Abstract
Soft-tissue conditioning due to posttraumatic oedema after complicated joint fractures is a central therapeutic aspect both pre- and postoperatively. On average, 6–10 days pass until the patient is suitable for surgery. This study compares the decongestant effect of vascular impulse technology (VIT) with that of conventional elevation. In this monocentric RCT, 68 patients with joint fractures of the upper (n = 36) and lower (n = 32) extremity were included and randomized after consent in a 1:1 ratio. Variables were evaluated for all fractures together and additionally subdivided into upper or lower extremity for better clinical comparability. Primary endpoint was the time in days from hospital admission to operability. Secondary endpoints were total length of stay, oedema reduction, pain intensity, complications, and revisions. The time from admission until operability was reduced by 1.4 (95% CI − 0.4; 3.1) days in the mITT analysis (p = 0.120) and was statistically significant with 1.7 (95% CI 0.1; 3.3) days in the as-treated sensitivity analysis (pAT = 0.038). Significantly less pain and a faster oedema reduction were found in the intervention group. Due to rare occurrences, nothing can be concluded regarding complications and revisions. Administration of VIT therapy did not lead to a significant reduction in time until operability in the whole population but was superior to elevation for soft-tissue conditioning and pain reduction. However, there was a significant reduction by 2.5 days (95% CI 0.7; 4.3) in the subgroup of lower extremity fractures. VIT therapy therefore seems to be a helpful tool in the treatment of posttraumatic oedema after complex joint fractures of the lower and upper extremity, especially in tibial head and lower leg fractures.
Similar content being viewed by others
Introduction
Soft-tissue swelling in the setting of complex joint fractures is a critical factor in clinical management1,2,3,4,5,6,7,8. The exerted mechanical forces due to the trauma cause direct damage to the surrounding soft tissues and vessels, leading to hematoma formation and primary swelling9,10,11,12. As a result of the developing inflammatory response, there is an increase in capillary permeability via the release of coagulative factors and inflammatory mediators, and thus a secondary swelling due to an accumulation of fluid in the interstitial space13. This gives rise to a clinical soft-tissue swelling, which leads to a rising tissue pressure and a reduced microcirculation due to venous stasis, and thus to tissue hypoxia and local acidosis13.
This impairs wound and bone healing, and the accumulation of metabolites causes increased pain14,15,16. If either the trauma is so severe that a vicious circle is formed from this cycle, or if an attempt is made to perform osteosynthesis in this setting, this can lead to the development of the maximal degree of soft-tissue trauma: compartment syndrome12,17,18. Because of this, joint fractures require rigorous soft-tissue conditioning until adequate decongestion has been achieved. Only then is surgical intervention possible without inadequately compromising the soft tissues. The traditional methods consisting of rest, elevation, cooling, and compression are mainly used as decongestant measures. These are effective and rheologically proven, especially for elevation19,20.
However, all these measures have a purely passive effect, by lowering the hydrostatic pressure, and are therefore both lengthy and only effective as long as they are consistently applied. In the 1970s, a venous plexus was demonstrated for both venous return from the foot and hand. Their physiology was proven in further studies using phlebography and duplex sonography21,22,23,24. In either case, the effect unfolds through a combination of passive and active moieties, which gave rise to the name venous hand or foot pump25,26,27. For the foot pump, the passive effect consists of weight bearing during the stance phase and the stretching of the plantar surface during roll-off, draining the contained blood volume proximally. In the hand, the superficial veins, both palmar and dorsal, pass over prominent bony structures and are compressed onto them during fist clenching24,28.
Synergistically with this passive moiety, the blood from the deep intermuscular veins in both regions is actively transported centrally by muscle pumps27. Since their development, there have been numerous studies on the use of intermittent pneumatic compression (IPC) devices, the Vascular Impulse Technology therapy (VIT therapy) being one form of these. This system consists of an air compressor connected to a pad placed into the palm of the hand or the sole of the foot with a bladder that is inflated every 20 s in less than 1 s to exert a pressure of 130 mmHg on the venous plexus of the foot and 80 mmHg on the hand, respectively. This causes a pulsatile blood flow and measurements have revealed that the effect reaches the right atrium21,22,23,24. These sufficiently demonstrate their effect on thromboprophylaxis in general and especially in the context of arthroplasty29,30,31,32,33. Less research has been done on their benefit in perioperative trauma care. Sufficient data for clinical benefit exist for foot and ankle fractures as most recently published by Schnetzke et al.34,35,36,37,38,39.
Only a few studies exist for other anatomical locations, further proximal on the lower leg and tibial plateau, as well as for joint fractures of the upper extremity. Their validity is further limited by methodological weaknesses, small case numbers or only a limited indication40,41,42,43,44. They all demonstrated significantly faster oedema resolution, an increased range of motion, and less pain10,45,46,47. In some cases, a volume reduction of 70–90% of the trauma-induced soft-tissue swelling could be achieved41,43. However, some studies, though having distinct methodologic flaws, showed no significant benefit48,49. Thus, evidence of the benefit of VIT therapy in this setting can be called ambiguous or sparse at best.
Therefore, and due to the lack of evidence for upper extremity fractures, the benefit of VIT therapy in complex fractures of the lower leg and the upper extremity was investigated with regard to decongestion, perioperative length of stay, pain intensity and medication, and complication and revision rates. It was hypothesized that VIT therapy would reduce the time in days until operability was achieved.
Results
The intervention and control groups were comparable in demographic variables (age, type of injury, gender, academic, comorbidities, smoker. See Tables 1 and 2). Upon evaluation of the primary endpoint in the mITT population of the entire study group, the intervention group showed a mean reduction of 1.4 (CI − 0.4; 3.1) days in the length of stay until operability, with 6.7 ± 2.9 days compared to 8.0 ± 3.8 days in the control group (p = 0.120, see Table 3). In the subsequent sensitivity analysis in the AT population, the difference was significant, with a reduction of 1.7 (CI 0.1; 3.3) days in the intervention group. (pAT = 0.038).
Analysis of the subgroups showed a significant reduction in favor of the intervention group in the lower extremity fractures with a difference of 2.5 (CI 0.7; 4.3) days (p = 0.009: see Table 3), but no significant difference could be detected with these numbers in the upper extremity with 0.9 (CI − 1.6; 3.4) days in favor of the intervention group (p = 0.490). The mean difference between the intervention and control group is shown in Fig. 1.
Taking the entire inpatient stay into account, there was also a reduction of 0.8 (CI − 2.6; 4.2) days in the intervention group, with 1.4 (CI − 3.0; 5.9) in the lower and 1.3 (CI − 2.3; 4.9) in the upper extremity respectively, but these were not significant (ptotal = 0.629; pLE = 0.512; pUE = 0.478).
Preoperatively a nearly two-fold increase in decongestion could be seen in the intervention group with a reduction of posttraumatic oedema of around 10.0 ± 10.5% per day instead of 5.6 ± 10.4% per day, which was not statistically significant (p = 0.163). Postoperatively there was an increase of about 50% in favor of the intervention group (10.7 ± 6.0%/d instead of 7.1 ± 9.2%/d, p = 0.148; see Fig. 2).
The overall mean increase in decongestion (pre- and postoperatively) by 3.9 (CI 0.001; 7.9; p = 0.050) was significant, with 10.3 ± 8.7% per day in the intervention group compared to 6.4 ± 9.8% per day in the control. In relation to mean use of the VIT therapy, this can be calculated as a reduction in swelling of 4.41 ± 15.41% per hour of VIT therapy.
A significant difference could be found in patients being pain-free preoperatively with a ratio of 40% (9/23) in the intervention group compared to 12% in the control group (3/26; p = 0.044) and upon discharge with 63% (12/19) compared to 4% (1/23; p < 0.001).
Only a slight numerical difference regarding opioids needed preoperatively could be seen in the intervention group with 26% (6/23) of the patients requiring pain medication according to step 3 of the WHO scheme, compared to 32% (8/25) in the control group (p = 0.756). Upon discharge, this difference rose to 5.6% (1/18) vs. 18.2% (4/22; p = 0.356).
Due to the low numbers, no statistical analysis could be performed concerning the complication and revision rates. There were two complications in each of the two groups resembling a rate of 11.8% in the intervention group and 15.4% in the control group. However, there was only one revision (fasciotomy) in one of the intervention group patients (5.9%, 1/17) due to noncompliance to the therapy protocol. The patient concerned used the VIT for only 1 h/day and regularly left the surgical ward to smoke cigarettes.
The multiple linear regression analysis (duration ~ group + demographic characteristics; Table 4) showed no difference in the primary endpoint regarding “age”, “gender”, “education level”, “comorbidities”, “smoker”, and “affected side”.
Discussion
This study aimed to explore if administering VIT therapy as a form of intermittent pneumatic compression would improve soft-tissue conditioning and could consequently reduce the time until operability would be achieved. Following the discovery of venous return from the foot and the hand by means of both an active and passive venous pump, studies were carried out that tried to stimulate these mechanisms and improve and accelerate soft-tissue conditioning as well as thromboprophylaxis, especially during the posttraumatic immobilization period. The focus was primarily on ankle fractures, since the soft-tissue envelope is rather thin and perfusion critical here, leading to high complication rates due to posttraumatic oedema. However, there have only been limited studies on other lower extremity fractures and even less on the upper extremity.
For tibial plateau and shaft fractures, only one published study with solely the abstract but not the full text in English could be found. This described a significantly faster reduction in swelling preoperatively in 68 cases of tibial and fibular fractures50. Seven studies could be identified in the last 40 years which focused on fractures of the upper extremity40,41,42,43,44,48,49,50. The effects of intermittent pneumatic compression were examined in lymphedema after mastectomy40, after operative treatment of Dupuytren’s contracture44, Colles’ fracture42, for 48 h perioperatively in distal radius fractures41, and only in one case after surgical treatment of distal radius fractures in the form of a prospective randomized trial43. In summary, a significantly improved postoperative decongestion, an improved venous return during therapy, and relieved pain with increased range of motion were found40,41,42,43,44.
Nonetheless, in two of these studies, posttraumatic oedema was not examined40,44. The work of Svensson et al. showed an effect in postoperative treatment, yet no therapy was given preoperatively42. In the study of Ramesh et al. the therapy was administered perioperatively, though no control group was included and the studied period covered just 48 h41. The first prospective randomized trial was conducted by Mader et al., but there was no control for elevation, nor a preoperative treatment. Additionally, it only included patients who were initially treated by an external fixator and presented preoperatively with an increased girth due to swelling of at least 3 cm43.
Only two recent studies could be identified48,49. Both could not demonstrate a significant or at least relevant benefit of the VIT therapy. In the first study, however, therapy was only applied after 4 weeks of postoperative immobilization in distal radius fractures48 and in the second study, a pressure of 20 mmHg was applied to the venous hand plexus, which does not resemble a physiologic stimulation49.
Thus, this study is the first to examine the effect of VIT therapy in the perioperative management of different complex fractures of the lower and upper extremity compared to elevation using a physiologic stimulation of the venous pump in the foot and hand in a prospective, controlled, and randomized setting.
This physiologic stimulation can be achieved by a fast pulsatile compression of the venous plexus as would result from weight bearing in case of the foot or clenching of the hand. This impulse induces shear stress, which in turn releases NO-reducing tissue hypoxia and improved blood flow21,22,23,24. Moreover, therapy was administered throughout the entire perioperative period right after hospital admission and so exactly that period in which an impaired micro- and macro-circulation might cause complications13,14,15,16. In previous studies, a continuous pressure was used or therapy only applied in a confined and not directly posttraumatic period.
It could be shown that the delay to surgery could be reduced by 1–2.5 days. The difference was higher in the lower extremity subgroup for two reasons: swelling was more severe and so the venous pump was more effective and, in general, the delay to surgery in the upper extremity was rather short with a mean of 5–6 days. Decongestion was significantly improved by approximately 50% from which a mean reduction in swelling of around 4.5% per hour use of the VIT therapy can be calculated. Hence, the beneficial effect of intermittent pneumatic compressions on posttraumatic oedema that could only be anticipated from former studies in these fractures could now be further substantiated and proven.
As described in a previous study, the improved microcirculation with fewer metabolites accumulating in the tissue is supposed to be the trigger of the lesser pain reported in the intervention group with three times as many patients reported being pain-free peroperatively and upon discharge13. No effect was found in regard to complication rates and revisions needed.
Limitations
A major limitation of the study in the case of upper extremity fractures is that only a fraction of the targeted group size of 20 patients per fracture could be achieved. Thus, only a limited power could be achieved in these groups with regard to the statistical analysis. On the one hand, this was because patients with such fractures were often too old or comorbid. On the other hand, and more importantly, this was because these patients were in most cases not admitted as inpatients for soft-tissue conditioning but were immobilized in a plaster cast and treated as outpatients. However, even in these cases, significant effects could be detected. The second limitation is the lack of blinding and hence the risk of performance bias. However, blinding was not feasible for either the observer or the participant due to the obvious intervention. This limitation was countered by selecting independent observers to assess the operability. A correlation analysis carried out in a preliminary study (κ = 0.816) revealed almost complete agreement in their assessments. Another limitation is that the pure circumference measurement performed to objectify the swelling was rounded to 5 mm increments. However, a volumetric analysis was not possible when patients were immobilized in a cast or external fixator and a more precise measurement was not possible due to the duration and size of the study and the fact that it was carried out in the context of normal inpatient daily routine.
Conclusion
Although no significant overall reduction in the delay until operability could be shown, there was a significant reduction by 2.5 days in complex fractures of the tibial head and lower leg. Significant improvement in decongestion by more than 50% and in pain-free patients preoperatively and upon discharge could be seen in both upper and lower extremity fractures. Due to the low incidence, no statement regarding complications and revisions could be made.
In short, Vascular Impulse Technology is an effective additional tool for soft-tissue conditioning, especially in fractures of the lower leg with a more profound swelling.
Methods
This prospective, randomized, controlled, monocentric study included a total of 68 patients with upper (n = 36) and lower limb (n = 32) fractures from 2016 to 2019. These were enrolled and randomized upon admission in permuted blocks of equal length to the intervention (n = 35) and control (n = 33) groups in a 1:1 ratio using the “Randoulette” program from the University of Munich (https://wwwapp.ibe.med.uni-muenchen.de/randoulette/index.jsp). Blinding was not possible due to the acoustically and visually obvious intervention. The study was pre-registered in the German Clinical Trials Registry (DRKS00010510; 18/07/2016) and approved by the Ethics Committee of the Rhineland-Palatinate Medical Association (837.155.16/10474) as the supervising committee for medical studies at the BG Trauma Center Ludwigshafen. The study protocol was published before the study was started51.
After informed consent, patients aged between 18 and 80 years with complex fractures of the lower leg shaft (n = 4), tibial plateau (n = 28), proximal (n = 13) and distal (n = 5) humerus, with ligamentous and bony elbow dislocations (n = 7) and distal radius fractures (n = 11) who could not be definitively treated by ORIF on the day of admission were included. Since patients with upper limb injuries were mostly managed as outpatients preoperatively, patients were also included from inpatient admission to surgery after decongestion (n = 5) due to the small number of cases.
Exclusion criteria were met if patients were younger than 18 or older than 80 years, had not signed an informed consent, had concomitant injuries to the contralateral side, or had open fractures with pre-existing soft-tissue complications, such as infections, stress blisters, necrosis or compartment syndrome. Patients with decompensated heart failure, thrombosis or pulmonary artery embolism, or acute phlebitis were not included. Participants in other studies, pregnant women, prison inmates, and drug or alcohol abusers were not included either.
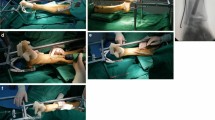
Figure 3 shows a flow chart of the study with data collection and drop-outs. After allocation to one of the groups, patients in the control group were only placed with the injured extremity in elevation; no further measures to reduce swelling, such as cooling or compression were carried out. Patients in the intervention group received a 15-min instruction in the VADOplex system (Fa. OPED GmbH, Oberlaindern, Germany), which they were required to use preoperatively without interruption if possible and postoperatively for at least 6–8 h daily. This air compressor inflates and deflates a pad with an air bladder attached to the sole of the foot or palm of the hand via Velcro straps to a pressure of 130 or 80 mmHg, respectively, within one second at intervals of 20 s. Stable fractures were immobilized in a splint or cast. Unstable fractures were reduced and immobilized with an external fixator until definitive surgical treatment.
The time from hospital admission to operability in days was defined as the study’s primary endpoint. Operability was evaluated by an independent observer (one of two senior physicians in trauma surgery who were not involved in the study) during the daily ward round. As practiced in preliminary studies, this was evaluated as soon as the normal skin wrinkling became apparent again52,53. In a preliminary study, almost complete agreement (κ = 0.816) was found between the two observers regarding the assessment of operability39.
The secondary endpoints (1) length of stay (days), (2) pain intensity (VAS), (3) pain medication (according to WHO step scheme), (4) soft-tissue swelling (cm) to calculate oedema reduction (%/d), (5) complications (n), and (6) revisions (n) were collected during a daily study visit. Swelling of the soft tissues was measured in cm circumference and, derived from this, reduction in swelling was evaluated as a percentage per day. For the evaluation of soft-tissue swelling in lower extremity fractures, the standardized measurement points at the level of the medial knee joint space and at the smallest tibial circumference were used. For proximal and distal humerus fractures, these points were 15 cm proximal and at the elbow level, and for elbow and distal radius fractures at the elbow level and 10 cm distally. These were the sites of greatest swelling and the measurement points closest to the fracture, thus the most clinically relevant. The mean percentage swelling at these points was assessed and further evaluated. Girth measurements were used since volumetric measurement was not suitable. This was because the unstable fractures included were reduced using an external fixator and measurements were obtained during daily work on the surgical ward.
In a previously conducted study, sample size calculation led to a group size of 17 patients per group to detect a clinically relevant and statistically significant difference of 2 ± 2 days induced by VIT therapy39,51. Although this number had been calculated for fractures of the ankle joint, it was also applied for the total number of patients in this study due to insufficient data for other anatomic regions.
Statistics
All data were described with suitable measurements of central tendency and dispersion. All confidence intervals (CIs) presented are 95% confidence intervals.
Data were analyzed for all fractures as a whole, as well as separately for the upper and lower extremities. All statistical tests were performed with a two-sided significance level of 5%. No correction for multiple testing was performed due to the non-confirmatory design.
All endpoints were evaluated in the modified intention-to-treat (mITT) population, which includes all patients who underwent surgery because of their fracture in the group they were allocated to by randomization. Patients who underwent conservative treatment after randomization and therefore did not reach the primary endpoint were excluded from analysis. For the primary endpoint, additional sensitivity analyses were performed in the as-treated (AT) population. In the sensitivity analysis, patients were allocated to the intervention group or control group according to the protocol they were treated after mainly (AT), correcting for treatment changers from the intervention group to the control.
Multiple linear regression was performed to analyze a possible influence of the demographic variables (a) age, (b) gender, (c) education level, (d) comorbidities, (e) smoker, or (f) affected side on the primary endpoint.
Interval scaled variables were analyzed using Welch’s t-test. Ordinal scaled variables were analyzed using the Mann–Whitney U test with continuity correction. Fisher’s exact test was used in the case of categorical variables.
The evaluation was carried out using the program R in version 3.6.3.
The graphics were created in Prism program from GraphPad Software, version 8.3.1.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ethics Committee of the State Medical Association of the Rhineland-Palatinate and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article. Data collection, coding, routing, and analysis were in accordance with legal data protection policy.
Data availability
The datasets generated and analyzed during the current study are available as a supplementary file 1.
References
Tull, F. & Borrelli, J. Jr. Soft-tissue injury associated with closed fractures: Evaluation and management. J. Am. Acad. Orthop. Surg. 11(6), 431–438 (2003).
Rueger, J. et al. Frakturen des distalen radius. Unfallchirurg 117(11), 1025–1036 (2014).
Reul, M. et al. Intraartikuläre Tibiakopffrakturen. Z. Orthop. Unfall. 155(03), 352–370 (2017).
Lambert, S. M. Ischaemia, healing, and outcomes in proximal humeral fractures. EFORT Open Rev. 3(5), 304–315 (2018).
Ott, N. et al. Komplikationsmanagement bei traumatischer Ellenbogeninstabilität. Arthroskopie 2, 1–9 (2020).
Culemann, U. Schaft- und distale Humerusfrakturen. Trauma Berufskrankheit 18(5), 468–473 (2016).
Bruder, A. M. et al. Prescribed exercise programs may not be effective in reducing impairments and improving activity during upper limb fracture rehabilitation: A systematic review. J. Physiother. 63(4), 205–220 (2017).
Ketterl, R. Vorgehen bei Unterschenkelfrakturen mit schwerem Weichteilschaden. Trauma Berufskrankheit 3(2), S167–S173 (2001).
Schaser, K.-D. et al. Effect of soft tissue damage on fracture healing: Intravital microscopic and biomechanical investigations in rats. In Chirurgisches Forum 2003 für experimentelle und klinische Forschung: 120. Kongress der Deutschen Gesellschaft für Chirurgie München, 29. 04. – 02.05.2003 (eds Menger, M. D. et al.) 9–11 (Springer, 2003).
Schaser, K. D. et al. Temporal profile of microvascular disturbances in rat tibial periosteum following closed soft tissue trauma. Langenbecks Arch. Surg. 388(5), 323–330 (2003).
Zhang, L. et al. Immediate microcirculatory derangements in skeletal muscle and periosteum after closed tibial fracture. J. Trauma 54(5), 979–985 (2003).
Tscherne, H. & Regel, G. Unfallchirugie: Trauma-Management. Tscherne Unfallchirurgie vol. 1 407 (Springer, 1997).
Schaser, K. D. et al. In vivo analysis of microcirculation following closed soft-tissue injury. J. Orthop. Res. 17(5), 678–685 (1999).
Blumberg, H., Griesser, H. J. & Hornyak, M. Distal post-traumatic edema—symptom of a sympathetic reflex dystrophy (Sudeck’s disease)?. Z. Orthop. Ihre Grenzgeb 130(1), 9–15 (1992).
Sandkühler, J. Schmerzgedächtnis: Entstehung, Vermeidung und Löschung. Dt Ärztebl 98(42), 2725–2730 (2001).
Lindahl, O. Experimental skin pain induced by injection of water-soluble substances in humans. Acta Physiol. Scand. Suppl. 179, 1–89 (1961).
Tscherne, H. & Lobenhoffer, P. Tibial plateau fractures. Management and expected results. Clin. Orthop. Relat. Res. 292, 87–100 (1993).
Tscherne, H., Lobenhoffer, P. & Russe, O. Proximal intra-articular tibial fractures. Unfallheilkunde 87(7), 277–289 (1984).
Giudice, M. L. Effects of continuous passive motion and elevation on hand edema. Am. J. Occup. Ther. 44(10), 914–921 (1990).
Tsang, K. K., Hertel, J. & Denegar, C. R. Volume decreases after elevation and intermittent compression of postacute ankle sprains are negated by gravity-dependent positioning. J. Athl. Train 38(4), 320–324 (2003).
Gardner, A. & Fox, R. The Return of Blood to the Heart: Venous Pumps in Health and Disease (Libbey, 1989).
Gardner, A. M. et al. Reduction of post-traumatic swelling and compartment pressure by impulse compression of the foot. J. Bone Jt. Surg. Br. 72(5), 810–815 (1990).
Gardner, A. M. N. & Fox, R. H. The venous footpump: Influence on tissue perfusion and prevention of venous thrombosis. Ann. Rheum. Dis. 51(10), 1173–1178 (1992).
Nicholas, J. S. The swollen hand. Physiotherapy 63(9), 285–286 (1977).
Horwood, A. The biomechanical function of the foot pump in venous return from the lower extremity during the human gait cycle: An expansion of the gait model of the foot pump. Med. Hypotheses 129, 109220 (2019).
Morris, R. J. & Woodcock, J. P. Evidence-based compression: Prevention of stasis and deep vein thrombosis. Ann. Surg. 239(2), 162–171 (2004).
Corley, G. J. et al. The anatomy and physiology of the venous foot pump. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 293(3), 370–378 (2010).
Simons, P. et al. Venous pumps of the hand: Their clinical importance. J. Hand Surg. 21(5), 595–599 (1996).
Petersen, W., Zantop, T. & Raschke, M. Tibiakopffraktur. Unfallchirurg 109(3), 219–234 (2006).
Ivanic, G. M. et al. Intermittent compression devices for swelling reduction and thrombosis prophylaxis—a pilot study after total hip replacement Is the 2 hour daily minimum application sufficient?. Unfallchirurg 109(9), 786–792 (2006).
Wilson, N. V. et al. Thrombo-embolic prophylaxis in total knee replacement. Evaluation of the A–V impulse system. J. Bone Jt. Surg. Br. 74(1), 50–52 (1992).
O’Connell, S. et al. The use of intermittent pneumatic compression in orthopedic and neurosurgical postoperative patients: A systematic review and meta-analysis. Ann. Surg. 263(5), 888–889 (2016).
Stannard, J. P. et al. Prophylaxis of deep venous thrombosis after total hip arthroplasty by using intermittent compression of the plantar venous plexus. Am. J. Orthop. (Belle Mead NJ) 25(2), 127–134 (1996).
Caschman, J., Blagg, S. & Bishay, M. The efficacy of the A–V impulse system in the treatment of posttraumatic swelling following ankle fracture: A prospective randomized controlled study. J. Orthop. Trauma 18(9), 596–601 (2004).
Dodds, M. K. et al. Effectiveness of ‘in-cast’ pneumatic intermittent pedal compression for the pre-operative management of closed ankle fractures: A clinical audit. Foot Ankle Surg. 20(1), 40–43 (2014).
Thordarson, D. B. et al. Facilitating edema resolution with a foot pump after calcaneus fracture. J. Orthop. Trauma 13(1), 43–46 (1999).
Thordarson, D. B., Ghalambor, N. & Perlman, M. Intermittent pneumatic pedal compression and edema resolution after acute ankle fracture: A prospective, randomized study. Foot Ankle Int. 18(6), 347–350 (1997).
Myerson, M. S. & Henderson, M. R. Clinical applications of a pneumatic intermittent impulse compression device after trauma and major surgery to the foot and ankle. Foot Ankle 14(4), 198–203 (1993).
Schnetzke, M. et al. Vascular impulse technology versus elevation for the reduction of swelling of lower extremity joint fractures: Results of a prospective randomized controlled study. Bone Jt. J. 103-b(4), 746–754 (2021).
Berlin, E. et al. Postmastectomy lymphedema. Treatment and a five-year follow-up study. Int. Angiol. 18(4), 294–298 (1999).
Ramesh, M. et al. Effectiveness of the AV impulse hand pump. J. Bone Jt. Surg. Brit. VoL. 81(2), 229–233 (1999).
Svensson, B. H. et al. Effect of pneumatic compression in connection with ergotherapeutic treatment of Colles’ fracture. A clinical controlled trial. Ugeskr Laeger 155(7), 463–466 (1993).
Mader, K. et al. Efficacy of the AV Impulse System versus cryotherapy in the reduction of postoperative oedema of the hand: A prospective randomised trial. Strat. Trauma Limb Reconstr. 1(1), 36–41 (2006).
Hazarika, E. Z., Knight, M. T. & Frazer-Moodie, A. The effect of intermittent pneumatic compression on the hand after fasciectomy. Hand 11(3), 309–314 (1979).
Abu-Own, A. et al. Effects of intermittent pneumatic compression of the foot on the microcirculatory function in arterial disease. Eur. J. Vasc. Surg. 7(5), 488–492 (1993).
Morgan, R. et al. Arterial flow enhancement by impulse compression. Vasc. Surg. 25(1), 8–15 (1991).
Credeur, D. P. et al. Effects of intermittent pneumatic compression on leg vascular function in people with spinal cord injury: A pilot study. J. Spinal Cord Med. 42(5), 586–594 (2019).
Alkner, B. A. et al. Effect of postoperative pneumatic compression after volar plate fixation of distal radial fractures: A randomized controlled trial. J. Hand Surg. (Eur. Vol.) 43(8), 825–831 (2018).
Yamazaki, H. et al. Venous perfusion assist system has no additional effect compared to simple elevation on post-operative edema in patients with distal radial fracture treated with volar locking plate fixation: A randomized controlled trial. J. Orthop. Sci. 24(3), 441–446 (2019).
Gan, X.-H. et al. Effect of AV impulse pump on pain and swelling-alleviating for patients with tibia and fibula fracture. J. Nurs. 7, 25 (2010).
Schnetzke, M. et al. Vascular Impulse Technology versus elevation in the treatment of posttraumatic swelling of extremity fractures: Study protocol for a randomized controlled trial. Trials 18(1), 73 (2017).
Chou, L. B. & Lee, D. C. Current concept review: Perioperative soft tissue management for foot and ankle fractures. Foot Ankle Int. 30(1), 84–90 (2009).
Harding, D. & Waddell, J. P. Open reduction in depressed fractures of the os calcis. Clin. Orthop. Relat. Res. 199, 124–131 (1985).
Funding
Open Access funding enabled and organized by Projekt DEAL. We gratefully acknowledge the funding of the BG Trauma Center Ludwigshafen and the company OPED. The sponsors had no influence on the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.F. and M.S.; methodology, M.S. and J.S.E.B.; validation, S.Y.V., P.A.G. and J.F.; formal analysis, L.B., M.S. and J.S.E.B.; investigation, M.S., M.B., B.S. and J.S.E.B.; resources, P.A.G.; data curation, L.B., J.S.E.B. and M.S.; writing—original draft preparation, J.S.E.B.; writing—review and editing, every author; visualization, J.S.E.B. and L.B.; supervision, J.F.; project administration, J.F.; all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The other authors declare an institutional grant from OPED GmbH but none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Barbari, J.S., Schnetzke, M., Bergmann, M.B. et al. Vascular impulse technology versus elevation for reducing the swelling of upper and lower extremity joint fractures. Sci Rep 13, 661 (2023). https://doi.org/10.1038/s41598-022-27231-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-27231-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.