Abstract
Venous thromboembolism (VTE) and major bleeding (MBE) are feared complications that are influenced by numerous host and surgical related factors. Using machine learning on contemporary data, our aim was to develop and validate a practical, easy-to-use algorithm to predict risk for VTE and MBE following total joint arthroplasty (TJA). This was a single institutional study of 35,963 primary and revision total hip (THA) and knee arthroplasty (TKA) patients operated between 2009 and 2020. Fifty-six variables related to demographics, comorbidities, operative factors as well as chemoprophylaxis were included in the analysis. The cohort was divided to training (70%) and test (30%) sets. Four machine learning models were developed for each of the outcomes assessed (VTE and MBE). Models were created for all VTE grouped together as well as for pulmonary emboli (PE) and deep vein thrombosis (DVT) individually to examine the need for distinct algorithms. For each outcome, the model that best performed using repeated cross validation was chosen for algorithm development, and predicted versus observed incidences were evaluated. Of the 35,963 patients included, 308 (0.86%) developed VTE (170 PE’s, 176 DVT’s) and 293 (0.81%) developed MBE. Separate models were created for PE and DVT as they were found to outperform the prediction of VTE. Gradient boosting trees had the highest performance for both PE (AUC-ROC 0.774 [SD 0.055]) and DVT (AUC-ROC 0.759 [SD 0.039]). For MBE, least absolute shrinkage and selection operator (Lasso) analysis had the highest AUC (AUC-ROC 0.803 [SD 0.035]). An algorithm that provides the probability for PE, DVT and MBE for each specific patient was created. All 3 algorithms had good discriminatory capability and cross-validation showed similar probabilities comparing predicted and observed failures indicating high accuracy of the model. We successfully developed and validated an easy-to-use algorithm that accurately predicts VTE and MBE following TJA. This tool can be used in every-day clinical decision making and patient counseling.
Similar content being viewed by others
Introduction
More than 540,000 total knee arthroplasties (TKA) and 230,000 total hip arthroplasties (THA) are annually performed in the United States, and these numbers continue to rise steadily1,2. Rates of venous thromboembolism (VTE) that consist of deep venous thrombosis (DVT) and pulmonary embolism (PE) range between 1 and 3% depending on a host of factors3,4. VTE is associated with immense morbidity and increased cost for episode of care and therefore has attracted much attention in recent years and with the introduction of bundled care5,6.
There are numerous risk factors for development of VTE that are either host or surgery related7,8,9,10,11,12. While it is important to know the individual risk factors associated with VTE, that alone does not always contribute to clinical decision making and overall risk stratification. In the era of personalized medicine, and taking into consideration the many existing pharmacological and non-pharmacological options for VTE prophylaxis, individualized risk scores are desperately needed. The Caprini score that is utilized in other surgical fields has never been validated on orthopedic surgical patients and is not applicable to patients undergoing TJA13. Subsequently—numerous risk stratification models have been suggested to be used following total joint arthroplasty (TJA), and while moving towards a more personalized approach for risk stratification they lack proper validation8,14,15,16.
Besides VTE, the risk of major bleeding events (MBE) in the postoperative period can also be consequential and occasionally fatal17,18,19,20,21,22. While similar rates of VTE and MBE are expected, the latter has received much less focus23,24. As evidence, none of the previous VTE scores take MBE into consideration, and as far as we are aware, there is currently no risk stratification model for MBE. VTE risk stratification and the influence on prevention modalities such as chemoprophylaxis have a direct influence on MBE17,18,19,20,21,22,25,26,27,28. Understanding this relationship may aid in providing an ideal risk‐benefit ratio to decide on the optimal VTE prophylaxis.
Many developments have occurred in the last decade in the field of joint arthroplasty including faster recovery and early mobilization, the use of tranexamic acid, spinal anesthesia, and the transition to aspirin for prophylaxis29,30,31. These factors have positively impacted the outcome of joint arthroplasty by minimizing complications and facilitating rapid recovery, to the point that some are now done as outpatient procedures32. In parallel with these changes in surgery, in recent years machine learning has been introduced into many areas within the healthcare system with the potential to revolutionize the medical landscape33,34,35. Recent developments in machine learning have facilitated a more comprehensive, accurate and user-friendly platform that may help clinicians in decision making.
Using a contemporary large institutional database with granular data, this study aimed to develop and validate an algorithm suitable for use in everyday clinical practice that could predict the probability of developing VTE and MBE in TJA patients, taking into account the influence of a large number of variables.
Methods
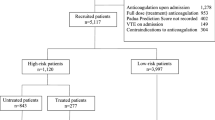
This was a single institution, retrospective cohort study. All methods were carried out in accordance with relevant guidelines and regulations. This study was reviewed and approved by the Institutional Review Board of Thomas Jefferson University with a waiver of informed consent. Following IRB approval, medical records of 37,948 patients who underwent either primary or revision total hip or knee arthroplasty (THA or TKA) between January 2009 and October 2020 were reviewed. STROBE reporting guidelines were followed throughout the data collection process36. Patients for whom a minimum 90-day follow-up was not available were excluded leaving us with 35,963 cases included in the study.
Sex, age, race, body mass index (BMI), patient-reported past medical history, Charlson Comorbidity and Elixhauser Comorbidity indexes were broken down to their individual components, as well as the American Society of Anesthesiologists (ASA) classification were collected. Variables that have shown an association with VTE in previous publications including hormone replacement therapy, rheumatoid arthritis, Sjogren’s syndrome, lupus, varicose veins, irritable bowel syndrome, history of stroke, myeloproliferative disease, and sleep apnea, were queried utilizing International Classification of Disease (ICD)-9 and ICD-10 codes37. Patients with any type of active cancer or a history of cancer were also identified and stratified based on a previously published VTE predictive model developed by Khorana et al.38,39. Keyword searches were conducted to identify coagulopathies and hypercoagulability in the patient population (Supplementary Table 1–2). Notes containing a keyword for coagulopathy or hypercoagulability (n = 74,886) were isolated and reviewed to enhance capture rates.
Clinical notes, hospital orders, and discharge summaries were reviewed to determine VTE prophylaxis prescribed to each patient postoperatively as well as identify chronic anticoagulation that the patient may be taking preoperatively. These were grouped into distinct groups—none, aspirin 81 mg (twice daily), aspirin 325 mg (twice daily), warfarin and others (including anti-factor Xa, Unfractionated Heparin, low molecular weight heparin, fondaparinux, Adenosine diphosphate receptor inhibitor and direct thrombin inhibitor). Information regarding the operation, including the specific joint operated on (knee versus hip), whether it was unilateral or bilateral, operative time, tourniquet use for only TKA procedures, surgical approach for only THA procedures, surgeon volume (dichotomized to normal versus high), use of cement, tranexamic acid administration, intraoperative blood transfusions (divided into 3 distinct categories: no transfusion, 1 unit transfusion and 2 or more unit transfusion) as well as type of anesthesia (regional versus general) was also collected from operative reports and anesthesia logs.
Two distinct outcomes were evaluated. The first was the occurrence of symptomatic DVT or PE within 90 days of surgery. To avoid including superficial clots that were not clinically significant, only patients that had a documented diagnosis, confirmatory study, and treatment for VTE were considered to have met the primary endpoint. The second main outcome was occurrence of major bleeding events (MBE) as defined by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis40. Symptomatic VTE and MBE occurring within 90 days of the operation were identified from medical records. To enhance the capture rate, comprehensive queries utilizing keywords for DVT, PE and MBE were conducted in clinical notes, physician dictations, and patient-provider phone-call logs (Supplementary Table 3 and 4). Notes containing a keyword for DVT (n = 44,752), PE (n = 14,878) and MBE (n = 9149) were isolated and manually reviewed. All readmissions within 90 days were also reviewed to detect any uncaptured VTE or MBE event.
Statistical analysis
Prior to running the predictive algorithms, a set of descriptive statistics were performed to understand the data distributions. Patients with VTE were compared to those who did not have VTE and those with MBE were compared to those who did not have MBE. Continuous data is presented as a mean (standard deviation) and categorical data is presented as a cell count (%). T-tests were used to calculate p values for continuous data and Chi-Square tests were used to calculate p values for categorical data. Due to the nature of comparisons in the first table, the alpha was adjusted to 0.001.
Following the descriptive breakdown, various machine learning methods were applied with the main objective of being able to determine specific variables that produced an increase likelihood chance of getting a DVT, PE, or MBE. The four models that were tested where Random Forest (RF), LASSO, Gradient Boosting Trees (XGB), and Support Vector Machines (SVM). Due to the nature of imbalance in the data as well, some of the models were also performed using down-sampling. In order to validate each model, both the VTE and MBE data sets were split out into a 70–30% split so we could properly train the data and test it.
Each training model analyzed used repeated cross validation (CV) techniques, where one fold was removed each time the model was fitted. The repeated CV was done 3 times with fivefold each time. To determine the best models from training, AUCs and Precision Curves were calculated. Once the “best” model was selected, the remaining data (test data) was tested on it to ensure it had the proper performance. All statistical analyses were done using R Studio (Version 3.6.3, Vienna, Austria).
Results
Of the 35,963 patients included in the study, 308 (0.86%) developed VTE (170 PE’s, 176 DVT’s) and 293 (0.81%) developed MBE. There were significant differences in patient demographics, characteristics, comorbidities, anticoagulation medications and operative factors between patients who developed VTE and MBE and those who did not (Table 1).
Venous thromboembolism
Risk factors for VTE in the initial univariate analysis were preoperative use of chronic anticoagulation other than aspirin (p < 0.001), older age (p < 0.001), higher BMI (p = 0.008), history of VTE (p < 0.001), hyper-coagulopathy (p < 0.001), higher ASA score (p < 0.001), heart failure (p = 0.002), atrial fibrillation (p < 0.001), other cardiovascular disease (p = 0.004), COPD (p = 0.004), dementia (p = 0.034), complicated DM (p = 0.021), CRF (p < 0.001), chronic anemia (p = 0.005), active malignancy (p < 0.001), metastatic disease (p = 0.002), knee joint (p < 0.001), simultaneous bilateral surgery (p < 0.001), underlying fracture (p < 0.001), operative duration (0.001), revision surgery (p < 0.001), cemented prosthesis (p < 0.001), direct lateral approach to the hip (p = 0.002), general anesthesia (p < 0.001), not using tranexamic acid (p < 0.001), allogenic blood transfusions (p < 0.001) and use of Warfarin for VTE prophylaxis (p < 0.001).
Separate models were developed for DVT and PE prediction and were tested using repeated cross-validation. Gradient boosting trees had the highest performance for both PE (AUC-ROC 0.774 [SD 0.055]) and DVT (AUC-ROC 0.759 [SD 0.039]) prediction and was chosen for algorithm development (Figs. 1 and 2 reflect performance on the validation cohort). Patients were grouped into categories according to predicted probability of VTE and the proportion of actual VTE in each category was examined; high agreement between expected and observed events was seen (Fig. 3).
Gradient boosting trees analysis showed the 10 most important factors associated with PE were the following (by order of importance): active cancer (very high risk), hyper-coagulopathy, blood transfusion, Warfarin for VTE prophylaxis, older age, operative duration, revision surgery, history of VTE, atrial fibrillation and underlying fracture (Fig. 4). For DVT, the 10 most important factors were the following (by order of importance): hyper-coagulopathy, older age, allogenic blood transfusions, revision surgery, Warfarin prophylaxis, simultaneous bilateral surgery, active cancer (very high risk), active or former smoking, underlying fracture and male sex (Fig. 5). Examples of different clinical scenarios and the algorithm predictions are demonstrated in Table 2.
Major bleeding events
Risk factors for MBE in the initial univariate analysis were preoperative use of chronic Warfarin (p < 0.001), older age (p < 0.001), higher BMI (p = 0.002), current or past smoking (p < 0.001), history of VTE (p < 0.001), hyper-coagulopathy (p < 0.001), higher ASA score (p < 0.001), Myocardial infarct (p < 0.001), heart failure (p = 0.002), atrial fibrillation (p < 0.001), complicated DM (p = 0.001), PUD (p < 0.001), CRF (p < 0.001), Sjogren syndrome (p = 0.015), chronic anemia (p = 0.009), varicose veins (p < 0.001), active and history of malignancy (p’s < 0.001), underlying fracture (p = 0.008), operative duration (p < 0.001), revision surgery (p < 0.001), cemented prosthesis (p < 0.001), tourniquet use (p < 0.001), direct lateral approach to the hip (p < 0.001), general anesthesia (p < 0.001), not using tranexamic acid (p < 0.001), allogenic blood transfusions (p < 0.001) and use of Warfarin for VTE prophylaxis (p < 0.001).
The MBE models were tested using repeated cross-validation. Lasso analysis had the highest AUC (AUC-ROC 0.803 [SD 0.035]) on the testing set and was chosen for MBE algorithm development (Fig. 6 reflect performance on the validation cohort). Patients were grouped into categories according to predicted probability of MBE and the proportion of actual MBE in each category was examined; high agreement between expected and observed events was seen (Fig. 7).
Lasso analysis for the entire cohort showed that the 10 most important factors associated with MBE were the following (by order of importance): revision surgery, chronic use of warfarin preoperatively, operative duration, general anesthesia, peptic ulcer disease (PUD), allogenic blood transfusions, older age, knee joint, varicose vein and current or past smoking (Fig. 8). Examples of different clinical scenarios and the algorithm predictions are demonstrated in Table 2.
Discussion
Due to the high morbidity and mortality associated with VTE and MBE, risk stratification prior to surgery is of great interest. Numerous studies have sought to identify specific risk factors that are associated with VTE and MBE41,42, and risk stratification models have also been proposed by prior studies reflecting its clinical importance8,14,15,16. The present study was designed and executed to take advantage of recent developments in machine learning algorithms that are patient-specific. We have successfully created an algorithm that not only accurately predicts VTE and MBE but also provides guidance on how different prophylactic measures may mitigate each individual’s risk. This study represents a major advancement in decision making prior to surgery and represents another major step towards patient-specific management.
Past studies have recognized multiple risk factors associated with VTE and MBE, including increasing age, previous VTE, revision surgery, cancer, type of procedure and more43,44,45. While the nature of the models used in this study were ‘black box’ which does not allow us to fully understand the decision trees generated to reach the final algorithm, looking at the random forest relative importance does provide insight into its decision making. All 10 of the most important variables that were pointed out for each one of the 3 outcomes—MBE, DVT and PE, have been previously reported as risk factors, providing reassurance that the algorithm is not only accurate, but consistent with accepted risk factors. One important finding was that while PE and DVT have many risk factors in common, one can gain from using different models in order to predict each of them and therefore we created and validated VTE and PE models separately.
In a recent systematic review of risk prediction scores for venous thromboembolism following joint replacement five observational cohort studies describing five risk scores were included46. The number of component variables in a single risk score ranged from 5 to 26. Three risk scores comprised 5–8 component variables. None of the studies reported calibration or discrimination statistics that can be directly compared to our model. Validation was lacking for all previous studies and they could be divided to ones in which scores are presented and evaluated within the same cohort or a holdout of patients9,15, and other studies specifically set out to validate an existing score or protocol16,47,48,49,50,51. Nam et al.51 were the only group prospectively evaluating their institutional protocol thus were able to take into account the influence of chemoprophylaxis in their evaluation. Using a simple protocol they categorized patients to “routine” (75.4%) and “high” (24.6%) risk groups and show 0.5% VTE rates in both. Whether their protocol does not capture high risk patients or the risk was mitigated by use of more aggressive anticoagulation in that group is debatable. In another study, Parvizi et al. used National Inpatient Sample (NIS) registry data to develop an individualized risk model for VTE which was based on 26 risk factors. The authors used scoring criteria to assess the performance of the model. The authors reported that their calibration curve showed a near perfect fit between the predicted VTE rate (using the risk model) and the actual rate of VTE in NIS data up to a 5% rate of VTE, beyond which point there was a clear divergence. Batelman et al.16 retrospectively evaluated the caprini and VTEstimator in a group of 363 TKA and THA patients. They failed to show an association between mean scores and risk for VTE. The study while interesting suffers from many methodological issues including small sample size and event rate (only 10 VTE), inability to assess the scores adequately due to missing data, and the evaluation of scores as continuous as opposed to categorical for risk stratification52. Krauss et al.47 compared a departmental protocol to the caprini score and showed that with a threshold of 10, the later was able to capture 7 out of 8 VTE compared to only 1 event that was captures using the departmental protocol. Notably, this threshold was evaluated and chosen to optimize the results of the caprini score within that specific cohort (using the youden index) and therefore does not reflect a true external validation of the score. More recently, Gold et al.48 in a cohort of 2155 TJA patients failed to find an association between high caprini scores both when evaluated continuously and categorically (with 11 as the threshold) when taking into account chemoprophylaxis in a multivariate analysis.
Several advantages make the present study stand out. The first is that the data that was used to develop the algorithm was derived from a single center and hence granular and contemporary. The Caprini score47 would automatically categorize patients undergoing TJA as high risk, however these surgeries have shifted tremendously over the years from having extended hospitalizations and limited postoperative weight bearing to becoming outpatient procedures with immediate mobilization. More and more patients receive spinal anesthesia, tranexamic acid, and aspirin as the primary chemoprophylaxis agent. These changes have reduced the rates of VTE during the last decade and our granular institutional data allowed us to capture these changes and take into account their influence48. A quick look at the univariate and relative importance analysis shows that modifiable factors such as tranexamic acid, type of anesthesia, and type of prophylaxis are important variables that previous studies/algorithms do not consider at all. The fact that these are modifiable variables is exciting as their influence could be tested in real time to examine whether they can change the individual patient’s risk. Previous works assumed that when patients have multiple risk factors and a calculated high risk they could benefit from more potent chemoprophylaxis—however recent studies have shown that is not the case which calls into question their practical use49. This algorithm could be used in everyday decision making prior to surgery and have an immense impact on treatment and hopefully lead to better outcomes and reduced VTE and MBE rates. Another important advantage is not only the assessment of VTE but also MBE that receives less attention in general but may also have a disastrous effect. Decisions that affect VTE result in a change in the MBE risk as well and vice versa, so the assessment should always include both outcomes.
This study is not without limitations. First, “black box” analyses as the ones used in this study are not easily interpretable and it remains unclear how the model predicts outcome. While relative importance and cross validation shed some light on what the model relies on more heavily, we are still left with some uncertainty. Second, while analyzing a cohort of almost 40,000 from a single institution, we may still have been underpowered to detect influence of some of the variables due to the low event rates of both VTE and MBE. This may have reduced the performance of all three models. Third, we were unable to detect whether the DVT cases were proximal or distal. One could assume the majority were proximal as all received treatment, however we cannot say for sure. Our conclusion therefore apply to clinically overt cases. Fourth, the design was retrospective hence mistakes in data collection and extraction may have occurred. Fifth, the model includes variables that are not always known prior to surgery and can only be assumed (such as operative time), limiting its utility for prospective risk stratification. Finally, there are many possible variables that were not assessed in the present study and their inclusion may have improved the predictive capabilities. Future studies to incorporate these factors into the algorithm may further refine this tool.
To conclude, we successfully created and validated an easy-to-use, practical and accurate tool for predicting VTE and MBE following TJA (which can be accessed through the following link: https://icmphilly.wordpress.com/ortho-applications). This algorithm can and should be used by clinicians in practice to improve decision making and patient counseling. There is currently an international multicenter study on the way to validate our findings externally.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available. Please contact research@rothmanortho.com to request data.
References
Maradit Kremers, H. et al. Prevalence of total hip and knee replacement in the United States. J. Bone Jt. Surg. Am. 97(17), 1386–1397 (2015).
Singh, J. A., Yu, S., Chen, L. & Cleveland, J. D. Rates of total joint replacement in the United States: future projections to 2020–2040 using the national inpatient sample. The J. Rheumatol. 46(9), 1134–1140 (2019).
Pedersen, A. B., Mehnert, F., Johnsen, S. P., Husted, S. & Sorensen, H. T. Venous thromboembolism in patients having knee replacement and receiving thromboprophylaxis: a Danish population-based follow-up study. JBJS 93(14), 1281–1287 (2011).
Keller, K. et al. Venous thromboembolism in patients hospitalized for knee joint replacement surgery. Sci. Rep. 10(1), 22440 (2020).
Secemsky, E. A., Rosenfield, K., Kennedy, K. F., Jaff, M. & Yeh, R. W. High burden of 30-day readmissions after acute venous thromboembolism in the United States. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 7(13), e009047 (2018).
Søgaard, K. K., Schmidt, M., Pedersen, L., Horváth-Puhó, E. & Sørensen, H. T. 30-year mortality after venous thromboembolism. Circulation 130(10), 829–836 (2014).
Zhang, Z. H. et al. Risk factors for venous thromboembolism of total hip arthroplasty and total knee arthroplasty: a systematic review of evidences in 10 years. BMC Musculoskelet. Disord. 16(1), 1–14 (2015).
Parvizi, J., Huang, R., Raphael, I. J., Arnold, W. V. & Rothman, R. H. Symptomatic pulmonary embolus after joint arthroplasty: stratification of risk factors. Clin. Orthop. 472(3), 903–912 (2014).
Parvizi, J., Huang, R., Rezapoor, M., Bagheri, B. & Maltenfort, M. G. Individualized risk model for venous thromboembolism after total joint arthroplasty. J. Arthroplasty 31(9 Suppl), 180–186 (2016).
Parvizi, J. Dvt prophylaxis: risk stratification solutions. Orthop. Proc. 99, 40 (2017).
Januel, J. M. et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA 307(3), 294–303 (2012).
Falck-Ytter, Y., Francis, C. W. & Johanson, N. A. Prevention of VTE in orthopedic surgery patients: American college of Chest Physicians evidence-based clinical practice guidelines. Chest 141(2 Suppl), e278S-e325S (2012).
Caprini, J. A., Arcelus, J. I., Hasty, J. H., Tamhane, A. C. & Fabrega, F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin. Thromb. Hemost. 17(Suppl 3), 304–312 (1991).
Dauty, M., Schmitt, X., Menu, P., Rousseau, B. & Dubois, C. Using the risk assessment and predictor tool (RAPT) for patients after total knee replacement surgery. Ann. Phys. Rehab. Med. 55(1), 4–15 (2012).
Bohl, D. D. et al. Development and validation of a risk stratification system for pulmonary embolism after elective primary total joint arthroplasty. J. Arthroplasty 31(9), 187–191 (2016).
Bateman, D. K., Dow, R. W., Brzezinski, A., Bar-Eli, H. Y. & Kayiaros, S. T. Correlation of the caprini score and venous thromboembolism incidence following primary total joint arthroplasty—results of a single-institution protocol. J. Arthroplasty 32(12), 3735–3741 (2017).
Arsoy, D., Giori, N. J. & Woolson, S. T. Mobile compression reduces bleeding-related readmissions and wound complications after THA and TKA. Clin. Orthop. 476(2), 381–7 (2018).
Anderson, D. R. et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann. Int. Med. 158(11), 800. https://doi.org/10.7326/0003-4819-158-11-201306040-00004 (2013).
Anderson, D. R. et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N. Engl. J. Med. 378(8), 699–707. https://doi.org/10.1056/NEJMoa1712746 (2018).
Hood, B. R. et al. Association of aspirin with prevention of venous thromboembolism in patients after total knee arthroplasty compared with other anticoagulants: a noninferiority analysis. JAMA Surg. 154(1), 65 (2019).
Muscatelli, S. R., Zheng, H., Hughes, R. E., Cowen, M. E. & Hallstrom, B. R. Non-inferiority of aspirin for venous thromboembolism prophylaxis after hip arthroplasty in a statewide registry. J. Arthroplasty 36(6), 2068–2075 (2021).
Bala, A., Huddleston, J. I., Goodman, S. B., Maloney, W. J. & Amanatullah, D. F. Venous thromboembolism prophylaxis after TKA: aspirin, warfarin, enoxaparin, or factor Xa inhibitors?. Clin. Orthop. 475(9), 2205–13 (2017).
Chan, N. C. et al. A systematic review of contemporary trials of anticoagulants in orthopaedic thromboprophylaxis: suggestions for a radical reappraisal. J Thromb. Thromb. 40(2), 231–239 (2015).
Lindquist, D. E. et al. Comparison of postoperative bleeding in total hip and knee arthroplasty patients receiving rivaroxaban, enoxaparin, or aspirin for thromboprophylaxis. Clin. Appl. Thromb. 24(8), 1315–1321 (2018).
Huang, R., Buckley, P. S., Scott, B., Parvizi, J. & Purtill, J. J. Administration of aspirin as a prophylaxis agent against venous thromboembolism results in lower incidence of periprosthetic joint infection. J. Arthroplasty 30(9), 39–41 (2015).
Lindquist, D. E. et al. Comparison of postoperative bleeding in total hip and knee arthroplasty patients receiving rivaroxaban, enoxaparin, or aspirin for thromboprophylaxis. Clin. Appl. Thromb. 24(8), 1315–21 (2018).
Nam, D. et al. Thromboembolism prophylaxis in hip arthroplasty: routine and high risk patients. J. Arthroplasty 30(12), 2299–303 (2015).
Cafri, G. et al. Comparative effectiveness and safety of drug prophylaxis for prevention of venous thromboembolism after total knee arthroplasty. J. Arthroplasty. 32(11), 3524–3528 (2017).
Abdel, M. P. & Berry, D. J. Current practice trends in primary hip and knee arthroplasties among members of the american association of hip and knee surgeons: a long-term update. J. Arthroplasty 34(7), S24–S27 (2019).
Fillingham, Y. A. et al. The safety of tranexamic acid in total joint arthroplasty: a direct meta-analysis. J. Arthroplasty 33(10), 3070-3082.e1 (2018).
Spinal anesthesia: The new gold standard for total joint arthroplasty?. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4515222/
Scully, R. D., Kappa, J. E. & Melvin, J. S. “Outpatient”—same-calendar-day discharge hip and knee arthroplasty. JAAOS-J. Am. Acad. Orthop. Surg. 28(20), e900–e909 (2020).
Rajkomar, A., Dean, J. & Kohane, I. Machine Learning in Medicine. N. Engl. J. Med. 380(14), 1347–1358 (2019).
Ascent of machine learning in medicine. Nat. Mater. 18(5), 407 (2019).
Shohat, N. et al. Frank stinchfield award: identifying who will fail following irrigation and debridement for prosthetic joint infection. Bone Jt. J. 102-B(7_Supple_B), 11–9 (2020).
von Elm, E. et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335(7624), 806–808 (2007).
Parvizi, J., Huang, R., Rezapoor, M., Bagheri, B. & Maltenfort, M. G. Individualized risk model for venous thromboembolism after total joint arthroplasty. J. Arthroplasty 31(9 Suppl), 180–186 (2016).
Khorana, A. A., Kuderer, N. M., Culakova, E., Lyman, G. H. & Francis, C. W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111(10), 4902–4907 (2008).
Ay, C. et al. Prediction of venous thromboembolism in cancer patients. Blood 116(24), 5377–5382 (2010).
Schulman, S. et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. JTH 8(1), 202–204 (2010).
Zhang, Z. H. et al. Risk factors for venous thromboembolism of total hip arthroplasty and total knee arthroplasty: a systematic review of evidences in 10 years. BMC Musculoskelet. Disord. 16(1), 24 (2015).
Dai, W. L., Lin, Z. M., Shi, Z. J. & Wang, J. Venous thromboembolic events after total knee arthroplasty: Which patients are at a high risk?. J Knee Surg. 33(10), 947–957 (2020).
Anderson, F. A. & Spencer, F. A. Risk factors for venous thromboembolism. Circulation 107(23_suppl_1), 1–9 (2003).
Abdol Razak, N. B., Jones, G., Bhandari, M., Berndt, M. C. & Metharom, P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers 10(10), 380 (2018).
Age is a major risk factor of venous thromboembolism (VTE) | European Respiratory Society. Available from: https://erj.ersjournals.com/content/38/Suppl_55/p3936
Kunutsor, S. K., Beswick, A. D., Whitehouse, M. R. & Blom, A. W. Systematic review of risk prediction scores for venous thromboembolism following joint replacement. Thromb. Res. 168, 148–155 (2018).
Krauss, E. S. et al. Implementation and validation of the 2013 caprini score for risk stratification of arthroplasty patients in the prevention of venous thrombosis. Clin. Appl. Thromb./Hemost. 25, 1076029619838066 (2019).
Gold, P. A. et al. Can the Caprini score predict thromboembolism and guide pharmacologic prophylaxis after primary joint arthroplasty?. J. Orthop. 21, 345–349 (2020).
Dashe, J., Parisien, R. L., Pina, M., De Giacomo, A. F. & Tornetta, P. III. Is the caprini score predictive of venothromboembolism events in orthopaedic fracture patients?. J. Orthop. Trauma 33(6), 269–275 (2019).
Saragas, N. P., Ferrao, P. N. F., Saragas, E. & Jacobson, B. F. The impact of risk assessment on the implementation of venous thromboembolism prophylaxis in foot and ankle surgery. Foot. Ankle Surg. Off. J. Eur. Soc. Foot. Ankle Surg. 20(2), 85–89 (2014).
Nam, D. et al. Thromboembolism prophylaxis in hip arthroplasty: routine and high risk patients. J. Arthroplasty 30(12), 2299–2303 (2015).
Diaz Quintero, L. A. et al. Letter to the editor on “correlation of the caprini score and venous thromboembolism incidence following primary total joint arthroplasty-results of a single-institution protocol”. J. Arthroplasty 33(8), 2697–2698 (2018).
Author information
Authors and Affiliations
Contributions
N.S.—Conceptualization, Data Collection, Investigation, Manuscript Writing & Editing L.L.—Data Collection, Investigation, Manuscript Writing M.B.S.—Data Analysis, Visualization, Manuscript Writing & Editing Y.F.—Analysis, Investigation, Manuscript Editing J.P.—Conceptualization, Analysis, Investigation, Manuscript Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shohat, N., Ludwick, L., Sherman, M.B. et al. Using machine learning to predict venous thromboembolism and major bleeding events following total joint arthroplasty. Sci Rep 13, 2197 (2023). https://doi.org/10.1038/s41598-022-26032-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26032-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.