Abstract
The lithium-pilocarpine rat model is a well-known model of temporal epilepsy. Recently we found that transcranial static magnetic stimulation (tSMS) delay and reduce the signs of EEG in this model. We aim to test the effect of combining the therapeutic action of tSMS and diazepam, a drug used to treat status epilepticus. We induce epilepsy in 12 Sprague–Dawley rats. Animals were classified as “magnet” when a magnetic neodymium cylinder was placed over the skull or “control” when a stainless-steel replica was used. Diazepam was injected 60-min after the second doses of pilocarpine injection. We found a reduction in the number of spikes/minute for magnet condition compared with sham condition, reaching significance at 60 min after diazepam injection. The Root-Mean-Square shown a significant reduction in magnet animals compared with those receiving diazepam (Tukey’s-test 30 and 60 min after diazepam injection, p < 0.01; 40 and 50 min after diazepam injection, p < 0.05). Furthermore, the power spectrum analysis shown a reduction in delta, theta, alpha and beta bands, on the diazepam + magnet animals compared to the diazepam + sham group. Analysis of high-frequency oscillations revealed an increased in the ripples due to pilocarpine being reduced by diazepam. Our results demonstrate that application of tSMS previously to diazepam potentiates the effect of the drug by reducing the electroencephalographic pattern associated with epileptiform discharges. We suggest a new synergistic cooperation between pharmacology and neuromodulation as a future treatment for epilepsy.
Similar content being viewed by others
Introduction
Epilepsy is a neurological disorder characterized by recurrent seizures, as a result of hypersynchronous excitatory electrical discharges from a group of neurons in the brain. Besides its severity and collateral comorbidities1, the epidemiological studies indicate that 6 million people in Europe2 and around 70 million people in the world3 are affected by this condition. Although the number of antiepileptic drugs (AEDs), including the group of benzodiazepines/barbiturates which interact with GABA-A receptors4, have grown in the last 40 years, and 60–70% of patients respond to them, there are still many people who cannot benefit from AEDs. For a number of cases surgery could be the option5, but considering that these procedures are not free of risk and complications, finding new treatments is a priority.
Over the past two decades, non-invasive brain stimulation (NIBS) emerged as valuable tool for treatment of neurological and psychiatric disorders6,7. In the particular case of epilepsy, the results have been inconclusive probably due to the variability in the stimulation protocols and etiological heterogeneity (for a comprehensive review see8). One of the most recently incorporated technique to the NIBS family is transcranial static magnetic stimulation (tSMS)9, whose application produces a clear and reproducible reduction of cortical excitability10,11,12,13,14,15. Following this line, in a recent paper we demonstrated that tSMS of moderate intensity (0.5 T) applied alone was able to delay and reduce the characteristic EEG signs of epileptiform discharges, both in rat and monkey13.
Given this background and taking into account studies demonstrating the synergistic effect of combination therapy regimens16, we wondered if the protective effects we have obtained using tSMS alone could enhance pharmacological strategies, even when the drug is used at very low doses. Based on that, the aim of the present study was to study the putative synergistic effect of combining tSMS with low doses of diazepam, a drug commonly used to treat status epilepticus, in the litio-pilocarpine rat model17.
Materials and methods
All the methods were carried out in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. The study was carried out following the rules of the Physiological Spanish Society, the International Council for Laboratory Animal Science, and the European Union guidelines (No. 2010/63/EU) for the protection of the laboratory animals used for scientific purposes. The ethics committee for animal research of the University Hospital of A Coruña approved the experimental protocol (P090). All efforts were made to minimize animal suffering and the number of animals used.
The animals used in this study were 12 male and female Sprague–Dawley rats, 2–3 months old. Animals were housed in standard cages on a 12/12-h light/dark cycle, having free access to food and tap water.
Electrode implantation for Electrocorticogram recording
Animals were introduced in a cage for anesthesia induction (5% sevofluorane). Once anesthetized, rats were placed in a stereotaxic frame and anesthesia was reduced to 3.5%. Once the animal was stabilized, the skin was opened, and the connective tissue removed until lambda and bregma landmarks were visible. Using a hand drill, two small craniotomies were made in the skull of each rat for the placement of two electrodes (stainless screws, 1.2-mm diameter, 4-mm length) to record electrocorticogram signals (ECoG). The active electrode was placed on the area of the right somatosensory cortex (AP − 1.5 mm from Bregma, L 2 mm from the midline) and the reference electrode was placed on the left primary visual cortex (AP -6.3 mm, L 3.5 mm).
Data acquisition
Continuous ECoG recordings started immediately after the surgery (at this stage the anesthesia level was set at 1.5–2% of sevofluorane). The ECoG signal was amplified using an A-M system differential amplifier (Model 1700 A-M System, LLC, Calsborg, WA, USA), band-pass filtered (gain 1000, range 1 Hz–500 Hz), digitized at 20 kHz (1401 CED A/D convertor card; Cambridge Electronic Design, UK) and stored (Spike 2 software; Cambridge Electronic Design, UK) in a PC for online checking and posterior analysis. The animals remained anaesthetized throughout the experiment.
Induction of status epilepticus and antiepileptic drug administration
We used the lithium-pilocarpine model to induce epilepsy18. 24 h before the surgery procedure described above the animals were intraperitoneally (i.p.) injected with 127 mg/kg of LiCl, and returned to their cages. Immediately after the surgery (always under anesthesia) we checked the quality of the ECoG signal, and after thirty minutes of stable recording, scopolamine (1 mg/kg) was injected (i.p.). After thirty minutes of scopolamine, two doses of pilocarpine (20 mg/kg each), thirty minutes apart, were administered (i.p.). Finally, diazepam (1.25 mg/kg) was i.p. injected 60 min after the second dose of pilocarpine. A schematic diagram showing the protocol timeline is illustrated in Fig. 1. In order to stablish the appropriate dose a preliminary experiment was carried out (n = 4) using 10.00, 2.50, 1.25 and 0.75 mg/kg of diazepam. 10.00 and 2.50 mg/kg doses of diazepam produced a practically total suppression of the oscillatory activity with long periods of isoelectric activity and with clear affectation of the respiratory function, and 0.75 mg/kg had not a noticeable effect on a visual inspection (data not shown). These pilot experiments showed us that the best doses for testing our hypothesis was 1.25 mg/kg of diazepam, as it was the lowest doses at which a pharmacological effect could be seen.
Schematic diagram showing the time schedule for the magnet and drug administration protocol during the recording session: T = 0 scopolamine (1 mg/kg), T = 30 pilocarpine (20 mg/kg), T = 60 pilocarpine (20 mg/kg), T = 120 Diazepam (1.25 mg/kg), all drugs were i.p. administered. Magnet or Sham were applied for 1 h, starting at T = 0.
Application of tSMS
Animals were randomly classified into two groups: One to be exposed to a magnetic field (Magnet group) and the other to be exposed to a stainless-steel replica (Sham group) without magnetic activity. We used a cylindrical nickel-plated (Ni–Cu–Ni) NdFeB magnet with a 45-mm diameter and a 30-mm thickness that weighed 360 g (NEUREK S.L., Toledo Spain). The maximum amount of magnetic energy stored in this magnet was 45 MGOe, with a nominal strength of 765 N (78 kg). Previous experiments, including actual measurements and computer models19,20,21,22 showed with a remarkable consistence a magnetic field of 0.5 T on the magnet surface and ∼0.3 T at 1 cm. A nonmagnetic replica of identical appearance and weight (i.e., indistinguishable from the magnet) was used for the sham stimulation (NEUREK S.L., Toledo Spain).
The magnet (or the replica) was placed on the rat's head (in contact with the skull) coinciding with the scopolamine injection, it was maintained for one hour and was removed when the second dose of pilocarpine was administered, hence the magnet did not overlap in time with diazepam administration. The magnet was placed with the field lines perpendicular to the cortical surface.
ECoG and data analysis
Data analyses were performed with Spike2 software (Cambridge Electronic Design, v8.03) and home-made routines in MATLAB.
The raw ECoG signal was downsampled to 1 kHz and recorded continuously from the beginning of the experiments. Every 30 min, an epoch of 60 s was automatically selected and analyzed in terms of epileptic spikes (normalized peak per minute) and root mean square (RMS), using specific tools included in the spike2 software. Epileptic spikes, considered as sudden change of the ECoG signal, have been long documented as a marker of the epileptic processes happening in the brain23. RMS is a common method to estimate the ECOG amplitude. For a given set of values, RMS is the square root of the arithmetic mean of the squares of these values. In the case of a DC signal or a non-sinusoidal signal, RMS provides an estimation of the averaged amplitude24.
We performed an analysis of the power spectral density (PSD) at the different experimental conditions, in four frequency bands separately: Delta (0 to 4 Hz), theta (4 to 8 Hz), alpha (8 to 12 Hz) and beta (12 to 30 Hz).
Finally, we also analyzed high frequency oscillations (HFOs). Raw ECoG signals were bandpass filtered between 80-200 Hz. In this frequency of 80-200 Hz, an oscillation with 4 or more consecutive peaks and with an amplitude higher than 3 SD of the mean was considered as a ripple25.
Statistical analyses were performed using RStudio (version 2022.07.1) software. Values were expressed as the mean ± standard error. Analysis of peaks per minute, RMS, PSD at the different frequency bands, and ripples, were analyzed using a repeated-measures analysis of variance (ANOVA). The degrees of freedom were corrected (if needed) with the Greenhouse–Geisser coefficient. Factors included CONDITION (sham and magnet) and TIME_drug_injection (baseline, prediazepam and postdiazepam). We used Tukey’s multiple comparison test for post hoc analysis. All results were considered significant at p < 0.05.
Results
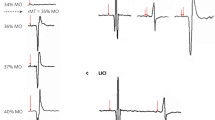
Figure 2 shows two ECoG samples obtained from two different animals, one with the magnet (Fig. 2a), and one with the replica (Fig. 2b), one hour after the second dose of pilocarpine (left panel), and thirty minutes after diazepam injection (right panel). As we have shown previously13 the magnet prevented the appearance of epileptic signs in the ECoG, this is, after pilocarpine injection the animal with the real magnet continued to show the up and down states characteristic of the anesthesia while the sham shows an altered ECoG with a spike pattern. We have considered the criterion to define the abnormal pattern of spikes a discharge that last at least 0.3 s, with an amplitude at least two times higher than the baseline. It should be noted that while in our previous manuscript the magnet was placed at the time of the first pilocarpine dose, here it was placed at the same time of the scopolamine injection. Diazepam, dispensed at a dose of 1.25 mg/kg (i.p.), was administered one hour after the second pilocarpine injection, and it is clear from the recording that thirty minutes after diazepam injection, there was a clear reduction in the number of ECoG spikes in both animals, being more pronounced in the animal with the magnet (for a population analysis, see Fig. 3).
(a) Average number of spikes at different time points after diazepam injection (doses 1.25 mg/kg, i.p.). Black dots represent data obtained from animals exposed to sham whereas red dots represent animals exposed to real magnet. Note that the data is normalized. (b) Bar histogram representing the percentage of reduction in the spontaneous Root Mean Square (RMS) at different time points after diazepam injection (doses 1.25 mg/kg, i.p.). Black bars, sham rats; red bars, magnet rats. In both cases (a, b), data represented 5 min epochs.
To assess the severity of the induced status epilepticus and the magnitude of the antiepileptic effect obtained with our manipulations, we measured the number of spikes/minute and the RMS. Regarding the number of spikes/minute, a repeated measures ANOVA revealed both, a significant interaction effect between group (sham and magnet) and time of drug injection (baseline, prediazepam and postdiazepam) (F (1, 14) = 5.039, p = 0.041), and also significant effect between groups (sham and magnet), being reduced significatively the number of spikes in the magnet condition (F (1, 14) = 6.535, p = 0.023). Comparison analysis revealed significant differences between groups, such that spikes/minute for the magnet condition at 60 min after diazepam injection were significantly reduced than for the sham condition at 60 min after diazepam injection (pairwise comparison with Tukey’s test, p < 0.01). Figure 3b shows the temporal evolution of the diazepam plus magnet (magnet animals) and the diazepam effect (sham animals) on the RMS of spontaneous ECoG activity, and as it can be seen, the RMS showed a greater reduction in the magnet group compared with those animals that only received the diazepam dose (pairwise comparison with Tukey’s test, 30 and 60 min after diazepam injection, p < 0.01; 40 and 50 min after diazepam injection, p < 0.05). Interestingly, as it is shown in Fig. 3a, while the effect mediated only by diazepam seems to reach the maximum about 30 min after the injection and keeps stable, on those animals pre-treated with the magnet the effect is also clear 30 min after the diazepam, but is even greater at 60 min since the effect continues to grow in the group exposed to the real magnet.
We also calculated the power of the ECoG activity, as it is a well stablish method for processing and quantifying the ECoG signal. Bands for delta (0–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz) rhythms were obtained averaging three one-hour period epochs, one obtained from baseline, other during the period of the second dose of pilocarpine injection, and the last one after diazepam injection. As it is shown in the Fig. 4, compared to the baseline situation, the power spectrum analysis showed a reduction in all the analysed bands, being this reduction more pronounced in the magnet animals. It is important to note that the reduction becomes bigger and more evident for the magnet animals after diazepam injection, reaching significance that difference in the theta band (pairwise comparison with Tukey’s test, p < 0.05).
Analysis of high-frequency oscillations, as ripples, could be seen in Fig. 5. In our recordings, ripples were evident after filtering the signal, a representative sample is shown in Fig. 5a (see methods) and were present before and during the seizures25. The ripples increased due to the effect of pilocarpine and were reduced by diazepam, however the magnet did not produce any effect on them, Fig. 5b. The mean frequency of the ripples in the different conditions was also analysed. The mean values obtained at baseline, pre and post diazepam, with the sham replica and with the magnet were respectively, for the sham condition 95.70 ± 7.38, 94.62 ± 3.43, 94.62 ± 6.60, and for the magnet condition 90.82 ± 3.20, 88.11 ± 2.17, 85.94 ± 0.00, and according to statistics there were not significant differences.
Discussion
Noninvasive brain stimulation techniques have recently emerged as useful tools for neuroscience research, clinical evaluation and treatment of various neurological and psychiatric conditions, among them, epilepsy. Based on previous data from our lab, the present study was designed to investigate whether tSMS -a novel NIBS technique- combined with low doses of diazepam can produce a synergistic effect modulating the EEG observed abnormalities in the pilocarpine epilepsy rat model. The idea was straightforward: Develop a combined treatment that allow effective control of epileptic seizures, minimizing the pharmacological doses used; a polytherapy with a single drug (at low doses) which allows reducing the danger of the addition of pharmacological side effects26. tSMS has, until date, no demonstrable side effects27.
We used the lithium-pilocarpine model of epilepsy in the anaesthetized rat because it is very reliable and produces recurrent seizures easily recorded with EEG28,29, which makes it a very suitable model to carry out pharmacological experiments such as the ones we have used in combination with neuromodulation. It does, however, have a limitation. It does not allow to study the behavior of the animals, which could have been useful to establish correlations between the different bands of the EEG and different behavioral stages30. However, if we consider our work as a proof of concept to demonstrate the synergistic effect between neuromodulation and pharmacology for the control of seizures, we believe that the experimental design is valid.
Our study yielded four main findings: (i) It is the confirmation that in this rat model of epilepsy, tSMS of moderate intensity prevents the appearance of epileptic signs in the EEG13. The magnet is able to produce this effect by reducing the cortical excitability altered in epilepsy. This effect, clear and reproducible, has been revealed by mean of electrophysiological techniques, ranging from single neuron recordings to evoked potentials, but it has also been observed through behavioral correlates. For example, when the magnet was applied to the visual cortex of behaving monkeys or humans, it produced a transient scotoma and a consequent alteration of the visual detection task10,11,12.
(ii) Our data demonstrate a synergistic action between magnetic fields and diazepam in reducing the severity of the epileptiform discharges, measured by the number of spikes and RMS of the ECoG, as compared to the sham-treated animals. Figure 3 shows this effect at diazepam dose of 1.25 mg/kg. To achieve cessation of acute epileptic signs in this model, usual doses are in the range of 5 to 10 mg/kg, 4 to 8 times higher than the ones we used in our experiments31.
Our results agree with previous research done in black Swiss mice, where audiogenic epileptic seizures treated with static magnetic fields and phenytoin produce a robust anticonvulsant effect32. Furthermore, in this manuscript we demonstrate that application of tSMS was able to develop a protective effect over time; it should be noted that the magnet was present during the pharmacological induction of epileptic status, but it was removed 1 h before the diazepam injection.
We do not know the mechanisms that mediate the effects of tSMS, but it has been postulated that it could affect voltage dependent calcium channels33, or that it could mechanically change membrane properties affecting to potassium channels34. Whatever the mechanism and whether or not it is more selective on a specific neuronal type (e.g. inhibitory interneurons/excitatory cells), what is well established is that, as a global outcome, it produces a reduction in cortical excitability. Diazepam, on the other hand, acts increasing GABAergic activity, and hence increasing the weight of the inhibition, furthermore, Diazepam could also inhibit voltage dependent sodium channels35,36 and voltage dependent calcium channels37, hence some mechanisms of action might be similar for tSMS and Diazepan while others are more specific for each of them.
An interesting aspect refers to the temporal course of the effects observed after tSMS. In previous studies it has been shown that some physiological measurements as motor evoked potentials or visual detection9,11 were completely recovered 20 or 30 min after being affected by magnet application. Here we have shown that after the injection of diazepam, 1 h apart from removing the magnet, there were still differences between the animals exposed to the magnet and those that were exposed to the sham, indicating that the effect of the magnet was maintained over time. This suggests the possible existence of a temporal window after the action of the magnet in which the action of other interventions is favored. These aspects will be the subject of future experiments.
(iii) Power spectral analysis, a measure related with the severity of the epilepsy38 shows a decrease in all bands studied (delta, theta, alfa, and beta) after applying the diazepam. This effect tends to be higher in those animals previously treated with magnet, reaching significant values at theta band. The reduction we observed in these bands agrees with other authors38 that found that any increase is associated to a greater number of seizures and the other way around. Our study showed that the theta band was the most affected frequency, and it fits well with proposals from other authors, namely, that the theta band is the preferred frequency for investigating the brain-network dysfunction26, and that this frequency band is a good epileptic marker39.
(iv) The results obtained with ripple analysis were somewhat surprising. The observed rate of ripples was similar between magnet and sham conditions, during the three different periods studied: baseline, prediazepam, and postdiazepam. For both conditions (sham and magnet), ripples were increased as a consequence of pilocarpine and decreased as a result of diazepam injection. We have not been able to verify any effect on them produced by the application of the magnet.
It has been demonstrated, using 4-aminopyridine treated rats, that seizures leading to status epilepticus show a higher number of ripples when compared to seizures leading to isolated seizures. As ripples appear to originate in the principal cells of the hippocampus, and inhibition of these neurons delays the initiation of status epilepticus40, our result suggest that diazepam could act on the firing of these hippocampal excitatory neurons and hence inhibiting the electrographic sings of epilepsy. However, the magnet did not affect ripples; it could be an indication of the limits of action of the magnetic field, since the intensity is highly reduced with the distance19.
Conclusions
In summary, our results demonstrate that application of tSMS previously to diazepam potentiates the effect of the drug by reducing the electroencephalographic pattern associated with epilepsy. There are several questions that have to be addressed in future experiments in order to establish a new antiepileptic protocol from our data: Optimal time interval between tSMS and drug injection, different dose/response relationships, increase or decrease in tSMS time and dose of diazepam. Even taking all this into account, we believe that our results open the door to a new kind of cooperation between pharmacology and neuromodulation as a future treatment for epilepsy.
Data availability
All data, and materials used in the analysis will be made available on request for proposals that set out to achieve aims specified in a methodologically and scientifically sound protocol. Applications to access data can be sent to c.labra@udc.es. Data access will be used for non-commercial, academic, and research use only.
References
Keezer, M. R., Sisodiya, S. M. & Sander, J. W. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 15, 106–115 (2016).
Forsgren, L., Beghi, E., Oun, A. & Sillanpaa, M. The epidemiology of epilepsy in Europe - a systematic review. Eur. J. Neurol. 12, 245–253 (2005).
Ngugi, A. K., Bottomley, C., Kleinschmidt, I., Sander, J. M. & Newton, C. R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 51, 883–890 (2010).
Macdonald, R. L. Cellular actions of antiepileptic drugs. In Antiepileptic Drugs. Handbook of Experimental Pharmacology Vol. 138 (eds Eadie, M. J. & Vajda, F. J. E.) (Springer, Berlin, 1999).
Sheng, J., Liu, S., Qin, H., Li, B. & Zhang, X. Drug-resistant epilepsy and surgery. Curr. Neuropharmacol. 16, 17–28 (2018).
Rajji, T. K. Noninvasive brain stimulation for the treatment of neurocognitive disorders: right for prime time?. Curr. Opin. Psychiatry 34(2), 129–135 (2021).
Pettorruso, et al. Non-invasive brain stimulation targets and approaches to modulate gambling-related decisions: A systematic review. Addict Behav. 112, 106657 (2021).
Gschwind, M. & Seeck, M. Modern management of seizures and epilepsy. Swiss Med. Wkly. 146, w14310 (2016).
Oliviero, A. et al. Transcranial static magnetic field stimulation of the human motor cortex. J Physiol. 589(Pt 20), 4949–4958 (2011).
Gonzalez-Rosa, J. J. et al. Static magnetic field stimulation over the visual cortex increases alpha oscillations and slows visual search in humans. J. Neurosci. 35, 9182–9193 (2015).
Aguilá, J., Cudeiro, J. & Rivadulla, C. Effects of static magnetic fields on the visual cortex: reversible visual deficits and reduction of neuronal activity. Cereb. Cortex. 26, 628–638 (2016).
Arias, P. et al. Transcranial static magnetic field stimulation of M1 reduces corticospinal excitability without distorting sensorimotor integration in humans. Brain Stimul. 10(2), 340–342 (2017).
Rivadulla, C. et al. Static magnetic fields reduce epileptiform activity in anesthetized rat and monkey. Sci. Rep. 8(1), 15985 (2018).
Nojima, et al. Transcranial static magnetic stimulation from bench to bedside and beyond. Neurosci. Res. 156, 250–255 (2020).
Viudes-Sarrion, et al. Static magnetic stimulation in the central nervous system: a systematic review. Neurol. Sci. 42, 1733–1749 (2021).
Stephen, L. J. & Brodie, M. J. Antiepileptic drug monotherapy versus polytherapy: pursuing seizure freedom and tolerability in adults. Curr. Opin. Neurol. 25(2), 164–172 (2012).
Loscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 20, 359–368 (2011).
Reddy, D. S. & Kuruba, R. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int. J. Mol. Sci. 14, 18284–18318 (2013).
Rivadulla, C., Foffani, G. & Oliviero, A. Magnetic field strength and reproducibility of neodymium magnets useful for transcranial static magnetic field stimulation of the human cortex. Neuromodulation 17, 438–441 (2014).
Kirimoto, H., Asao, A., Tamaki, H. & Onishi, H. Non-invasive modulation of somatosensory evoked potentials by the application of tanscranial static magnetic field stimulation over the primary and supplemental motor cortices. Sci. Rep. 6, 34509 (2016).
Tharayil, J. J., Goetz, S. M., Bernabei, J. M. & Peterchev, A. V. Field distribution of transcranial static magnetic stimulation in realistic human head model. Neuromodulation Technol. Neural Interface 21(4), 340–347 (2017).
Park, J., Lee, C., Lee, S. & Im, C. Comparison of magnetic field distributions generated by various permanent magnets for transcranial static magnetic stimulation: A simulation study. Comput. Biol. Med. 114, 103476 (2019).
Matsumoto, H. & Ajmone-Marsan, C. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol. 9, 286–304 (1964).
Zahan, S., & Islam, Md.R. Epileptic seizure detection and classification using support vector machine from scalp EEG signal. In 1st International Conference on Advances in Science, Engineering and Robotics Technology (ICASERT), pp. 1–5 (2019).
Wang, S., Lévesque, M. & Avoli, M. Effects of Diazepam and ketamine on pilocarpine-induced status epilepticus in mice. Neuroscience 421, 112–122 (2019).
Li, Y. et al. Evaluation of brain network properties in patients with MRI-negative temporal lobe epilepsy: an MEG study. Brain Topogr. 34, 618–631 (2021).
Oliviero, A. et al. Safety study of transcranial static magnetic field stimulation (tSMS) of the human cortex. Brain Stimul. 8(3), 481–485 (2015).
Rattka, M., Brandt, C. & Loscher, W. The intrahippocampal kainate model of temporal lobe epilepsy revisited: Epileptogenesis, behavioral and cognitive alterations, pharmacological response, and hippoccampal damage in epileptic rats. Epilepsy Res. 103, 135–152 (2013).
Robel, S. et al. Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci. 35, 3330–3345 (2015).
Sharma, S., Puttachary, S., Thippeswamy, A., Kanthasamy, A. G. & Thippeswamy, T. Status epilepticus: Behavioral and electroencephalography seizure correlates in Kainate. Exp. Models. Front. Neurol. 9, 7 (2018).
Jones, D. M. et al. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res. 50, 301–312 (2002).
McLean, M. J., Engström, S., Qinkun, Z., Spankovichc, C. & Pollety, D. Effects of a static magnetic field on audiogenic seizures in black Swiss mice. Epilepsy Res 80, 119–131 (2008).
Rosen, A. D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem. Biophys. 39, 163–173 (2003).
Hernando, A. et al. Effects of moderate static magnetic field on neural systems is a non-invasive mechanical stimulation of the brain possible theoretically?. Front Neurosci. 14, 419 (2020).
Willow, M., Kuenzel, E. A. & Catterall, W. A. Inhibition of voltage-sensitive sodium-channels in neuro-blastoma cells and synaptosomes by the anticonvulsant drugs diphenylhydantoin and carbamazepine. Mol. Pharmacol. 25(2), 228–234 (1984).
Mclean, M. J. & Macdonald, R. L. Benzodiazepines, but not beta-carbolines, limit high-frequency repetitive firing of action-potentials of spinal-cord neurons in cell-culture. J. Pharmacol. Exp. Ther. 244(2), 789–795 (1988).
Taft, W. C. & Delorenzo, R. J. Micromolar-affinity benzodiazepine receptors regulate voltage-sensitive calcium channels in nerve-terminal preparations. Proc. Natl. Acad. Sci. USA 81(10), 3118–3122 (1984).
Chen, J. et al. Can spectral power be used as a candidate seizure marker of the periodic discharges pattern?. Front Neurol. 12, 642669 (2021).
Douw, L. et al. Epilepsy is related to theta band brain connectivity and network topology in brain tumor patients. BMC Neurosci. 11, 103 (2010).
Sukhotinsky, et al. Optogenetic delay od status epilepticus onset in an in vivo rodent epilepsy model. PLoS ONE 8(4), e62013 (2013).
Funding
This work was supported by Instituto de salud Carlos III (ISCIII) PI21/00151 co-funded by the European Union and Xunta de Galicia, grupos de referencia competitiva 2022. ED431C 2022/05.
Author information
Authors and Affiliations
Contributions
C.d.L., C.R. and J.C. designed the experiments, C.d.L., and C.R. performed the experiments, C.d.L. performed the analysis, and C.d.L., C.R., and J.C. contributed to the interpretation of data. C.d.L drafted the manuscript. All authors contributed to the discussion, revised the final version of the manuscript and approved its submission to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Labra, C., Cudeiro, J. & Rivadulla, C. Synergistic effects of applying static magnetic fields and diazepam to improve EEG abnormalities in the pilocarpine epilepsy rat model. Sci Rep 13, 214 (2023). https://doi.org/10.1038/s41598-022-26870-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26870-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.