Abstract
Orthodontic tooth movement (OTM) occurs through proteolytic remodelling within the periodontium following the application of external force to the tooth. This study describes the first characterization of the salivary peptidome and protease profile during the alignment stage of fixed appliance orthodontic treatment. Unstimulated whole mouth saliva from 16 orthodontic patients (10 males, 6 females, mean (SD) age 15.2 (1.6) years) was collected prior to fixed appliance placement (T1), 1-h (T2), 1-week (T3) following fixed appliance placement and on completion of mandibular arch alignment (T4). Salivary peptides were extracted using filtration followed by mass spectrometry to identify amino acid sequences. Protease prediction was carried out in silico using Proteasix and validated with gelatin zymography and enzyme-linked immunosorbent assay. A total of 2852 naturally-occurring peptides were detected, originating from 436 different proteins. Both collagen and statherin-derived peptide levels were increased at T2. Proteasix predicted 73 proteases potentially involved in generating these peptides, including metalloproteinases, calpains and cathepsins. Changes in predicted activity of proteases over time were also observed, with most metalloproteinases showing increased predicted activity at T2–T3. Increased gelatinolytic activity and MMP8/MMP9 levels were detected at T3. Collectively, multiple protein targets and changes in protease-predicted activity during OTM have been identified.
Similar content being viewed by others
Introduction
Orthodontic tooth movement (OTM) provides the basis of orthodontic treatment and involves co-ordinated tissue remodelling in the periodontal ligament and alveolar bone following force application to the tooth1. Force transduction within the periodontium triggers a localised aseptic inflammation mediated by cytokines, prostaglandins, tissue necrosis factors and proteases, followed by degradation or synthesis of extracellular matrix (ECM) in the periodontal ligament and alveolar bone. These processes, in turn promote the secretion of many proteins and enzymes into the gingival crevicular fluid (GCF) and whole mouth saliva (WMS)1,2.
Several enzymes have been identified in WMS, including amylase, carbonic anhydrase, catalase, and proteases3. Proteases are one of the largest families, with more than 500 encoded in the human genome4. Initiation, progression, and resolution of inflammation, as well as ECM remodelling are regulated by proteases5. Cathepsins and matrix metalloproteinases (MMPs) are involved in remodelling of periodontal ligament, ECM and alveolar bone1, in particular GCF MMP1–3, MMP8–9, MMP13, MMP142,6,7,8,9 and WMS MMP8–9, MMP12 concentrations all increasing during OTM10.
WMS is a complex fluid that contains secretions from all the major and minor salivary glands, constituents from GCF, the oral microbiome and dietary inputs. More recently, proteomic and peptidomic non-targeted approaches of WMS have provided biomarkers for the early detection, progression, monitoring, and responses to many oral and systemic disorders treatments11,12. In silico prediction of proteases using the open-source software Proteasix (www.proteasix.org) has predicted proteases potentially involved in the generation of the peptides in patients with periodontitis13, wound infection14, diabetic nephropathy15 and cardiorenal syndrome16. Proteasix uses information about naturally-occurring peptides corresponding to the protein using the UniProt ID as identified by mass spectrometry to predict potential cleaving proteases. Retrieving information about cleavage sites from protease databases (MEROPS, BRENDA) allows the generation of a list of predicted proteases17. The analysis of cleavage site-specificity by Proteasix can predict multiple proteases from a single sample revealing the proteolytic events underlying physiological and pathological processes taking place within the oral cavity18.
To our knowledge, the WMS natural peptidome generated during OTM has not previously been investigated. This retrospective longitudinal study has used a peptidomic approach, supplemented with mass spectrometry and bioinformatics to predict the profile and activity pattern of proteases responsible for WMS peptide generation. Susceptible protein targets have been identified, and time-dependent changes in the salivary peptidome and predicted proteases have been assessed during the alignment stage of orthodontic treatment with fixed appliances. Further targeted approaches used zymography and ELISA to validate the prediction results.
Results
This study investigated WMS from 16 participants (10 male, 6 female) with a mean (SD) age of 15.2 (1.6) years and a mean (SD) crowding of 6.4 (2.2) mm, with only three participants having extraction-based treatment. Mean plaque index was: T1 0.57 (0.3); T3 0.76 (0.32); T4 1.09 (0.40); whilst gingival index was T1 0.73 (0.31); T3 0.84 (0.22); T4 1.25 (0.35). Plaque and gingival indices were increased significantly at T4 compared with T1 (p < 0.001). Mean WMS flow-rate (ml/min) was: T1 0.67 (0.29); T2 0.85 (0.37); T3 1.03 (0.45); T4 0.93 (0.37). In addition, mean protein content (mg/ml) was: T1 1.51 (0.48); T2 1.55 (0.34); T3 1.65 (0.68); T4 1.14 (0.48). No statistically significant differences were detected in either measurement between time-points (assessed using repeated measures ANOVA).
Peptidome characteristics of WMS
A total of 2852 naturally-occurring peptides were identified by mass spectrometry originating from 436 different proteins with 49 common to all time-points (Fig. 1a). Percentage of peptides identified for each common protein was calculated (Fig. 1b) (Supplementary Appendix Table S1). The most abundant belonged to the major salivary proteins, mainly proline-rich proteins, statherin, histatins and P-B peptide. When the percentages of peptides identified for each common protein were compared over time, no statistically significant changes were found for PIGR, PRP1, PRB2, PRB3, PRBC, SMR3B, HIS1 at all time-points. Statistically significant degradation of STAT, PROL4, CO1A1 (all p < 0.05) and CO2A1 (p < 0.01) were observed at T2 compared with T1, with this degradation returning to baseline levels at T4. Peptides belonging to PRR27 were significantly increased at T2 and T3 (both p < 0.05), also returning to baseline at T4. Conversely, the percentages of peptides belonging to PRB4 were significantly decreased at T2 (p < 0.01), returning to T1 levels by T4. Percentages of peptides belonging to HIS3 were significantly decreased at both T2 (p < 0.01) and T3 (p < 0.05) (Supplementary Appendix Fig. S1).
(a) Venn diagram showing the distribution of proteins of origin of the identified peptides by mass spectrometry at four time-points. T1 baseline (before placement of orthodontic appliance), T2 1 h after placement of orthodontic appliance, T3 1 week after placement of orthodontic appliance, T4 end of the alignment. (b) Heatmap displaying the percentages of peptides for the most abundant common proteins among all the participants (p1, p2, p3, p4, and p5) at four time-points. The scale refers to the percentages of peptides of each protein in the total number of peptides for each participant.
Prediction of protease activity
The profile of all predicted proteases is shown in Fig. 2. In total, 73 were predicted to be active in WMS of participants (Table 1). For each protease, the percentage of cleavage from total cleavage events for each participant was calculated and a percentage threshold of cleavage set at 1% to consider activity of a particular protease (Fig. 2)16. Twenty-four proteases had a percentage threshold of cleavage > 1% and amongst these, calpains, MMPs and cathepsins were the most prevalent groups and potentially implicated in the generation of WMS peptides at all time-points. When the predicted activity of these proteases was compared over time, there was a statistically significant increase of CTSG (cathepsin G) (p < 0.05, p < 0.05), ELANE (neutrophil elastase) (p < 0.001, p < 0.01), MMP13 (p < 0.01, p < 0.05), MMP3 (p < 0.01, p < 0.05), MMP8 (p < 0.001, p < 0.01), PGA3 (pepsin) (p < 0.01, p < 0.05) at both T2 and T3 compared to T1. Predicted activity of MME (neprilysin) (p < 0.05), MMP25 (p < 0.05), MMP9 (p < 0.05) was significantly increased at T2, whereas MMP12 (p < 0.05) predicted activity was significantly increased at T4 compared to T1. In contrast, predicted activity of CAPN1 (p < 0.001, p < 0.001), CAPN2 (p < 0.001, p < 0.001), CTSK (cathepsin K) (p < 0.01, p < 0.05), MEP1A (p < 0.01, p < 0.05), TMPRSS7 (p < 0.01, p < 0.05) was significantly decreased at both T2 and T3 compared to T1. Furthermore, predicted activity of MMP7 (p < 0.01), KLK4 (kallikrein 4) (p < 0.05) was significantly decreased at T3 and T2. No changes in level of predicted activity of ADAMTS4, CTSB (cathepsin B), CTSL (cathepsin L), CTSS (cathepsin S), KLK6 (kallikrein 4), MMP14, PLG (plasminogen) were found at any time-points.
Graph showing the profile of all proteases as predicted by Proteasix. The bars represent the percentage of cleavages for each predicted protease at four time-points. T1 baseline (before placement of orthodontic appliance), T2 1 h after placement of orthodontic appliance, T3 1 week after placement of orthodontic appliance, T4 end of the alignment. The interrupted line represents a percentage threshold (1%) to consider the activity of the proteases. Data are shown as mean ± SD. Data were analysed by repeated measures ANOVA; *p < 0.05; **p < 0.01; ***p < 0.001.
Targeted approaches to validate proteases predictions
WMS from all 16 participants was used with gelatin zymography to directly assess gelatinolytic activity and with ELISA to assess protease abundance. Three distinct bands were identified in all samples at approximately 190 kDa (band 1), 72 kDa (band 2) and 62 kDa (band 3) (Fig. 3a) (Supplementary Appendix Fig. S2). Gelatinolytic activity increased over time and returned to baseline levels at T4, being around 1.8× more elevated at T3 than T1 for band 2 (p < 0.01) and 1.4× more at T3 than T1 for band 3 (p < 0.05) (Fig. 3b–d). ELISA demonstrated that MMP8 levels increased over time but were only statistically significant at T3 and T4 compared with T1 (p < 0.001; p < 0.05, respectively) (Fig. 4a). MMP9 levels were significantly increased at T3 compared with T1 (p < 0.01) (Fig. 4b).
Gelatin zymography for the investigation of gelatinolytic activity in unstimulated whole mouth saliva of 16 participants at four time-points. (a) Representative example of Coomassie-stained zymogram gel demonstrating bands with gelatinolytic activity at three different molecular weights (uncropped gel with original arrangement of lanes is presented in Supplementary Appendix Fig. S2). (b–d) Relative quantification of band intensity of bands 1, 2, and 3. The fold change of gelatinolytic activity at T2, T3, and T4 for each band was assessed relative to T1. T1 baseline (before placement of orthodontic appliance), T2 1 h after placement of orthodontic appliance, T3 1 week after placement of orthodontic appliance, T4 end of the alignment, M molecular weight markers, MMP-9 std matrix metalloproteinase-9 standard; *p < 0.05; **p < 0.01.
Graphs showing the levels of matrix metalloproteinase-8 (MMP8) (a) and matrix metalloproteinase-9 (MMP9) (b), as measured by ELISA (Enzyme-linked immunosorbent assay), in unstimulated whole mouth saliva of 16 participants at four time-points. T1 baseline (before placement of orthodontic appliance), T2 1 h after placement of orthodontic appliance, T3 1 week after placement of orthodontic appliance T4 end of the alignment. Data were analysed by repeated measures ANOVA; *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
This study describes the first characterisation of the salivary peptidome and protease profile during OTM using a peptidomic approach aided by mass spectrometry and bioinformatics. Proteolytic activity plays a crucial role in ECM remodelling and several enzymes have been implicated including serine, aspartate, and cysteine proteases19. Our non-targeted approach identified 73 predicted proteases responsible for producing peptides of which only 57 matched to a previous study of WMS using the same software13. The additional 16 novel proteases could be attributed to changes in the Proteasix algorithm since the original publication4, age-related differences in the WMS proteome and peptidome20 or more likely the effect of OTM. From the 73, 24 had > 1% cleavage16 with calpains, MMPs, cathepsins the most prevalent groups.
OTM occurs through remodelling of the periodontium mediated by acute inflammation characterized by vascular changes, leukocyte1,8,19 and neutrophil infiltration21 as early as 1-h following the application of orthodontic force22. Thereby, the time-points used in this study were chosen in an attempt to observe the different biological changes that underpin OTM23. A statistically significant increase in predicted activity of CTSG, ELANE, PGA3 was found at 1-h and 1-week following appliance placement. Neutrophil azurophilic granules contain ELANE and CTSG, which regulate inflammation and modulation of the immune response14,24 through retention of pro- and anti-inflammatory activities21. These proteases have not previously been investigated in relation to OTM; however, they seem to have an important role in regulation of the acute inflammatory reaction during initial movement.
MMPs play a pivotal role in ECM remodelling and are involved in inflammatory regulation19 and MMP8, MMP9 and MMP13 are produced by polymorphonuclear leukocytes8,24,25. MMPs have been extensively investigated in GCF and to a lesser extent WMS, during OTM. Our results reveal significantly increased predicted activity of MMP3, MMP8, MMP13 at 1-h and 1-week, whilst MMP9, MMP25, MME were significantly increased 1-h after appliance placement and MMP12 only at the end of alignment. These predictions were validated by ELISA, which confirmed MMP8 levels were significantly increased at 1-week until end of alignment, whereas MMP9 was significantly increased only at 1-week. In contrast to the predicted results, no statistically significant difference was identified in MMP8 or MMP9 levels 1-h after appliance placement when assessed by ELISA possibly because ELISA detects total MMPs (both pro- and active forms)26 and actual activity might be masked by total protein measurement. Notably, WMS MMP8, MMP9, MMP12 levels increased at 1-h10 and 1-week27 and GCF MMP3, MMP8, MMP9, MMP13 levels have previously positively correlated with OTM2,6,7,8,9. It has also been shown that orthodontic force application significantly increases MMP88, MMP3, MMP13, and MMP9 levels28 in GCF 1-h after orthodontic appliance activation, which agrees with our predicted results.
MME has never previously been studied in OTM; however, one previous study reported that MME mRNA levels were upregulated in periodontitis-affected gingival tissues compared with healthy tissues and that MME expression was detected in neutrophils and fibroblasts in those tissues. In addition, they reported that MME contributes to the regulation of inflammation by degrading IL-1β, which is a crucial inflammatory cytokine29. Therefore, MME may have a role in the aseptic inflammatory reaction associated with OTM. Additionally, our predicted results showed that MMP7 activity was significantly decreased 1-week after appliance placement, which contradicts previous data30,31.
Our results showed that CAPN1, CAPN2, MEP1A, TMPRSS7 and KLK4 predicted activity was significantly decreased during OTM however, no information is available concerning the production of these proteases during this process. There are few studies on the role of cathepsin during OTM in humans whilst in rats OTM induced a statistically significant increase in CTSK gene expression32. Periodontal cells express CTSB (cathepsin B) but not CTSK33; Previous studies reported contradictory results on the level of CTSB in GCF; one study reported on an increase in the level of CTSB after 24-h but no change after 1-h and 1-week of OTM33 and the second study reported on a decrease in the level of CTSB after 24-h34. Therefore, further investigations are necessary to establish the link between cathepsins and OTM in humans.
Proteolysis is the primary source of peptides and significant efforts have been made to identify resultant fragments, cleavage sites and implicated proteases. The most abundant peptides belonged to the major salivary proteins, mainly proline-rich statherin, histatins and P-B peptide25,35. The ECM of soft and hard periodontal tissues is comprised mainly of type I collagen. Collagen breakdown is considered critical for periodontal and alveolar bone remodelling associated with OTM36,37,38 and total type I collagenase activity in GCF is increased 10-times in GCF of orthodontic patients36. In the present study, peptides derived from COL1A1, COL1A2 were significantly increased 1-h after appliance placement, suggesting that these proteins display high susceptibility to proteolysis during OTM. This is supported by our protease activity predictions, which linked with the increased levels of detected collagen-derived peptides suggest that MMPs play roles in the breakdown of collagen and ECM remodelling during OTM. MMP8 and MMP13 are members of the collagenase group and MMP9 is a member of the gelatinase group; these proteases are effective in degrading type I collagen and gelatin8,36 and our data demonstrated increased predicted activity of both. Moreover, our gelatin zymography results showed increased gelatinolytic activity for two bands identified at 72 and 62 kDa over time but was statistically significant only 1-week after appliance placement. Differences in methodology could justify the inability to detect gelatinolytic activity at T2 but possibly suggests that a peptidomic approach might provide a better tool to detect the activity of proteases. Additionally, the increase in statherin-derived peptides 1-h after appliance placement maybe because fixed orthodontic appliance placement involving acid-etching of the teeth results in demineralization, and statherin is known to be involved in calcium homeostasis and teeth remineralization25,35. It also has a strong affinity for the tooth surface and was identified previously in the pellicle formed on metallic brackets39.
There were no statistically significant changes in plaque and gingival indices after 1-h and 1-week of orthodontic force application compared to baseline levels, and linear regression results showed no statistically significant association between the levels of MMP8, MMP9 with plaque and gingival indices over-time. Therefore, since changes in the proteolytic activity were observed in this study 1-h and 1-week after orthodontic appliance placement, we may assume that these changes were induced by orthodontic force rather than by bacterial plaque or gingival inflammation, suggesting that orthodontic forces modulate the proteolytic activity in the periodontal tissues.
Limitations of this study include being a retrospective in design, and using a small sample size with the mass spectrometry and bioinformatics approaches. Therefore, prospective clinical trials with a larger sample size should be ideally conducted. Additionally, further research focusing on the detailed characterization of the role of each identified protease and its substrates may enhance our understanding of the biology of OTM and possibly reveal novel biomarkers associated with OTM.
In conclusion, the profile and activity pattern of proteases responsible for salivary peptide generation have been mapped and susceptible protein targets have been identified during the alignment stage of fixed appliance orthodontic treatment. The proteases detected in WMS showed changes over time, with most MMPs and proteases associated with inflammation showing increases as early as 1-h after appliance placement, supported by increased collagen-derived peptides levels. Protease prediction from peptidome data demonstrates a potential tool for identifying and discriminating between different phases of OTM.
Materials and methods
Study design and participants
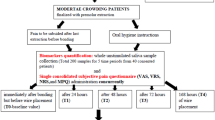
This retrospective longitudinal study evaluated WMS from 16 participants during the alignment phase of orthodontic treatment with fixed appliances. Participant inclusion was based upon the following criteria: undergoing fixed appliance orthodontic treatment (with or without tooth extractions); mandibular arch incisor irregularity of 4–12 mm; 12–18 years old at treatment-start; medically fit and healthy; taking no prescription medication and normal-weight body mass index.
Ethical approval was obtained from the United Kingdom National Research Ethics Service, NRES Committee foundation (14/LO/0769), and written informed consent was received from all parents, guardians, and children before sample collection. All methods were performed in accordance with the approved guidelines and regulations. All participants had their fixed appliances (Victory-APC 0.022-inch brackets, MBT prescription; 3M-Unitek) placed in the Department of Orthodontics, Faculty of Dentistry, Oral & Craniofacial Sciences, King’s College London (Guy’s and St Thomas; NHS Foundation Trust). A specific arch wire sequence was followed (0.014-inch nickel titanium; 0.018-inch nickel titanium; 0.017 × 0.025-inch nickel titanium and 0.019 × 0.025-inch stainless steel) and participants were seen every 6 weeks. Participants were followed up to completion of alignment between January 2015 and June 2016.
Sample size calculation was based on a previous study investigating time-dependent changes in salivary levels of MMP8 and MMP9 during orthodontic treatment. In this study, differences in levels of salivary biomarkers between different time-points were identified with a mean effect size of 0.8740. Using G*Power 3.1.9.7 software41, a sample size of 13 was estimated to be sufficient to detect a significant difference in salivary biomarker levels between the different time-points (assuming a power of 80% and a significance level of 5%). A sample size of 16 was used to compensate for power underestimation between the biomarkers.
WMS collection and processing
Unstimulated WMS was collected at 4 time-points: (T1) start-of-treatment; (T2) 1-h and (T3) 1-week following fixed appliance placement; and (T4) completion of alignment (0.019 × 0.025-inch stainless steel rectangular archwire placed in the lower arch). WMS samples were centrifuged at 9200×g for 5-min, aliquoted, labelled and stored at − 80 °C. Samples were defrosted on ice and total protein concentration measured using a Bicinchoninic Acid (BCA) Protein Assay (Thermo-Scientific). For all participants, plaque levels and gingival health were measured at T1, T3 and T4 using established validated indices. The thickness of dental plaque adjacent to the gingival margin was measured using Silness and Löe criteria42, where a score from 0 to 3 is ascribed to each of the four surfaces of the tooth; these scores are then added and divided by four to provide the plaque index for each tooth. The plaque index for the individual is then calculated by adding the individual scores from each tooth and dividing them by the number of teeth examined. The gingival index was assessed using the same approach following Löe and Silness criteria43.
Separation of naturally occurring peptides from WMS
Naturally-occurring peptides were collected from WMS of 5 participants at each time-point (20 samples). Ten kDa cut-off spin-filters (Merck-Millipore) were washed and conditioned with 500 µL 10 mM ammonium bicarbonate solution by centrifugation at 4000 rpm for 25-min. Spin-filters were loaded with 1 mL WMS, centrifuged at 4000 rpm for 25-min with the resulting filtrate peptides collected and sent for mass spectrometry (Cambridge Institute for Medical Research Proteomics Centre, UK).
Mass spectrometry
A 100 µL WMS peptide sample was dried-down using a Savant SpeedVac Concentrator (Thermo-Scientific), solubilized in a 50 µL loading-solvent (3% acetonitrile; 0.1% trifluoroacetic acid) and 1 µL analysed by LC-MSMS using a Q-Exactive-Plus coupled to an RSLCnano3000 (Thermo-Scientific). Peptides were resolved on a 50 cm EASY-spray column (Thermo-Scientific) using a gradient rising from 3 to 40% solvent B (80% MeCN, 0.1% formic acid) by 90-min. MS-spectra were acquired at 70,000 (fwhm) between m/z 400 and 1500. Filtrate peptides were analysed by LC-MSMS using a Q-Exactive-Plus coupled to an RSLCnano3000 (Thermo-Scientific). Peptides were resolved on a 50 cm EASY-spray column (Thermo-Scientific) using a gradient rising from 10 to 40% solvent B (80% MeCN, 0.1% formic acid) by 42-min. The S-lens FR level was set to 50. MS-spectra were acquired at 70,000 (fwhm) between m/z 200 and 2000. Data were processed using PEAKS Studio (version X, Bioinformatics Solutions) with the following parameters: no enzyme; human database (UniProt reference proteome downloaded 18 Dec 2018 containing 21,066 proteins) or bacterial database (Uniprot proteome downloaded 5 Apr 2019 containing 161,286 proteins) with additional contaminant database (containing 246 common contaminants); oxidation (M), carbamidomethylation (C) as variable modifications at the PEAKS-DB stage, LFQ was carried out using PEAKS-LFQ using normalization by total protein intensity. Protein and protein-peptide information were exported from PEAKS-Studio.
Zymography
Samples were analysed using 10% zymogram gelatin gel electrophoresis. Equal amounts of non-reducing Tris–Glycine SDS sample buffer (2×) and samples (10 µg) were loaded into the gel wells and run at 125v constant for 90-min with 10× Tris–Glycine SDS running. The gel was then placed in a zymogram renaturing buffer for 30-min, zymogram developing buffer (Life Technologies) for another 30-min (later replaced with a fresh developing buffer) and the gel incubated overnight at room temperature. Thereafter, the gel was stained with Coomassie Brilliant Blue and de-stained. Protease digestion appeared as transparent bands against a darkly stained blue background. Zymogram gel was scanned with ChemiDoc™ MP Imaging System (BioRad). Densitometric analysis was performed with Image J44.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) (R&D Systems) was used to assess human total MMP8 and MMP9 (active and pro-active forms).
Bioinformatic analysis
Protease prediction was carried out using the Proteasix tool in function of the peptides identified by mass spectrometry. Proteasix is an open-source peptide-centric tool that can be used to predict in silico the proteases involved in naturally occurring peptide generation and returns all possible proteases at a cleavage site (http://www.proteasix.org)17. Jvenn (http://bioinfo.genotoul.fr/jvenn/example.html) is an online Venn diagram tool used to find unique proteins at each time-point as well as those common to T1, T2, T3, T4.
Statistical analysis
Data were tested for normality using Shapiro–Wilk and Kolmogorov–Smirnov tests. Repeated measures ANOVA was used to analyse the normally-distributed data followed by correction for multiple testing using Dunnett’s multiple-comparisons test. Friedman test was used to analyse the non-normally distributed data followed by correction for multiple testing using Bonferroni. Regression-analysis was performed of the outcome (MMP8, MMP9-levels), explorative factor (plaque index or gingival index) and variation through time-points. Statistical analysis was performed using GraphPad Prism version 9.0 (GraphPad Software). The differences were considered statistically significant if p < 0.05.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
References
Li, Y., Jacox, L. A., Little, S. H. & Ko, C. C. Orthodontic tooth movement: The biology and clinical implications. Kaohsiung J. Med. Sci. 34, 207–214 (2018).
Garlet, T. P., Coelho, U., Silva, J. S. & Garlet, G. P. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur. J. Oral Sci. 115, 355–362 (2007).
Castagnola, M., Cabras, T., Vitali, A., Sanna, M. T. & Messana, I. Biotechnological implications of the salivary proteome. Trends Biotechnol. 29, 409–418 (2011).
Mulkern, D. et al. Predicted salivary human protease activity in experimental gingivitis revealed by endoProteo-FASP approach. Eur. J. Oral Sci. 128, 386–394 (2020).
Marshall, N. C., Finlay, B. B. & Overall, C. M. Sharpening host defenses during infection: Proteases cut to the chase. Mol. Cell Proteom. 16, S161-s171 (2017).
Bildt, M. M., Bloemen, M., Kuijpers-Jagtman, A. M. & Von den Hoff, J. W. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in gingival crevicular fluid during orthodontic tooth movement. Eur. J. Orthod. 31, 529–535 (2009).
Zhang, B., Yang, L., Zheng, W. & Lin, T. MicroRNA-34 expression in gingival crevicular fluid correlated with orthodontic tooth movement. Angle Orthod. 90, 702–706 (2020).
Apajalahti, S., Sorsa, T., Railavo, S. & Ingman, T. The in vivo levels of matrix metalloproteinase-1 and -8 in gingival crevicular fluid during initial orthodontic tooth movement. J. Dent. Res. 82, 1018–1022 (2003).
Cantarella, G. et al. Levels of matrix metalloproteinases 1 and 2 in human gingival crevicular fluid during initial tooth movement. Am. J. Orthod. Dentofacial Orthop. 130(568), e511–e566 (2006).
Xu, X. et al. Levels of matrix metalloproteinases in saliva during orthodontic tooth movement. Int. J. Clin. Exp. Med. 13, 1564–1571 (2020).
Zhang, J. et al. Magnetic bead-based salivary peptidome profiling analysis during orthodontic treatment durations. Biochem. Biophys. Res. Commun. 421, 844–849 (2012).
Pappa, E. et al. Downregulation of salivary proteins, protective against dental caries, in type 1 diabetes. Proteomes 9, 33 (2021).
Trindade, F. et al. Toward the definition of a peptidome signature and protease profile in chronic periodontitis. Proteom. Clin. Appl. 9, 917–927 (2015).
Hartman, E. et al. Bioinformatic analysis of the wound peptidome reveals potential biomarkers and antimicrobial peptides. Front. Immunol. 11, 620707 (2021).
Krochmal, M. et al. Urinary peptidomics analysis reveals proteases involved in diabetic nephropathy. Sci. Rep. 7, 15160 (2017).
Petra, E. et al. Urine peptidome analysis in cardiorenal syndrome reflects molecular processes. Sci. Rep. https://doi.org/10.1038/s41598-021-95695-z (2021).
Klein, J. et al. Proteasix: A tool for automated and large-scale prediction of proteases involved in naturally occurring peptide generation. Proteomics 13, 1077–1082 (2013).
Trindade, F. et al. EndoProteoFASP as a tool to unveil the peptidome-protease profile: Application to salivary diagnostics. Methods Mol. Biol. 1719, 293–310 (2018).
Krishnan, V. & Davidovitch, Z. E. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofacial Orthop. 129, e461–e469 (2006).
Cabras, T. et al. Age-dependent modifications of the human salivary secretory protein complex. J. Proteome Res. 8, 4126–4413 (2009).
Korkmaz, B., Horwitz, M. S., Jenne, D. E. & Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 62, 726–759 (2010).
Jayaprakash, P. K. et al. Elevated levels of Interleukin (IL)-1β, IL-6, tumor necrosis factor-α, epidermal growth factor, and β2-microglobulin levels in gingival crevicular fluid during human orthodontic tooth movement (OTM). J. Fam. Med. Prim. Care 8, 1602–1606 (2019).
Saloom, H. F., Papageorgiou, S. N., Carpenter, G. H. & Cobourne, M. T. Impact of obesity on orthodontic tooth movement in adolescents: A prospective clinical cohort study. J. Dent. Res. 96, 547–554 (2017).
Pisano, E. et al. Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS. Eur. J. Oral Sci. 113, 462–468 (2005).
Amado, F., Lobo, M. J., Domingues, P., Duarte, J. A. & Vitorino, R. Salivary peptidomics. Expert Rev. Proteom. 7, 709–721 (2010).
Cheng, X.-C., Fang, H. & Xu, W.-F. Advances in assays of matrix metalloproteinases (MMPs) and their inhibitors. J. Enzyme Inhib. Med. Chem. 23, 154–167 (2008).
Sioustis, I.-A. et al. Salivary metalloproteinase-8 and metalloproteinase-9 evaluation in patients undergoing fixed orthodontic treatment before and after periodontal therapy. Int. J. Environ. Res. Public Health 18, 1583 (2021).
Capelli Junior, J. et al. Matrix metalloproteinases and chemokines in the gingival crevicular fluid during orthodontic tooth movement. Eur. J. Orthod. 33, 705–711 (2011).
Nezu, A. et al. Expression of neprilysin in periodontitis-affected gingival tissues. Arch. Oral Biol. 79, 35–41 (2017).
Patil, A., Sable, R., Aphale, S., Moge, A. & Oswal, D. Levels of matrix metalloproteinase-7 and osteopontin in human gingival crevicular fluid during initial tooth movement. APOS Trends Orthod. 5, 77 (2015).
Canavarro, C., Teles, R. P. & Capelli Júnior, J. Matrix metalloproteinases -1, -2, -3, -7, -8, -12, and -13 in gingival crevicular fluid during orthodontic tooth movement: A longitudinal randomized split-mouth study. Eur. J. Orthod. 35, 652–658 (2012).
Baloul, S. S. et al. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am. J. Orthod. Dentofacial Orthop. 139, S83–S101 (2011).
Sugiyama, Y. et al. The level of cathepsin B in GCF during human orthodontic tooth movement. Eur. J. Orthod. 25, 71–76 (2003).
Rhee, S. H., Kang, J. & Nahm, D. S. Cystatins and cathepsin B during orthodontic tooth movement. Am. J. Orthod. Dentofacial Orthop. 135, 99–105 (2009).
Vitorino, R. et al. Towards defining the whole salivary peptidome. Proteom. Clin. Appl. 3, 528–540 (2009).
Ingman, T., Apajalahti, S., Rice, D. & Sorsa, T. Gingival crevicular fluid, matrix metalloproteinases, and their bioactive regulators as potential adjunctive chair-side point-of-care biomarkers in orthodontic tooth movement. Semin. Orthod. 18, 270–277 (2012).
Takahashi, I. et al. Expression of MMP-8 and MMP-13 genes in the periodontal ligament during tooth movement in rats. J. Dent. Res. 82, 646–651 (2003).
Terajima, M. et al. Glycosylation and cross-linking in bone type I collagen. J. Biol. Chem. 289, 22636–22647 (2014).
Siqueira, W. L., Canales, M. P., Crosara, K. T. B., Marin, L. M. & Xiao, Y. Proteome difference among the salivary proteins adsorbed onto metallic orthodontic brackets and hydroxyapatite discs. PLoS ONE 16, e0254909 (2021).
Sioustis, I. A. et al. Salivary metalloproteinase-8 and metalloproteinase-9 evaluation in patients undergoing fixed orthodontic treatment before and after periodontal therapy. Int. J. Environ. Res. Public Health 18, 1583 (2021).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Silness, J. & Loe, H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol. Scand. 22, 121–135 (1964).
Löe, H. & Silness, J. Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol. Scand. 21, 533–551 (1963).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Author information
Authors and Affiliations
Contributions
F.W., contributed to conception, design, data acquisition, analysis, interpretation, drafted and critically revised the manuscript; H.S., contributed to data acquisition and critically revised the manuscript; J.H., contributed to data acquisition and critically revised the manuscript; M.T.C., contributed to conception, design, data interpretation, drafted and critically revised the manuscript; G.H.C., contributed to conception, design, data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wazwaz, F., Saloom, H., Houghton, J.W. et al. Salivary peptidome analysis and protease prediction during orthodontic treatment with fixed appliances. Sci Rep 13, 677 (2023). https://doi.org/10.1038/s41598-022-26969-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26969-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.