8 h of light and darkness, 30:25 °C day and night temperature). During the growth period, necessary care such as irrigation and other operations was taken regularly. After the full growth of the leaves of the seedlings, the treatments were carried out. To investigate the effects of foliar application of chitosan-salicylic acid nanocomposites (CS-SA NCs) on the physiological and biochemical properties of ‘Sultana’ cultivar grape in salinity conditions, a factorial experiment was used in completely randomized design (CRD) with three replications. One month after the establishment of the plants, salt stress was applied for one month, and during the stress period, the root environment of the plants was completely washed with salt-free water once every five days to minimize the changes in EC and pH due to washing, and nanocomposite foliar spraying Chitosan-salicylic acid was applied in two stages (the first stage two weeks after salt stress and the second stage at the end of salt stress). Treatments included: salinity stress at three levels (0, 50, and 100 mM NaCl) and foliar application of CS-SA NCs at three levels (0, 0.1, and 0.5 mM).
Synthesis of chitosan-salicylic acid nanocomposite (CS-SA NCs)
To prepare CS-SA NCs, a biopolymer was used to load salicylic acid. In this study, 0.1 g of low molecular weight nanocomposite powder (25 ml of 1 wt% acetic acid solution was added and stirred for 2 h at 70 °C using a magnetic stirrer to obtain a clear CS solution. 100 μl SA of the prepared solution was added to the CS solution, then stirred rapidly for 1 h Sodium tripolyphosphate (TPP) was used as a cross linker with a ratio of 2.5 to CS content. TPP was dissolved in 5 ml of distilled water and then slowly added to the CS-SA solution, then rinsed several times with distilled water to remove the reaction material from the supernatant.
Chitosan-salicylic acid nanocomposite (CS-SA NCs) characterization
Figure 7a represents the TEM image of fabricated CS-SA NCs. As shown, the successful octahedron nanoparticles are around 70–100 nm, which agrees with the DLS data presented in Fig. 7b.
Chlorophyll Fluorescence Indices
Chlorophyll fluorescence was measured by a fluorometer (model: PAM 2500-WALZ, Germany) from the last fifth of leaves in the light. Minimal fluorescence (F0), photochemical quantum yield of photosystem II (Y(II)), electron transport rate (ETR), maximal fluorescence (Fm), Variable fluorescence (Fv), and maximum photochemical quantum yield of photosystem II (Fv/Fm) were assayed.
Chlorophyll and carotenoid content
0.5 g of the sample was digested with 5 cc of 80% acetone and centrifuged for 10 min at 6000 rpm and read at 663, 645, and 470 nm. Chlorophyll content was determined according to Dere et al.74.
where V = volume of the extract (ml); W = Weight of fresh leaves (g).
Proline content
Using Bates et al.75 method, 0.5 g of plant sample was first digested with 10 ml of 3% sulfosalicylic acid and after centrifugation, 2 ml of the extract, 2 ml of ninhydrin acid, and 2 ml of glacial acetic acid were mixed and placed in a bain-marie. Then, 4 ml of toluene was added, and it was read at 520 nm.
Malondialdehyde
0.2 g of the plant sample was homogenized in 2 ml of 20% Trichloroacetic acid containing 0.05% TBA. The samples later were incubated atat 95 °C for 30 min and transferred to the ice. The samples were then centrifuged at 10,000 rpm for 10 min and the absorbance was measured at 532 and 600 nm. The extent of lipid peroxidation was obtained from the difference between the absorption wavelengths in the darkness coefficient of 155 mmol cm−176.
Electrolyte leakage content
Ion leakage was measured according to the Nayyar method77. The electrical conductivity of the samples was measured by reaching the ambient temperature as the initial EC by EC model CC-501. To measure the secondary EC, the samples were placed at 100 °C for 10 min. After reaching the EC ambient temperature again, the samples were measured as EC2 by EC meter, and finally the percentage of ion leakage from the product. The division of the primary EC into the secondary EC was calculated.
Carbohydrate soluble content
To measure soluble carbohydrates, 3 ml of the alcoholic extract obtained was mixed with 1 ml of freshly prepared anthrone (330 mg of anthrone and 300 ml of 15% sulfuric acid). After cooling, the adsorption of the samples with the device the spectrophotometer (UV-1800 Shimadzu, Japan) was read at a wavelength of 653 nm78.
Total soluble protein content
Reaction solution contained 100 μl of enzyme solution, 200 μl of Bradford reagent, and 700 μl of deionized water. 2 min after the complex formation; Bradford regent shows the highest integration with the amino acids. Absorbance was evaluated at 535 nm. The protein content of the samples was calculated based on a standard curve obtained from the defined amounts of bovine serum albumin79.
Hydrogen peroxide
0.2 g of the plant material was homogenized in 2 ml of 0.1% Trichloroacetic acid and centrifuged at 12,000 g for 15 min. 0.5 ml supernatant was added to 0.5 ml of phosphate buffer (10 mmol, pH = 7) and 1 ml of Iodide potassium (1 mol). The sample's absorbance was measured at 390 nm. Standard curves were established with the different concentrations of Hydrogen peroxide80.
Total antioxidant capacity
The antioxidant capacity of the extracts was calculated as the inhibition percentage of DPPH using the method of Chiou et al.81. 0.2 g of leaf tissue was digested with 2 ml of 80% methanol, then the resulting extract was centrifuged for 30 min at 4 °C and 5000 rpm. 100 μl of leaf extract was mixed with 1900 μl of DPPH solution and homogenized. Absorbance was evaluated at 520 nm.
Antioxidant enzymes assay
For the extraction of Guaiacol peroxidase (GPX) and soluble proteins, 0.2 g of the sample was homogenized in liquid nitrogen. 2 ml phosphate buffer (pH = 7.5) containing EDTA (0.5 mol) was added. The samples were incubated at 4 °C for 15 min and were centrifuged at 15 rpm. Due to the instability and very low half-life of ascorbate peroxidase with ex-vivo conditions and for the keeping structure of the compound; we tried to use polyvinylpyrrolidone 5% and ascorbate (2 ml) to the respected enzyme solution.
Guaiacol peroxidase (GPX) activity
For GPX activity, the reaction mixture was containing 1 ml phosphate buffer (100 mmol, pH = 7) along with EDTA (0.1 mmol), 1 mL guaiacol (15 mmol), 1 ml H2O2 (3 mmol), and 50 μL of the extracted enzyme solution. The reaction response was measured at 470 nm for 1 min. Enzymatic activity, based on the amount of tetraguaiacol, was obtained using a darkness coefficient of 26/6 m cm−182.
Ascorbate peroxidase (APX) activity
APX was assayed as; the reaction mixture was containing 250 μL phosphate buffer (pH = 7) along with EDTA, 10 μL H2O2 (1 mmol), 250 μL sodium ascorbate (0.25 mmol) and 50 μL enzyme solution. The absorbance was measured at 290 nm for 1 min. Enzymatic activity was calculated using the darkness coefficient of 2.8 mmol−1 cm−1. The resulting number indicates the activity of Ascorbate Peroxidase based on micromoles of oxidized Ascorbate per minute82.
Superoxide dismutase (SOD) activity
SOD activity was determined by measuring the inhibition of light reduction of nitroblue tetrazolium at a wavelength of 560 nm. Doing this, 50 ml of 50 mM potassium phosphate buffer, pH: 7.5, was used. Then, 75 μM nitroblue tetrazolium, 13 μM methionine, 0.1 μM EDTA solution, and 4 μM riboflavin were added to the buffer and the solution was stored in a dark place83.
Catalase (CAT) activity
0.5 g of grape leaf samples were homogenized with 0.1 M cold potassium phosphate buffer (pH: 7.5) with 0.5 mM EDTA based on the method of Dezar et al.84. From the resulting supernatant, 0.05 ml was added to 1.5 ml of 0.1 mM phosphate buffer (pH: 7) and 1.45 ml of double distilled water. The reaction was started by adding 0.5 ml of 75 mM hydrogen peroxide and a decrease in adsorption was recorded at 240 nm for 1 min.
Leaves nutrient elements content
The flame photometric method (Corning, 410, England) was employed to measure the amount of sodium and potassium. The atomic absorption spectrometer (Corning, 410, England) was used to measure Zn, Fe, Ca, P, Mg, and Mn content (Corning, 410, England) and N content was quantified by Kjeldahl methods85,86.
The present experiment was performed as a factorial based on a completely randomized design with three replications. MSTATC (ver. 2.1, Michigan University), Minitab (ver. 17), and R software (ver. 3.6.3) were used for the ANOVA, cluster, biplot, and correlation analysis of data, respectively and, Excel (2016) was used to draw the figures. The means were compared using Duncan’s multiple range tests at 5 and 1% probability levels.
Conclusion
The results of this research demonstrated that photosynthetic pigments decreased in ‘Sultana’ cultivar grape plants with increasing salinity stress, but it increased the content of osmolytes and antioxidant enzymes. Salinity interrupted ionic homeostasis and reduced nutrients. The application of the CS-SA NCs in stress and non-stress conditions positively affected the improvement of the studied traits of grape plants, improved nutrient levels, and reduced the Na level. Consequently, this nanocomposite represents an innovative approach that can be successfully used in grape plants to improve the yield under salinity stress. However, further validation is needed to determine their effectiveness in other plant species.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAT:
-
Catalase activity
- APX:
-
Ascorbate peroxidase activity
- GPX:
-
Guaiacol peroxidase activity
- SOD:
-
Superoxide dismutase activity
- EL:
-
Electrolyte leakage
- F0:
-
Minimal fluorescence
- MDA:
-
Malondialdehyde
- Protein:
-
Total soluble protein
- RWC:
-
Relative Water Content
- Chlb:
-
Chlorophyll b
- Totalchl:
-
Total chlorophyll
- Chla:
-
Chlorophyll a
- Fm:
-
Maximal fluorescence
- PAR:
-
Photosynthetically active radiation
- F0:
-
Minimal fluorescence
- ETR:
-
Electron transport rate
- Fv:
-
Variable fluorescence
- Fv/Fm:
-
The ratio of variable fluorescence to maximal fluorescence
- N:
-
Nitrogen
- P:
-
Phosphor
- K:
-
Potassium
- Mg:
-
Magnesium
- Zn:
-
Zinc
- Fe:
-
Iron
References
Hoque, T. S., Sohag, A. A. M., Burritt, D. J. & Hossain, M. A. Salicylic acid-mediated salt stress tolerance in plants. In Plant Phenolics in Sustainable Agriculture (eds Lone, R. et al.) (Springer, 2020).
Arif, Y., Singh, P., Siddiqui, H., Bajguz, A. & Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 156, 64–77 (2020).
Keutgen, A. J. & Pawelzik, E. Impacts of NaCl stress on plant growth and mineral nutrient assimilation in two cultivars of strawberry. Environ. Exp. Bot. 65(2), 170–176 (2009).
Heidari, M. & Jamshidi, P. Effects of salinity and potassium application on antioxidant enzyme activities and physiological parameters in pearl millet. Agric. Sci. China 10(2), 228–237 (2011).
Tiwari, J. K. et al. Effect of salt stress on cucumber: Na+/K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol. Plant. 32(1), 103–114 (2010).
Hanafy Ahmed, A. H., Darwish, E., Hamoda, S. A. F. & Alobaidy, M. G. Effect of putrescine and humic acid on growth, yield and chemical composition of cotton plants grown under saline soil conditions. Am. Eurasian J. Agric. Environ. Sci. 13(4), 479–497 (2013).
Ahmad, I., Ahmad, T. K. A., Basra, S. M., Hasnain, Z. & Ali, A. Effect of seed priming with ascorbic acid, salicylic acid and hydrogen peroxide on emergence, vigor and antioxidant activities of maize. Afr. J. Biotechnol. 11(5), 1127–1137 (2012).
Lotfi, R., Pessarakli, M., Gharavi-Kouchebagh, P. & Khoshvaghti, H. Physiological responses of Brassica napus to fulvic acid under water stress: Chlorophyll a fluorescence and antioxidant enzyme activity. Crop J. 3(5), 434–439 (2015).
Aazami, M. A., Vojodi Mehrabani, L., Hashemi, T., Hassanpouraghdam, M. B. & Rasouli, F. Soil-based nano-graphene oxide and foliar selenium and nano-Fe influence physiological responses of “Sultana” grape under salinity. Sci. Rep. 12(1), 1–13 (2022).
Serraj, R. & Sinclair, T. R. Osmolyte accumulation: Can it really help increase crop yield under drought conditions?. Plant Cell Environ. 25, 333–341 (2002).
Hasanuzzaman, M. et al. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 18, 2–28 (2017).
Saleem, M. H. et al. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 27, 5211–5221 (2020).
Aazami, M. A., Mahna, N. & Naghshband Hasani, R. Salicylic acid affects antioxidant system of some grape cultivar under cold stress conditions. J. Biodivers. Environ. Sci. 5(5), 280–290 (2014).
Oraei, M., Gohari, G., Panahirad, S., Zareei, E. & Zaare-Nahandi, F. Effect of salicylic acid foliar application on Vitis vinifera L. cv. ‘sultana’ under salinity stress. Acta Sci. Pol. Hortorum Cultus 18(2), 155–169 (2019).
Hidangmayum, A., Dwivedi, P., Katiyar, D. & Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 25(2), 313–326 (2019).
Cataldo, E., Fucile, M. & Mattii, G. V. Biostimulants in viticulture: A sustainable approach against biotic and abiotic stresses. Plants 11(2), 162 (2022).
Krupa-Małkiewicz, M. & Fornal, N. Application of chitosan in vitro to minimize the adverse effects of salinity in Petunia× atkinsiana D. don. Ecol. Eng. 19(1), 143–149 (2018).
Ji, J., Hao, S., Wu, D., Huang, R. & Xu, Y. Preparation, characterization and in vitro release of chitosan nanoparticles loaded with gentamicin and salicylic acid. Carbohydr. Polym. 85(4), 803–808 (2011).
Choudhary, R. C. et al. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 7(1), 1–11 (2017).
Singh, R. K. et al. Viewpoint of chitosan application in grapevine for abiotic stress/disease management towards more resilient viticulture practices. Agriculture 12, 1369. https://doi.org/10.3390/agriculture12091369 (2022).
Kumaraswamy, R. V. et al. Salicylic acid functionalized chitosan nanoparticle: A sustainable biostimulant for plant. Int. J. Biol. Macromol. 123, 59–69 (2019).
Khalili, N. et al. Chitosan-enriched salicylic acid nanoparticles enhanced anthocyanin content in grape (Vitis vinifera L. cv. Red Sultana) berries. Polymers 14, 3349. https://doi.org/10.3390/polym14163349 (2022).
Sampedro-Guerrero, J., Vives-Peris, V., Gomez-Cadenas, A. & Clausell-Terol, C. Encapsulation reduces the deleterious effects of salicylic acid treatments on root growth and gravitropic response. Int. J. Mol. Sci. 23, 14019. https://doi.org/10.3390/ijms232214019 (2022).
Alston, J. M. & Sambucci, O. Grapes in the world economy. In The Grape Genome (eds Cantu, D. & Walker, M. A.) (Springer, Cham, 2019).
Ma, Y., Qin, F. & Tran, L. S. Contribution of genomics to gene discovery in plant abiotic stress responses. Mol. Plant 5(6), 1176–1178 (2012).
Sivritepe, N., Özkan Sivritepe, H., Çelik, H. & Vahap Katkat, A. Salinity responses of grafted grapevines: Effects of scion and rootstock genotypes. Not. Bot. Horti Agrobot. Cluj Napoca 38(3), 193–201 (2010).
Desouky, I. M., Shaltout, A. D., Haggag, L. F., Shahin, M. F. M. & El-Hady, E. S. Salinity tolerance of some grapevine cultivars as affected by salt creek and freedom rootstocks. Middle East J. Agric. Res. 4(1), 112–122 (2015).
Fozouni, M., Abbaspour, N. & Doulati Baneh, H. Short term response of grapevine grown hydroponically to salinity: Mineral composition and growth parameters. Vitis 51(3), 95–101 (2012).
Kalaji, H. M. et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 38, 102. https://doi.org/10.1007/s11738-016-2113-y (2016).
Wright, H., DeLong, J., Lada, R. & Prange, R. The relationship between water status and chlorophyll a fluorescence in grapes (Vitis spp.). Postharvest Biol. Technol. 51(2), 193–199 (2009).
Kalaji, H. M., Bosa, K., Kościelniak, J. & Żuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 73, 64–72 (2011).
Na, Y. W. et al. Chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hortic. Environ. Biotechnol. 55(4), 280–286 (2014).
Azizpour, K. et al. Physiological response of spring durum wheat genotypes to salinity. J. Plant Nutr. 33(6), 859–873 (2010).
Mehta, P., Jajoo, A., Mathur, S. & Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 48(1), 16–20 (2010).
Alkahtani, M. D. et al. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy 10(8), 1180 (2020).
Osman, M. S., Badawy, A. A., Osman, A. I. & Latef, A. A. H. A. Ameliorative impact of an extract of the halophyte Arthrocnemum macrostachyum on growth and biochemical parameters of soybean under salinity stress. J. Plant Growth Regul. 40(3), 1–12 (2020).
Latef, A. A. H. A., Alhmad, M. F. A., Kordrostami, M., Abo-Baker, A. B. A. E. & Zakir, A. Inoculation with Azospirillum lipoferum or Azotobacter chroococcum reinforces maize growth by improving physiological activities under saline conditions. J. Plant Growth Regul. 39(3), 1293–1306 (2020).
Wasti, S., Mimouni, H., Smiti, S., Zid, E. & Ben Ahmed, H. Enhanced salt tolerance of tomatoes by exogenous salicylic acid applied through rooting medium. OMICS 16(4), 200–207 (2012).
Karlidag, H., Yildirim, E. & Turan, M. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci. Agric. 66(2), 180–187 (2009).
Neelam, M., Rahul, M., Ajiboye, M., Kafayat, Y. & Lateefat, Y. Salicylic acid alters antioxidant and phenolics metabolism in Catharanthus roseus grown under salinity stress. Afr. J. Tradit. Complement. Altern. Med. 11(5), 118–125 (2014).
Khan, W. M., Prithiviraj, B. & Smith, D. L. Effect of foliar application of chitin and chitosan oligosaccharides on photosynthesis of maize and soybean. Photosynthetica 40(4), 621–624 (2002).
Ullah, N. et al. Mitigation the adverse effect of salinity stress on the performance of the tomato crop by exogenous application of chitosan. Bull. Natl. Res. Cent. 44(1), 1–11 (2020).
Attia, M. S. et al. Impact of foliar application of chitosan dissolved in different organic acids on isozymes, protein patterns and physio-biochemical characteristics of tomato grown under salinity stress. Plants 10(2), 388 (2021).
Sofy, A. R. et al. Improving regulation of enzymatic and non-enzymatic antioxidants and stress-related gene stimulation in Cucumber mosaic cucumovirus-infected cucumber plants treated with glycine betaine, chitosan and combination. Molecules 25(10), 2341 (2020).
Khayyat, M., Tehranifar, A., Davarynejad, G. H. & Sayyari-Zahan, M. H. Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of ‘Malas-e-Saveh’and ‘Shishe-Kab’ pomegranates in response to salinity stress. Photosynthetica 52(2), 301–312 (2014).
Farouk, S., Ghoneem, K. M. & Ali, A. A. Induction and expression of systemic resistance to downy mildew disease in cucumber by elicitors. Egypt J. Phytopathol 36(1–2), 95–111 (2008).
Bakhoum, G. S., Sadak, M. S. & Badr, E. A. E. M. Mitigation of adverse effects of salinity stress on sunflower plant (Helianthus annuus L.) by exogenous application of chitosan. Bull. Natl. Res. Cent. 44, 1–11 (2020).
Ashraf, M., Akram, N. A., Arteca, R. N. & Foolad, M. R. The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit. Rev. Plant Sci. 29(3), 162–190 (2010).
Shahba, Z., Baghizadeh, A., Vakili, S. M. A., Yazdanpanah, A. & Yosefi, M. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl). J. Struct. Biol. 2(3), 35–41 (2010).
Manaa, A. et al. Simultaneous application of salicylic acid and calcium improves salt tolerance in two contrasting tomato (Solanum lycopersicum) cultivars. S. Afr. J. Bot. 95, 32–39 (2014).
Martínez González, L., Reyes Guerrero, Y., Falcón Rodríguez, A. & Núñez Vázquez, M. Effect of seed treatment with chitosan on the growth of rice (Oryza sativa L.) seedlings cv. INCA LP-5 in saline medium. Cult. Trop. 36(1), 143–150 (2015).
Jumali, S. S., Said, I. M., Ismail, I. & Zainal, Z. Genes induced by high concentration of salicylic acid in 'Mitragyna speciosa’. Aust. J. Crop Sci. 5(3), 296 (2011).
Shahba, Z., Baghizadeh, A., Vakili, S. M. A., Yazdanpanah, A. & Yosefi, M. The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) sugar, protein and proline contents under salinity stress (NaCl). J. Biophys. Struct. Biol. 2(3), 35–41 (2010).
Shakirova, F. M., Sakhabutdinova, A. R., Bezrukova, M. V., Fatkhutdinova, R. A. & Fatkhutdinova, D. R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 164(3), 317–322 (2003).
Rabêlo, V. M. et al. The foliar application of a mixture of semisynthetic chitosan derivatives induces tolerance to water deficit in maize, improving the antioxidant system and increasing photosynthesis and grain yield. Sci. rep. 9(1), 1–13 (2019).
Chamnanmanoontham, N., Pongprayoon, W., Pichayangkura, R., Roytrakul, S. & Chadchawan, S. Chitosan enhances rice seedling growth via gene expression network between nucleus and chloroplast. Plant Growth Regul. 75(1), 101–114 (2015).
Aazami, M. A., Rasouli, F. & Panahi Tajaragh, R. Influence of salinity stress on morphological, nutritional and physiological attributes in different cultivars of Prunus amygdalus L.. J. Plant Nutr. 44(12), 1758–1769 (2021).
Idrees, M., Naeem, M., Aftab, T. & Khan, M. M. A. Salicylic acid mitigates salinity stress by improving antioxidant defense system and enhances vincristine and vinblastine alkaloids production in periwinkle [Catharanthus roseus (L.) G. Don]. Acta Physiol. Plant. 33(3), 987–999 (2011).
Sadak, M. S. & Talaat, I. M. Attenuation of negative effects of saline stress in wheat plant by chitosan and calcium carbonate. Bull. Natl. Res. Cent. 45, 136. https://doi.org/10.1186/s42269-021-00596-w (2021).
Singh, R. K. et al. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of grape (Vitis vinifera L. ‘Touriga Franca’ and ’Tinto Cão’) Tissues. Antioxidants 8, 525. https://doi.org/10.3390/antiox8110525 (2019).
Molassiotis, A. N. et al. Antioxidant and anatomical responses in shoot culture of apple rootstock MM 106 treated with Nacl, KCl, mannitiol or sorbitol. Biol. Plant. 50(1), 61–68 (2006).
Peykani, L. S. & Sepehr, M. F. Effect of chitosan on antioxidant enzyme activity, proline, and malondialdehyde content in Triticum aestivum L. and Zea mays L. under salt stress condition. Iran J. Plant Physiol. 9, 2661–2670 (2018).
Mohseni, Z., Moradian, F. & Rahdari, P. The Study of Activity of Antioxidant Enzymes, Guaiacol Peroxidase and Ascorbate Peroxidase and the amount of Na, K and pigment content in Spinach oleracea L. under NaCl Salinity Stress. J. Plant Res. 32 (4), 915–924 (2020).
Hussin, S., Geissler, N. & Koyro, H. W. Effect of NaCl salinity on Atriplex nummularia (L.) with special emphasis on carbon and nitrogen metabolism. Acta Physiol. Plant. 35, 1025–1038 (2013).
Parihar, P., Singh, S., Singh, R., Singh, V. P. & Prasad, S. M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 22(6), 4056–4075 (2015).
Jayakannan, M., Bose, J., Babourina, O., Rengel, Z. & Shabala, S. Salicylic acid in plant salinity stress signaling and tolerance. Plant Growth Regul. 76(1), 1–16 (2015).
Yildirim, E., Turan, M. & Guvenc, I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J. Plant Nutr. 31(3), 593–612 (2008).
Jayakannan, M., Bose, J., Babourina, O., Rengel, Z. & Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 64(8), ert085 (2013).
Abdel-Mawgoud, A. M. R., Tantawy, A. S., El-Nemr, M. A. & Sassine, Y. N. Growth and yield responses of strawberry plants to chitosan application. Eur. J. Sci. Res. 39(1), 161 (2010).
Dzung, N. A. Enhancing crop production with chitosan and its derivatives. In Chitin, Chitosan, Oligosaccharides and Their Derivatives (ed. Kim, S.-K.) 643 (Taylor and Francis, Boca Raton London New York, 2011).
Jamali, B., Eshghi, S. & Shahidi-Rad, K. Growth and fruit characteristics of strawberry cv. selva as affected by different application timing of salicylic acid under saline conditions. Int. J. Fruit Sci. 15, 339–352 (2015).
Tiwari, J. K. et al. Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol. Plant. 32(1), 103–114 (2010).
Jayakannan, M., Bose, J., Babourina, O., Rengel, Z. & Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 64(8), 2255–2268 (2013).
Dere, Ş, Gunes, T. & Sivaci, R. Spectrophotometric determination of chlorophyll a, b and total carotenoid content of some algae species using different solvent. Tr. J. Bot. 22, 13–17 (1998).
Bates, L. S., Waldren, R. P. & Tear, I. D. Rapid determination of free proline for water stress studies. Plant Soil. 39, 205–208 (1973).
Health, R. L. & Packer, L. Photo peroxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198 (1968).
Nayyar, H. Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ. Exp. Bot. 50, 253–264 (2003).
Irigoyen, J. J., Emerich, D. W. & Sanchez-Diaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in modulated alfalfa (Medicago sativa L.) plants. Physiol. Plant. 84, 55–60 (1992).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254 (1976).
Velikova, V., Yordanov, J. & Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants-protective role of exogenous polyamines. Plant Sci. 151, 59–66 (2000).
Chiou, A. et al. Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem. 102(2), 516–522 (2007).
Yoshimura, K., Yabute, Y., Ishikawa, T. & Shigeoka, S. Expression of spinach ascorbate per-oxidase isoenzymes in response to oxidative stresses. Plant Physiol. 123, 223–233 (2000).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutase II. Purification and quantitive relationship with water soluble protein in seedling. Plant Physiol. 50, 315–318 (1977).
Dezar, C. A., Gago, G. M., González, D. H. & Chan, R. L. Hahb-4, a sunflower Homeobox-Leucine Zipper gene, confers drought tolerance to Arabidopsis thaliana plants. Transgenic Res. 14, 429–440 (2005).
Tandon, H. L. S., Cescas, M. P. & Tyner, E. H. An acid-free vanadate-molybdate reagent for the determination of total phosphorus in Soils. Soil Sci. Soc. Am. J. 32, 48–51 (1968).
Jones, J. B. Plant tissue analysis for micronutrients. In Micronutrients in Agriculture (ed. Mortvedt, J. J.) 319–346 (Soil Science Society America, Madison, 1972).
Acknowledgements
These results were from MSc thesis of Maryam Maleki and this work was supported by the University of Maragheh, Iran.
Author information
Authors and Affiliations
Contributions
M.A.A. Study conception and design performed experiments, Drafting of manuscript done. M.M. Methodology. F.R. Analysis of data. G.G. Improvement of the manuscript done. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aazami, M.A., Maleki, M., Rasouli, F. et al. Protective effects of chitosan based salicylic acid nanocomposite (CS-SA NCs) in grape (Vitis vinifera cv. ‘Sultana’) under salinity stress. Sci Rep 13, 883 (2023). https://doi.org/10.1038/s41598-023-27618-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-27618-z
This article is cited by
-
Monitoring plant responses in field-grown peanuts exposed to exogenously applied chitosan under full and limited irrigation levels
Scientific Reports (2024)
-
An overview of biostimulant activity and plant responses under abiotic and biotic stress conditions
Systems Microbiology and Biomanufacturing (2024)
-
Fascinating Role of Silicon dioxide Nanoparticles and Co-inoculation of Mycorrhiza and Rhizobacteria to Combat NaCl Stress: Changes in Physiological Characteristics, Uptake of Nutrient Elements, and Enhancing Photosystem II Activities in Wheat
Journal of Soil Science and Plant Nutrition (2024)
-
Unraveling the Synergistic Potential of Mycorrhizal Consortium in Augmenting Salinity Stress Tolerance in Wheat by Advancing Physiological, Metabolic, Nutrient, and Ultrastructural Attributes
Journal of Soil Science and Plant Nutrition (2024)
-
Efficient strategies for controlled release of nanoencapsulated phytohormones to improve plant stress tolerance
Plant Methods (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.