Abstract
Diagnosis of calcified nodules (CNs) is critical in the proper management of coronary artery disease, but CNs can be detected only using intracoronary imaging modalities. This study aimed to investigate the ability of coronary computed tomography angiography (CCTA) in predicting CNs detected using optical coherence tomography (OCT). From 138 patients who underwent OCT-guided percutaneous coronary intervention (PCI) after CCTA evaluation, 141 PCI target vessels were retrospectively enrolled and classified into CN (12 vessels/11 patients; CNs in the PCI culprit lesion) and non-CN (129 vessels/127 patients; without CNs) groups based on the OCT analysis. Retrospective CCTA analysis revealed significantly higher coronary artery calcification score (CACS), calcified plaque volume (CPV), and maximum calcified plaque area (MCPA) of the target vessel in the CN group than in the non-CN group. Receiver operating characteristic curve indicated that CACS ≥ 162 (area under the ROC curve (AUC 0.76, sensitivity 83.3%, specificity 54.2%), CPV ≥ 20.1 mm3 (AUC 0.83, sensitivity 100%, specificity 57.3%), and MCPA ≥ 4.51 mm2 (AUC 0.87, sensitivity 91.7%, specificity 78.3%) were the best cutoff values for predicting CNs. MCPA showed the highest AUC among all the CCTA parameters. In conclusion, CCTA is useful for predicting OCT-detected CNs in PCI target vessels.
Similar content being viewed by others
Introduction
Calcified nodules (CNs), which are protrusions into the blood vessel lumens, are pathologically associated with acute coronary syndrome (ACS)1,2,3 and other major cardiovascular events, such as target lesion revascularization, stent thrombosis, and myocardial infarction (MI) after percutaneous coronary intervention (PCI)4,5. The diagnosis of CNs is critical in the proper management of patients with coronary artery disease. At present, invasive intracoronary imaging modalities, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT), are generally implemented for the in vivo diagnosis of CNs, but non-invasive methods are lacking.
Coronary computed tomography angiography (CCTA) is a non-invasive tool for evaluating the coronary plaque morphology; coronary plaques can be classified on the basis of computed tomography (CT) values as low-attenuation, fibrous, and calcified plaques, with quantitative assessment of plaque volumes6,7,8. CCTA-calculated coronary artery calcium score (CACS) is widely used to quantitatively evaluate the coronary artery calcium burden9, where a higher CACS is related to poorer clinical outcomes as compared with lower CACS10,11.
OCT is an intravascular imaging modality with a maximal spatial resolution of 10 mm, which is tenfold higher than that of IVUS12,13. Because near-infrared light from the OCT system can penetrate calcium, OCT provides a more detailed characterization of the morphology of calcified coronary plaques compared with IVUS14,15. Several studies employing OCT have reported that CNs are frequently observed in lesions with large amounts of calcified plaques5,16,17. Since CCTA quantitatively evaluates calcium plaques, estimation of coronary calcium burden by CCTA may help identify the presence of CNs. This study aimed to investigate the diagnostic ability of CCTA to identify CNs in patients undergoing PCI.
Methods
Study population
This single-center, retrospective, and observational study was conducted at Nara Medical University, Japan. A total of 341 patients who underwent OCT-guided PCI for de novo target vessels after CCTA evaluation between January 2014 and December 2018 were screened. Patients with any of the following conditions were excluded from the study: (1) ST elevation or non-ST elevation MI, (2) vessels with PCI before CCTA evaluation, (3) > 1 year duration between CCTA and OCT, (4) absence of OCT images before stent implantation, and (5) unanalyzable CCTA or OCT images. Finally, 141 PCI target vessels from 138 patients were included in this study analysis and were classified (based on OCT analysis) into the following two groups: CN (12 vessels/11 patients with OCT-detected CNs in the PCI culprit lesion) and non-CN (129 vessels/127 patients without CNs) groups (Fig. 1). Patient characteristics were collected from the medical records, while the results of blood investigations conducted within 3 months of OCT were analyzed.
This study was approved by the Ethics Committee of Nara Medical University (reference no. 1759-2) and complied with the Declaration of Helsinki’s ethical principles. Informed consent was obtained in the form of an opt-out option from the Department of Cardiovascular Medicine, Nara Medical University website.
CCTA procedure and analysis
CCTA was performed using a dual-source CT scanner (Somatom Definition, Siemens, Forchheim, Germany). The detailed scan protocol is described in Supplementary methods. CCTA analysis was performed using the SYNAPSE VINCENT version 5.1. Images were independently evaluated by 3 investigators familiar with the CCTA analysis, and any disagreements were resolved by consensus. Retrospective CCTA studies were performed for each vessel evaluated with OCT. Coronary plaques were defined as structures with a minimum 1 mm2 area within or adjacent to the arterial lumen, clearly distinguishable from the vessel lumen, and surrounded by pericardial tissue; tissue with signal intensity below -30 Hounsfield units (HU) was considered pericardial fat and excluded from the analysis. Based on the results of our previous study with OCT7,8,9, each tissue was classified by its CT value (< −30, −30 to 50, 51 to 200, 201 to 500, and > 500 HU indicated pericardial fat, low-attenuation plaque, fibrous plaque, enhanced media, and calcified plaque, respectively). Non-calcified plaques were defined as low-attenuation or fibrous plaques. Previous studies have reported that high-risk plaque features, such as positive remodeling (a lesion with a remodeling index of ≥ 1.1), very low-attenuation plaque (minimum CT value of ≤ 30 HU), napkin-ring sign (a central low-attenuation portion surrounded by a ring-like higher attenuation), and spotty calcifications (a small calcification of 3 mm or less on curved multiplanar reformation images and occupying only one side on the cross-sectional images) are predictive factors for cardiac events8,18,19,20.
First, each vessel was classified into four segments (right coronary artery, left main trunk, left anterior descending, and left circumflex). Each segment vessel was evaluated for the CACS (evaluated using the Agatston score17), calcified plaque volume (CPV; in mm3), non-calcified plaque volume (NCPV; mm3), low-attenuation plaque volume (LAPV; mm3), fibrous plaque volume (mm3), maximum calcified plaque area (MCPA) on the cross-sectional images (mm2), and presence of positive remodeling, very low-attenuation plaque, napkin-ring sign, and spotty calcifications. MCPA was measured at the largest calcified plaque area on cross-sectional measurements at 1-mm intervals through the entire target vessel. Volumetric assessment for each classified plaque (calcified, non-calcified, low-attenuation, and fibrous plaques) by CT value was completed automatically with SYNAPSE VINCENT version 5.1.
The inter-observer reproducilibity measured by Pearson's coefficient were r = 0.994 to 0.999 for CACS, r = 0.999 for CPV, and r = 0.999 for MCPA (among three investigators; J.S, S.N, and A.O.). The intra-observer reproducibility measured by Pearson's coefficient were r = 0.999 for CACS, r = 0.999 for CPV, and r = 0.999 for MCPA.
OCT study and analysis
OCT imaging was performed using a frequency-domain OCT system (C8 System, Dragonfly Imaging Catheter and ILUMIEN OPTIS; St. Jude Medical, St. Paul, MN, USA) or an optical frequency-domain imaging (OFDI) system (Terumo, Tokyo, Japan). The OCT imaging technique has been previously described21.
An OCT examination was performed for the PCI target vessel as distally as possible before PCI to evaluate the morphology of the calcified plaque. For lesions with severe narrowing precluding passage of the OCT or OFDI catheter, removal of blood from the field for clear visualization of the vessel wall, or lesions with an angiographically visible thrombus, the baseline OCT examination was performed after pre-dilatation using a balloon catheter, rotational atherectomy, or thrombectomy.
Plaque tissue characterization22 was performed at PCI culprit lesions based on OCT findings. Lipid plaques were defined as plaques with signal‐poor region with diffuse borders. Thin-cap fibroatheroma was defined as a lipid plaque with a fibrous cap thickness ≤ 65 μm and lipid arc > 90°. A calcified plaque is defined as a low-signal intensity area with sharply delineated borders5,23. OCT-detected CN was defined as a high-backscattering mass protruding into the lumen with the strong signal attenuation and irregular surface5,23. Among the lesions where OCT examination was performed after rotational atherectomy, calcified sites ablated by rotational atherectomy on cross-sectional OCT images were excluded from analysis while identifying the presence of CN. Representative OCT and CCTA images of calcified plaques are shown in Fig. 2.
Representative CCTA and OCT images of calcified plaques. (a) A representative image of calcified nodule. CCTA image showing a large area of calcification. The area of calcified plaque on the cross-sectional image (at blue arrow heads) is 21.77 mm2. The OCT image of this lesion demonstrates calcified nodules (plus symbols). (b) A representative image of eccentric calcified plaque. The area of calcified plaque on the cross-sectional image (at blue arrow heads) is 2.8 mm2. The OCT image of this lesion demonstrates a superficial calcific sheet (asterisks). (c) A representative image of circumferential calcified plaque. CCTA image showing a circumferential calcification. The aread of calcified plaque on the cross-sectional image (at blue arrow heads) is 3.8 mm2. The OCT image of this lesion demonstrates a circumferential calcification (white arrow heads). (d) A representative image of spotty calcification. CCTA image showing a spotty calcification. The area of calcified plaque on the cross-sectional image (at blue arrow heads) is 0.8 mm2. The OCT image of this lesion demonstrated superficial small calcification (white arrow head). CCTA coronary computed tomography angiography, OCT optimal coherence tomography.
In addition, we evaluated the lesion length (mm), minimal lumen diameter (mm), % diameter stenosis, minimal lumen area (mm2), and % area stenosis. % diameter stenosis and % area stenosis are calculated as mean reference lumen diameter minus the minimal lumen diameter divided by the mean reference lumen diameter and mean reference lumen area minus the minimal lumen area divided by the mean reference lumen area in the cross-sectional images, respectively9. Mean reference diameter and mean reference area are defined as (proximal + distal reference diameters) divided by 2 and (proximal + distal reference areas) divided by 2, respectively. The cross-section analysis was performed at every 1 mm interval.
The OCT images were analyzed by two independent observers (S.I. and M.W.) in a blinded manner using a dedicated offline review system (St. Jude Medical). Two observers blinded to each patient’s clinical and lesion characteristics independently performed the OCT measurements. Any discordance between the two observers was resolved by consensus with a third reviewer (A.O.).
Statistical analysis
Depending on the distribution, categorical and continuous variables were represented as numbers/percentages and means ± standard deviations or medians/interquartile ranges, respectively. Student’s t-test and Wilcoxon test were performed to examine the nominal variables and continuous values with normal and non-normal distributions, respectively. Further, the nominal variables were examined using the Fisher's exact test. Receiver operating characteristic (ROC) curve analysis and area under the ROC curve (AUC) were used to determine the best cutoff values for quantitative CCTA parameters for predicting the OCT-detected CNs. The best cutoff value was defined as the value with the highest sum of Youden's index (sensitivity + specificity − 1). Significant superiority tests were two-sided and P values < 0.05 were considered significant. JMP version 14 software (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis.
Results
Table 1 shows the patient characteristics and results of laboratory findings. Hypertension was significantly higher in the CN group than that in the non-CN group. Further, patients of the CN group were more frequently treated with maintenance dialysis and insulin than those of the non-CN group. Furthermore, significant differences were observed in the hemoglobin levels, estimated glomerular filtration rates, and products of calcium and phosphate levels between the two groups. No significant difference was observed between the two groups for the duration from CCTA to OCT examination (CN group: 2 [interquartile range (IQR) 1 − 6] months; non-CN group: 1 [IQR 1 − 3] months: P = 0.24).
Table 2 shows a comparison of OCT findings between CN and non-CN group vessels. An OCT examination was performed after pre-dilatation using a balloon catheter or thrombectomy for four vessels in the CN group and for 21 vessels in the non-CN group. Incidence of lipid rich plaque was significantly higher in the non-CN group than that in the CN group. Incidence of calcified plaque was significantly higher in the CN group than that in the non-CN group. Minimum lumen diameter and area were significantly larger in the CN group than those in the non-CN group. % diameter stenosis was significantly smaller in the CN group than that in the non-CN group.
Table 3 shows a comparison of CCTA findings at the PCI target vessels between the CN and non-CN groups. Significant differences were observed between the CN and non-CN groups in CACS (342 [IQR 203 − 855] vs. 147 [IQR 33 − 318]: P = 0.002), CPV (77.4 mm3 [IQR 29.3 − 212.8] vs. 14.5 mm3 [IQR 0 − 38.9]: P = 0.0002), and MCPA (8.12 mm2 [IQR 5.32 − 12.30] vs. 1.44 mm2 [IQR 0 − 3.98]: P < 0.0001). No significant differences were observed in NCPV and the incidences of positive remodeling and napkin-ring signs. On the other hand, the presence of very low-attenuation plaque and spotty calcification were significantly higher in the non-CN group.
Figure 3 shows the ROC curve analysis for CCTA parameters to predict OCT-detected CNs. The optimal cutoff values to predict the presence of CNs were CACS ≥ 162 (AUC = 0.76), CPV ≥ 20.1 mm3 (AUC = 0.83), and MCPA ≥ 4.51 mm2 (AUC = 0.87).
ROC curve analysis for CCTA findings to predict CN; CACS (a), CPV (b), MCPA (c). The optimal cutoffs to predict CNs are CACS ≥ 162 (AUC = 0.76), CPV ≥ 20.1 mm3 (AUC = 0.83), and MCPA ≥ 4.51 mm2 (AUC = 0.87). AUC area under the curve, CACS coronary artery calcification score, CCTA coronary computed tomography angiography, CN calcified nodule, CPV calcified plaque volume, MCPA maximum calcified plaque area on cross-sectional image, ROC receiver operating characteristic curve.
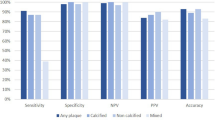
Table 4 summarizes the diagnostic accuracies of CCTA parameters for predicting CNs. MCPA showed the highest AUC among all the CCTA parameters. This AUC was significantly higher than that of CACS, but no significant differences were observed in AUCs between MCPA and CPV (Supplementary Table S1). Furthermore, AUCs of combinations of MCPA with other CCTA parameters did not yield improvements in CN predictions. All CCTA parameters showed negative predictive values (NPVs) of > 90%, but low positive predictive values (PPVs). Among all the CCTA parameters, MCPA (≥ 4.51 mm2) also showed the highest diagnostic accuracy (79.4%) and PPV (28.2%). CPV (≥ 20.1 mm3) demonstrated highest sensitivity and NPV. Table 5 shows the CCTA findings of vessels with CNs. Eleven of the 12 vessels with CNs showed MCPA of ≥ 4.51 mm2. Moreover, all CNs were present within the calcified plaques, including the cross-sectional slices with MCPA. CACS ≥ 162, CPV ≥ 20.2 mm3, and MCPA ≥ 4.51 mm2 in the culprit vessel were the best cutoff values for discriminating CN.
As plaque volume is related to the analysis length, we compared each plaque volume/target vessel length ratio between the CN and non-CN groups (Supplementary Table S2). The CACS/target vessel and CPV/target vessel length ratios were significantly higher in the CN group than those in the non-CN group. Furthermore, ROC curve analysis indicated that CACS/target vessel length ratio ≥ 2.2 and CPV/target vessel length ratio ≥ 0.18 ratio were optimal cutoff values for predicting CNs (AUC 0.83, sensitivity 83.3%, specificity 69.8%; AUC 0.87, sensitivity 100%, specificity 61.2%, respectively) (Supplementary Table S3 and Supplementary Figure).
As a significant difference was observed in the incidence of calcified plaque between two groups, we also evaluated the diagnostic accuracy of CCTA parameters for predicting CN in the subgroup with calcified plaque detected using OCT. The results revealed that CACS, CPV, and MCPA were significantly larger in the CN group than those in the non-CN group (Supplementary Table S4). ROC curve analysis indicated that CACS ≥ 310, CPV ≥ 20.2 mm3, and MCPA ≥ 4.51 mm2 were optimal cutoff values for predicting CNs (AUC 0.70, sensitivity 41.7%, specificity 67.0%; AUC 0.79, sensitivity 91.7%, specificity 50.5%; AUC 0.83, sensitivity 91.7%, specificity 70.3%, respectively) (Supplementary Table S5).
Discussion
Non-invasive methods for the diagnosis of CNs, which are among the causative factors for ACS, are not available; this study investigated the possibility of using CCTA, a quantitative method of evaluating the calcium burden in vessels, in predicting the presence of CNs by analyzing patients who underwent OCT-guided PCI. The results indicate that CCTA-driven quantitative evaluation of the calcified plaques may be advantageous for predicting OCT-detected CNs.
CCTA-measured CACS is widely used for the quantitative evaluation of calcium burden9 and prediction of coronary events10,11, and is calculated based on regions with CT values ≥ 130 HU9. On the other hand, CPV is calculated by the total volume of regions with CT values > 500 HU, which was defined as the threshold to detect calcified plaques based on the results of previous studies with intravascular imaging6,7,8. The present study demonstrated that a CACS ≥ 162 or CPV ≥ 20.2 mm3 was a superior predictor for identifying the presence of CNs. Furthermore, recent OCT studies have demonstrated that the maximal calcium arc and thickness on cross-sectional analysis were larger in lesions with CNs than that in those without CNs16,17,18. Therefore, the calcified plaque area can be considered as a useful predictor for the presence of CNs. The fact that AUC in MCPA was the highest among all the tested CCTA parameters and all the CNs were observed within calcified plaques with MCPA support the usefulness of measuring MCPA for identifying the calcified lesions, including CNs. In a pathological study, CN was close to the lesion with large calcium burden24. Therefore, MCPA is also helpful for identifying the CN lesion site.
In a sub-analysis of the PROSPECT study carried out using IVUS, Xu et al. reported that the coronary vessels in patients with CNs had a more necrotic core, thick-cap fibroatheroma, and dense calcium than in those without CNs25. In the sub-analysis of the CLIMA study26, which is a large prospective registry on the plaque vulnerability assessed by OCT, Prati et al. reported that the patients with CNs showed coronary plaques with a thinner fibrous cap and macrophage in comparison to those without CNs. These previous observations suggest that in addition to having a large calcium burden, coronary arteries with CNs also represent vulnerability and advanced atherosclerotic features. The present study demonstrated similar LAPVs in both the groups, but the incidence of very low-attenuation plaque and spotty calcification, which are the vulnerable plaque feature, was significantly higher in the non-CN group than that in the CN group. The difference in the vulnerable feature of the target vessel between the above studies and the present study may be partly due to the difference in the spatial resolution of the imaging modality used in these studies. The partial volume effect and blooming artifact of calcified plaque in CCTA evaluation might disturb the visualization of the non-calcified plaque around the calcified plaque.
Recent intravascular imaging studies have shown the association between calcified lesions with CNs and higher stent failure rates in patients who underwent newer-generation DES with modern devices4,5,27. Moreover, Prati et al. reported that the presence of CNs in non-culprit coronary plaques was associated with worse clinical outcomes, including cardiac death and target-vessel MI26. Hence, calcified lesions with CNs can be considered high-risk lesions. There is no established treatment for improving the clinical outcomes after PCI in calcified lesions with CNs and to prevent CNs-associated future coronary events; however, proper diagnosis of CNs is essential to stratify coronary events risks. To the best of our knowledge, the present study is the first to examine the CCTA predictors of OCT-detected CNs.
Study limitations
This study has some limitations; first, the sample size was small, with only 12 vessels in the CN group. The cutoff values of CACS ≥ 162, CPV ≥ 20.1 mm3, and MCPA ≥ 4.51 mm2 for discriminating CN had the diagnostic accuracies with very low PPV due to very small sample size of the CN group compared to that of the non-CN group. Second, a potential selection bias exists as patients who underwent both the CCTA and OCT evaluations were included. At Nara Medical University, CCTA is generally avoided in patients with severe renal dysfunction, not on dialysis, and in unstable conditions, such as cardiogenic shock or congestive heart failure. Third, OCT assessments after pre-dilatation using a balloon catheter, rotational atherectomy, or thrombectomy were performed in approximately 20% of cases with severe narrowing not amenable to OCT or OFDI catheter passage or removal of blood from the field of view, which may have affected the visualization of the calcified plaques. Since calcified plaques ablated by rotational atherectomy may resemble the CNs, we excluded the calcified sites ablated by rotational atherectomy from analysis while identifying the presence of CN. Fourth, multivariate analysis of predictors of CNs could not be performed because of the small number of patients with CNs. Fifth, overestimation of CPV or MCPA due to partial volume effect and blooming artifact is inevitable. Finally, given the very small sample size of the CN group compared to that of the non-CN group, the results of this study should be interpreted with caution. The present findings are considered exploratory in nature. A large-scale study is needed to verify the present results.
Conclusions
CCTA is useful for predicting OCT-detected CNs in PCI target vessels, and quantitative measurements of the calcium burden in coronary vessels can help to identify the presence of CNs.
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
Virmani, R., Kolodgie, F. D., Burke, A. P., Farb, A. & Schwartz, S. M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Jia, H. et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 62, 1748–1758 (2013).
Sugiura, J. et al. Progression of a calcified nodule causing acute myocardial infarction in a patient on hemodialysis—Serial optical coherence tomography. Circ. J. 83, 490 (2019).
Morofuji, T. et al. Clinical impact of calcified nodule in patients with heavily calcified lesions requiring rotational atherectomy. Catheter. Cardiovasc. Interv. 97, 10–19 (2021).
Iwai, S. et al. Prognostic impact of calcified plaque morphology after drug eluting stent implantation—An optical coherence tomography study. Circ. J. 85, 2019–2028 (2021).
Komatsu, S. et al. Detection of coronary plaque by computed tomography with a novel plaque analysis system, ‘Plaque Map’, and comparison with intravascular ultrasound and angioscopy. Circ. J. 69, 72–77 (2005).
Soeda, T. et al. Diagnostic accuracy of dual-source computed tomography in the characterization of coronary atherosclerotic plaques: comparison with intravascular optical coherence tomography. Int. J. Cardiol. 148, 313–318 (2011).
Soeda, T. et al. Intensive lipid-lowering therapy with rosuvastatin stabilizes lipid-rich coronary plaques—Evaluation using dual-source computed tomography. Circ. J. 75, 2621–2627 (2011).
Sugiura, J. et al. Clinical course of optical coherence tomography-detected lipid-rich coronary plaque after optimal medical therapy. Circ. Rep. 4, 29–37 (2022).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832 (1990).
Budoff, M. J. et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J. Am. Coll. Cardiol. 49, 1860–1870 (2007).
Detrano, R. et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 358, 1336–1345 (2008).
Kataiwa, H., Tanaka, A., Kitabata, H., Imanishi, T. & Akasaka, T. Safety and usefulness of non-occlusion image acquisition technique for optical coherence tomography. Circ. J. 72, 1536–1537 (2008).
Kume, T. et al. Visualization of neointima formation by optical coherence tomography. Int. Heart J. 46, 1133–1136 (2005).
Sugawara, Y. et al. Plaque modification of severely calcified coronary lesions byscoring balloon angioplasty using lacrosse non-slip element: Insights from an optical coherence tomography evaluation. Cardiovasc. Interv. Ther. 34, 242–248 (2019).
Kume, T. et al. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention 6, 768–772 (2011).
Lee, T. et al. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc. Imaging 10, 883–891 (2017).
Sugiyama, T. et al. Calcified plaques in patients with acute coronary syndromes. JACC Cardiovasc. Intv. 12, 531–540 (2019).
Finck, T. et al. Long-term prognostic value of morphological plaque features on coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging. 21, 237–248 (2020).
Andreini, D. et al. Coronary plaque features on CTA can identify patients at increased risk of cardiovascular events. JACC Cardiovasc. Imaging. 13, 1704–1717 (2020).
Lee, S. E. et al. Differences in progression to obstructive lesions per high-risk plaque features and plaque volumes with CCTA. JACC Cardiovasc. Imaging. 13, 1409–1417 (2020).
Fujii, K. et al. Expert consensus statement for quantitative measurement and morphological assessment of optical coherence tomography. Cardiovasc. Interv. Ther. 35, 13–18 (2020).
Watanabe, M. et al. Side branch complication after a single-stent crossover technique: Prediction with frequency domain optical coherence tomography. Coron. Artery Dis. 25, 321–329 (2014).
Torii, S. et al. Eruptive calcified nodules as a potential mechanism of acute coronary thrombosis and sudden death. J. Am. Coll. Cardiol. 77, 1599–1611 (2021).
Xu, Y. et al. Prevalence, distribution, predictors, and outcomes of patients with calcified nodules in native coronary arteries: A 3-vessel intravascular ultrasound analysis from Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT). Circulation 126, 537–545 (2012).
Prati, F. et al. Clinical outcomes of calcified nodules detected by optical coherence tomography: A sub-analysis of the CLIMA study. EuroIntervention 16, 380–386 (2020).
Watanabe, Y. et al. Comparison of clinical outcomes of intravascular ultrasound-calcified nodule between percutaneous coronary intervention with versus without rotational atherectomy in a propensity-score matched analysis. PLoS ONE 15, e0241836 (2020).
Acknowledgements
Authors thank the patients, participating cardiologists, and the staff who contributed to this study.
Author information
Authors and Affiliations
Contributions
J.S. wrote the main manuscript text and prepared the Figures. All authors, mainly M.W., reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sugiura, J., Watanabe, M., Nobuta, S. et al. Prediction of optical coherence tomography-detected calcified nodules using coronary computed tomography angiography. Sci Rep 12, 22296 (2022). https://doi.org/10.1038/s41598-022-26599-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26599-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.