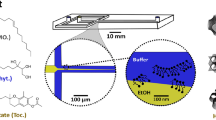
Abstract
Compartmentalization can serve different purposes such as the protection of biological active substances from the environment, or the creation of a unique combination of biomolecules for diagnostic, therapeutic, or other bioengineering applications. We present a method for direct encapsulation of molecules in biocompatible and semi-permeable microcapsules made from low-molecular weight poly(ethylene glycol) diacrylate (PEG-DA 258). Microcapsules are produced using a non-planar PDMS microfluidic chip allowing for one-step production of water-in-PEG-DA 258-in-water double-emulsions, which are polymerized with UV light into a poly-PEG-DA 258 shell. Semi-permeable microcapsules are obtained by adding an inert solvent to the PEG-DA 258. Due to the favorable hydrophilicity of poly-PEG-DA 258, proteins do not adsorb to the capsule shell, and we demonstrate the direct encapsulation of enzymes, which can also be dried in the capsules to preserve activity. Finally, we leverage capsule permeability for the implementation of a two-layer communication cascade using compartmentalized DNA strand displacement reactions. This work presents the direct encapsulation of active biomolecules in semi-permeable microcapsules, and we expect our platform to facilitate the development of artificial cells and generating encapsulated diagnostics or therapeutics.
Similar content being viewed by others
Introduction
Recent advances in microfluidics, material science, synthetic biology, and bioengineering enable precision engineering of micron sized compartments, allowing the manipulation of increasingly complex biomolecular systems1,2. Droplet microfluidic technology enables the production of polymeric compartments with precisely defined size and structure, templated from double-emulsions or higher order emulsions with a polymerizable phase as reviewed by Datta et al.3. One drawback with polymer precursors used as a middle-phase is that the obtained polymerized compartments are in general hermetic to the permeation of solutes, however Kim et al.4 demonstrated that semi-permeable polymeric shells can be fabricated by introducing an inert diluent, or porogen, in the polymer precursor phase. Upon UV polymerization, the non-reactive porogen is excluded from the polymerizing polymer in a process called polymerization-induced phase separation (PIPS). However, the drawback of the polymers used in this study and of most polymers obtained from water-immiscible precursors is the hydrophobicity of the polymerized capsule shell leading to protein adsorption, and therefore preventing direct encapsulation of proteins or enzymes.
Recently it was shown that PEG-diacrylate with a molecular weight of 258 g/mol PEG-DA 258 can be used as a polymerizable middle phase for the production of double-emulsions with a microfluidic capillary device5. The researchers also showed that poly-PEG-DA 258 has a hydrophilicity similar to hydrogels made from water soluble PEG-DA derivatives of higher molecular weight. Such favorable characteristics allowed them to directly encapsulate fibrinogen without noticeable adsorption to the polymer shell. However, the produced capsules were also hermetic and prevented even small molecule dyes (erioglaucine disodium salt: 793 g/mol, oil red O: 408 g/mol) to diffuse out of the capsules, which would severely limit the use and application range of such capsules.
To directly encapsulate biologically active macromolecules in biocompatible semi-permeable capsules, we combined the favorable characteristics of the low molecular weight PEG-DA 258 with pore formation by PIPS. In this study, we used a PDMS microfluidic device with a flow-focusing, co-axial, and non-planar geometry which does not require surface treatment to produce double-emulsions of water-in-PEG-DA 258-in-water. In order to obtain semi-permeable capsules, we used PIPS to form small pores in the capsule shells. By encapsulating fluorescent cargoes of different sizes or placing empty capsules in fluororophore containing solutions, we showed that the capsules were semi-permeable with a size cutoff allowing the direct encapsulation of proteins and enzymes of 32.7 kDa and above, while permitting transport of smaller molecules. Biomolecules were retained in the capsules without degradation, and were homogeneously distributed inside the capsules. Encapsulated enzymes survive air drying in trehalose and had enzymatic activity after rehydration. The semi-permeable capsules were able to communicate with their environment allowing us to implement a two-layer signalling cascade by immobilizing DNA strand displacement reactions inside the liquid core of two different microcapsule populations6. Altogether, this work describes the development and characterization of semi-permeable microcapsules with a biocompatible polymeric shell that can be used for the encapsulation of different biomolecules for use in diagnostic, therapeutic, or synthetic biology applications.
Results
Production of semi-permeable PEG-DA 258 microcapsules using PIPS
As PEG derivatives are generally considered biocompatible, bioinert, and biodegradable7, we tested various PEG-DAs of low molecular weight, such as PEG-DA MW 700, PEG-DA MW 575 and PEG-DA MW 258 as precursors for the production of microcapsules after photopolymerization by UV illumination. For PEG-DA with MW 575 or higher, their water miscibility prevented us to readily form double-emulsions. Leonavicene et al. recently showed that it is possible to obtain a two-phase system by combining PEG-DA MW 575 and high molecular weight PEG-DA MW 8000 in the inner phase and form core-shell capsules using PEG-DA as a capsule material8. However, we considered an alternative approach in using PEG-DA MW 258 as a potential polymer precursor due to its water immiscibility, as was also reported by Nam et al.5. This property of PEG-DA MW 258 allowed us to use it as the middle phase in double-emulsions and was compatible with droplet generation in a PDMS device (Fig. 1A). To form the double-emulsions, we used an aqueous continuous phase supplemented with 10% PVA. The PEG-DA 258 middle phase is supplemented with a photoinitiator (HMPP) and surfactant (Span80), with the optional addition of a mild organic solvent. We produced monodisperse double-emulsions in a jetting regime with flow rates of 2500 \(\upmu \)L/h for the aqueous continuous phase, 200 \(\upmu \)L/h for the PEG-DA MW 258 middle phase, and 250 \(\upmu \)L/h for the aqueous inner phase (Fig. 1B). Interestingly, the double-emulsions could be generated without requiring any surface treatment of the device owing to the non-planar geometry of the PDMS device which prevents wetting of the collection channel by the hydrophobic middle-phase.
Production of semipermeable microcapsules in a PDMS device with 3D geometry. (A) Schematic representation of the PDMS device with 3D geometry. W/O/W double-emulsions are generated with a PEG-DA 258 middle phase encapsulating an inner aqueous core. (B) Micrographs of the PDMS device operation. (C) Brightfield image of microcapsules obtained after UV polymerization of the collected double-emulsion. (D) Size distribution of a representative batch of polymerized microcapsules. (E) Schematic representation of PIPS upon UV illumination. 15% Butyl-acetate (porogen) was mixed with PEG-DA 258 to form semi-permeable microcapsules. (F) In the collected double-emulsion, both high molecular weight 500 kDa FITC-dextran and lower molecular weight 40 kDa RITC-dextran are retained in the inner aqueous phase. (G) After UV polymerization and PIPS, pores are formed in the capsule shell and capsules become semi-permeable.
We collected double-emulsions in a UV-transparent cuvette for 15 minutes, after which the capsules were polymerized in batch by UV exposition. After polymerization we obtained monodisperse capsules with a mean diameter of 62 \(\upmu \)m (Fig. 1C,D). The coefficient of variation was close to 5%, in accordance with previous results using similar PDMS devices9. The capsule shell was estimated from inspecting microscope images to be between 5 and 10 \(\upmu \)m thick. Our results not only confirm the observation from Nam et al.5 that water-immiscible PEG-DA MW 258 is a suitable middle phase, but demonstrated its compatibility with double-emulsion generation in a PDMS device which does not require any surface treatment, and resulted in the production of monodispersed poly-PEG-DA 258 microcapsules with porous thin shells (Fig. 1E–G).
PEG-DA 258 microcapsules are semi-permeable and the pore size can be adjusted by changing the porogen. Schematic representation of PEG-DA 258 microcapsules produced using (A) 15% butyl-acetate or (C) 15% 1-decanol as porogen. 15% butyl-acetate microcapsules selectively allowed the permeation of 10 kDa RITC-dextran while excluding 32.7 kDa EGFP. The larger pore size of microcapsules produced with 15% 1-decanol as porogen allowed the diffusion of both fluorescent molecules. Microcapsules produced using (B) 15% butyl-acetate or (D) 15% 1-decanol porogen were immersed in a solution containing 10 kDa RITC-dextran and 32.7 kDa EGFP. The evolution of the fluorescent signal in the Cy3 and FITC channels was observed after 5 min, 1 h and 24 h. While microcapsules produced using 15% 1-decanol were permeable to both fluorescent molecules, we clearly observed the selective permeability of microcapsules produced using 15% butyl-acetate as a porogen.
To produce semi-permeable capsules, we used the mild inert solvent butyl-acetate added to the middle phase serving as a porogen in PIPS4,10. Butyl-acetate was used by Kim et al.4 to form nanopores with diameter below 30 nm in a thin shell composed of a cross-linked network of ethoxylated trimethylolpropane triacrylate (ETPTA) and glycidyl methacrylate (GMA). To evaluate the permeability of the semi-permeable capsules, we added 500 kDa FITC-dextran and 40 kDa TMR-dextran to the inner aqueous phase, and observe that almost 90% of the polymerized capsules retained the 500 kDa FITC-dextran after the capsules were extensively washed by successive centrifugation in aqueous buffer and followed by a 24h incubation in buffer at 4 \(^{\circ }\)C (Fig. 1G). On the other hand, only about 40% of the capsules retained the lower molecular weight 40 kDa TMR-dextran. These results showed that some capsules are semi-permeable, as evidenced by capsules in which only the higher molecular weight fluorophore is retained. Even though the size cut-off could not be precisely determined from this experiment, we estimated it to be well below 500 kDa and potentially close to the size of the smaller fluorophore of 40 kDa.
To better characterize the semi-permeability, we prepared empty capsules and placed them in a solution of fluorescent molecules of smaller molecular weights (Fig. 2). We placed empty capsules produced using 15% butyl-acetate porogen in a solution containing both 10 kDa RITC-dextran and 32.7 kDa EGFP (Fig. 2A,B). We observed that most capsule showed a relatively rapid increase in signal in the red fluorescent channel, corresponding to the lower molecular weight 10 kDa RITC-dextran. After 1 h incubation, most capsules showed a red fluorescent signal, and all capsules displayed a red fluorescent signal after 24 h incubation. At the same time, the majority of these capsules showed no signal increase in the green fluorescent channel corresponding to the 32.7 kDa EGFP. By superimposing the signal from the two fluorescent channels, we could clearly see that a majority of the capsules were semi-permeable and excluded EGFP for at least 24 h while allowing diffusion of the 10 kDa RITC-dextran into their interior. We observed however some variability in the permeability of the capsules, with some capsules appearing permeable to EGFP already after a few minutes of incubation, while a few capsules still excluded 10 kDa RITC-dextran after 1 h.
We obtained semi-permeable capsules with a different size cutoff using 1-decanol as a porogen. The use of 1-decanol was reported by Kim et al.4 to create larger pores in the ETPTA/GMA polymer shell due to a different interaction parameter of 1-decanol with the forming polymer. Here, we show that capsules produced with 15% 1-decanol porogen in PEG-DA 258 also resulted in higher permeability than capsules produced with the butyl-acetate porogen (Fig. 2C,D). We observed an increase in green fluorescent signal corresponding to 32.7 kDa EGFP in the capsule interior after only a few minutes incubation and all capsules were fluorescent after 24 h. In addition, all capsules were completely permeable to 10 kDa RITC-dextran. To modify capsule permeability, we also explored the use of a smaller fraction of porogen with 10% butyl-acetate. We observe that a significant proportion of the capsules had no fluorescent signal corresponding to 10 kDa RITC-dextran after 1 h of incubation, suggesting a lower permeability cut-off (Supplementary Fig. S1). We also used different solvents as porogen such as 15% octanol (Supplementary Fig. S2) which yielded capsules of intermediate permeability between what was observed using 15% butyl-acetate or 15% decanol porogen. Using 15% 2-ethyl-1-hexanol (Supplementary Fig. S3), we produced capsules with a permeability comparable to 15% butyl-acetate. These results demonstrate that it is possible to modify capsule permeability by varying porogen content and composition, and that different solvents are compatible with our microfluidic production of semi-permeable capsules.
Direct encapsulation of proteins inside semi-permeable PEG-DA 258 capsules. EGFP was added to the aqueous inner phase for direct encapsulation. (A–C) Microscope images of double-emulsions showing a fluorescent signal in the FITC channel. (D–F) After polymerization, the fluorescent signal was still present in the interior of most capsules. Variability in the pore size or a size cutoff close to the 32.7 kDa EGFP resulted in some protein leakage. (G) Fluorescent intensity profile of the capsule indicated in panel E. The fluorescent profile suggests a homogeneous distribution of EGFP in the interior of the capsule without protein adsorption to the shell material. (H–J) Microscope images of polymerized capsules containing FITC-streptavidin after direct encapsulation. Fluorescent signal is present in all capsules, suggesting that the pore size is too small for FITC-streptavidin leakage. (K) Fluorescent profile across two capsules from panel I. The profile suggests a homogeneous distribution of FITC-streptavidin in the interior of the capsule without protein adsorption to the shell material.
Direct encapsulation of proteins
Next, we show that we can directly encapsulate proteins in semi-permeable poly-(PEG-DA 258) microcapsules without adsorption to the polymeric shell or loss of function. We selected 15% butyl-acetate as porogen to allow the retention of proteins and enzymes in the interior of the capsules, while allowing the transport of smaller biomolecules across the capsule shell. We encapsulated 32.7 kDa EGFP inside our microcapsules by adding it to a final concentration of 2 \(\upmu \)g/mL to the inner aqueous phase. EGFP fluorescence signal was observed in the double-emulsion without any sign of precipitation(Fig. 3A–C), and, once polymerized, the capsules displayed a homogeneous fluorescent profile without any sign of protein adsorption to the capsule material (Fig. 3D–G). We also encapsulated 60 kDa FITC-labelled streptavidin in the aqueous inner phase to a final concentration of 50 \(\upmu \)g/mL, and the fluorescent signal profile in the polymerized capsules indicated no sign of protein adsorption on the capsule shell (Fig. 3H–K). After incubation in PBS for 24 h, we saw that a proportion of the EGFP-containing capsules did not contain any fluorescent signal (Fig. 3D–F). This observation suggests a permeability cutoff close to the size of these fluorescent biomolecules. We also expect some variability in the pore size of the capsules due to the batch UV polymerization process, during which the UV intensity could slightly differ depending on the position of the capsule in the cuvette. Also, some capsules might have been broken or damaged which would lead to the release of their cargo. With the larger molecular weight 60 kDa FITC-streptavidin, we saw that most polymerized capsules contained fluorescent signal after aqueous washes, indicating a size cutoff below the size of FITC-streptavidin.
We demonstrated that the poly-PEG-DA 258 shell obtained after UV polymerization is compatible with direct protein encapsulation. The materials used did not lead to adsorption of proteins to the capsule shell and the polymerization-induced phase separation process formed pores sufficiently small to retain proteins with molecular weights of 32.7 kDa and above.
Direct encapsulation of functional enzymes in semi-permeable PEG-DA 258 microcapsules. A luciferase-GFP fusion protein was directly encapsulated in semi-permeable capsules. The fluorescent fusion protein allows for the visualization of the enzyme (A) in the double-emulsion, and (B), in the polymerized capsules. (C) The encapsulated econoLuciferase shows a strong signal in a bioluminescent assay. Direct encapsulation of \(\beta \)-galactosidase. (D,E) Enzyme-containing capsules were dispersed in trehalose and air-dried at 37 \(^{\circ }\)C. (F) After rehydration with a solution containing CPRG, the substrate was hydrolyzed to chlorophenol red. (G) The solution was imaged with a color camera mounted on an inverted microscope with \(\times \) 4 magnification. Capsules displayed a purple color in their interior, indicative of \(\beta \)-galactosidase enzymatic activity.
After successful encapsulation of fluorescent proteins, we encapsulated enzymes and performed enzymatic assays with the produced capsules. We used a recombinant GFP-luciferase fusion protein (econoLuciferase, Biosynth), which allowed confirmation of encapsulation by measuring its fluorescent signal. The molecular weight of the fusion protein being over 90 kDa led to retention of the enzyme in the interior of the microcapsules. Indeed, we saw that both the double-emulsion and polymerized capsules contained a fluorescent signal from the econoLuciferase fluorescent fusion protein. In both cases, we observed a speckled distribution of the fluorescent signal which might be due to some precipitation in the 10% PVA inner solution (Fig. 4A,B). The econoLuciferase-containing capsules were placed in a solution containing D-luciferin, and we measured a bioluminescent signal two orders of magnitude higher than the signal observed for empty capsules (Fig. 4C). These results demonstrated that an active enzyme can be encapsulated in poly-PEG-DA 258 microcapsules, with the semi-permeable shell allowing diffusion of the substrate D-Luciferin into the core of the microcapsules to generate a bioluminescent signal.
In a second example, we showed that capsules can be dried and rehydrated while preserving enzyme function. We encapsulated \(\beta \)-galactosidase, a tetrameric enzyme with a molecular weight of 465 kDa. To show that enzyme-containing capsules can be dried and retain activity upon rehydration, we dispersed \(\beta \)-galactosidase-containing capsules in a 0.5 M trehalose solution and dried small drops overnight in an incubator at 37 \(^{\circ }\)C resulting in trehalose pellets (Fig. 4D,E). After rehydrating the dried pellets with a Chlorophenolred-\(\beta \)-d-galactopyranoside (CPRG) solution, we observed a change in color upon enzymatic conversion of the yellow CPRG to chlorophenol red. We observed that the color change occurred at the location of the capsules, and visualization with a 4× objective showed that the interior of some caspules turn to an intense purple red color (Fig. 4F,G). Although the images were not used as a quantification of the chlorophenol red concentration, they clearly showed that conversion of CPRG to chlorophenol red occurred inside the \(\beta \)-galactosidase-containing capsules. These results demonstrated the possibility for the direct encapsulation of active enzymes into semi-permeable microcapsules, and we additionally showed that capsules can be air-dried in a lyoprotective solution of trehalose and still retain their activity after rehydration.
Immobilization of DNA strand displacement reaction network in semi-permeable microcapsules and implementation of a two-layer signalling cascade. (A) Schematic representation of the two-layer signalling cascade as developed by Joesaar et al.6 (B) Implementation of the two-layer signalling cascade in poly-PEG-DA 258 capsules. The two capsule populations were mixed together and imaged on a cell-counting slide immediately after addition of 50 nM input strand (A). An increase in Cy5 and Cy3 fluorescent signals was observed corresponding to the activation of the first and second populations, respectively. (C) Median intensity of detected particles. An increase in the Cy5 signal was observed corresponding to the activated first population of capsules. After release and diffusion of the signal strand (\(Q_1\)) to the second capsule population, an increase in Cy3 signal was observed corresponding to their subsequent activation. The larger symbols correspond to the median of all detected particles in a given fluorescent channel.
Encapsulated DSD reaction networks and implementation of a two-layer signalling cascade
We investigated the possibility of encapsulating more complex biomolecular systems, as this could be used for sensing and responding to a stimulus in diagnostic, therapeutic, or theranostic applications. It was recently demonstrated that DNA strand displacement (DSD) reactions can be performed in proteinosome microcompartments11 as a model of protocellular communication and distributed biomolecular computation6. Here, we immobilized biotinylated DNA strands in our poly-(PEG-DA 258) capsules containing streptavidin, following the design from Joesaar et al.6.
We implemented a two-layer signalling cascade by functionalizing a first population of capsules with a transducer DSD gate that activates after toehold displacement by a ssDNA input strand (A), which leads to the release of a signal strand (Q1) and unquenching of a Cy5 fluorophore. The (Q1) signal strand can in turn activate a second population of capsules functionalized with a transducer-amplifier DSD gate releasing a second signal strand (Q2), this time unquenching a Cy3 fluorophore. We also added a fuel strand which acts as an amplifier (Fig. 5A). The two capsule populations were mixed together and the two-layer signalling cascade was activated upon addition of the input strand (A). In this experiment, we mixed the two capsule populations, added 50 nM input strand (A), and loaded the freely moving capsules into a cell counting chamber. We first measured a Cy5 signal increase corresponding to the first population of capsules containing the first DSD transducer gate being activated. After a time lag of a few minutes, we saw a subsequent increase in Cy3 signal corresponding to the second population of capsules containing the transducer-amplifier DSD gate (Fig. 5B,C). We observed a Cy5 increase in about 10 capsules of the first population and Cy3 increase in about 15 capsules of the second population. The activation of the two-layer cascade could be modified by changing the concentration of input strand (A). By increasing its concentration to 100 nM, the activation of the first population was greatly accelerated, and it was difficult to capture the initial signal increase (Supplementary Fig. S4). On the other hand, reducing concentration of (A) to 10 nM led to a much lower level of activation (Supplementary Fig. S4). In all cases, we observed a lag of 5 to 10 min between activation of the first population and activation of the second population. While this is a succinct implementation of the recently developed compartmentalized DSD reactions, these results demonstrated that DSD reactions can be efficiently encapsulated in our semi-permeable microcapsules and employed to build communicating biomolecular systems.
Discussion
In this work, we present the production of biocompatible and semipermeable poly-PEG-DA 258 microcapsules and their use for encapsulation of proteins or DNA networks while retaining functionality. First, we show that PEG-DA 258 can be used as a polymerizable middle phase for the production of microcapsules templated from water-in-PEG-DA 258-in-water double-emulsion. The generation of such double-emulsion does not require the use of a glass capillary device5, but can be produced in a PDMS device with 3D geometry which does not require any surface treatment12. The relatively simple method and reproducibility in the fabrication and use of untreated PDMS devices for the production of poly-PEG-DA 258 microcapsules should make this method available to many labs with access to basic soft lithography fabrication.
By adding an inert diluent to the PEG-DA 258 middle phase, we show that semipermeable microcapsules can be formed by PIPS. This technique was previously used in the formation of sermi-permeable microcapsules made from other acrylate based polymers such as blends of ETPTA/GMA4 or EDGMA/GMA10. While the semi-permeability of capsules made from such polymers was demonstrated, no direct encapsulation of biological components was achieved, apart from the encapsulation of plasmid DNA in the ETPTA/GMA microcapsules in Niederholtmeyer et al.13. It is interesting to note that the plasmid DNA contained in these capsules could still serve as a protein expression template after immersion in a cell-free transcription translation system. However, the ETPTA/GMA capsule had to be reacted first with amino-PEG12-alcohol to prevent the adsorption of proteins on the capsule shell, which also indicates that direct encapsulation of proteins or enzymes would have been precluded by the use of such polymers.
Here, we confirm previous observation from Nam et al.5 that poly-PEG-DA 258 microcapsules do not lead to the adsorption of biomolecules, including fluorescent proteins or enzymes, allowing their direct encapsulation in the interior of the semi-permeable microcapsules. Moreover, the small pores obtained by PIPS when using butyl-acetate as a porogen were estimated to be close to or even smaller than the hydrodynamic radius of the 32.7 kDa small EGFP. This will allow for the encapsulation of a variety of proteins, RNA, DNA or other (bio)-molecule of interest as long as their size is not smaller than this cut-off. We show that the encapsulation of active enzymes is possible and that the semi-permeability of the capsules overcomes the challenges of stable encapsulation without release of the enzyme, while at the same time allowing diffusion of reactants and products in and out of the capsules. Alternatively, we propose that the encapsulation of streptavidin can serve as an immobilization partner for smaller molecules. Also, encapsulation of functionalized magnetic beads or particles of appropriate size, could be used for the same purpose. In future developments, this could serve to immobilize peptides and small proteins with affinity tags, or even small molecules, which could be activated or released upon sensing of an external stimulus14. Here we applied this concept by immobilizing short strands of DNA modified with a biotin handle on encapsulated streptavidin, which served in the implementation of a two-layer signalling cascade from DNA strand displacement (DSD) reactions. While the implementation of DSD reactions in microcompartments proposed by Joesaar et al.6 was originally performed in proteinosomes, we show that our semi-permeable microcapsules are also capable of implementing such complex synthetic biology application.
This work is the first demonstration of the direct encapsulation of active biomolecules in semi-permeable biocompatible poly-PEG-DA 258 capsules, but with some limitations. While the encapsulation method and parameters selected were suitable for the applications chosen, we recognize that improvements could be made to increase the yield of intact microcapsules and to obtain a specific semi-permeability cut-off. Effective removal of porogen in aqueous washes and other downstream processes affecting capsule permeability can be further improved to produce more homogeneous capsules. As controlling UV illumination initiating the polymerization process is critical for PIPS and generating the desired permeability, future developments could use in-line polymerization15 to produce capsules to ensure uniform illumination of each double-emulsion droplet. Finally, matching the density between the different phases, modifying the shell thickness, as well as exploring different porogen, surfactant, and photoinitiator compositions will be required to produce capsules with tailored properties for other specific applications.
In conclusion, we demonstrated the one-step encapsulation of a variety of biomolecules in poly-PEG-DA 258 microcapsules templated from double-emulsions. Fluorescent molecules, proteins, enzymes, and DNA strands with biological activity can be loaded in the core of the biocompatible semi-permeable capsules. This opens doors towards building complex and robust multi-component biomolecular artificial cells with diagnostic, therapeutic, and synthetic biology applications.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Rideau, E., Dimova, R., Schwille, P., Wurm, F. R. & Landfester, K. Liposomes and polymersomes: A comparative review towards cell mimicking. Chem. Soc. Rev. 47, 8572–8610 (2018).
Laohakunakorn, N. et al. Bottom-up construction of complex biomolecular systems with cell-free synthetic biology. Front. Bioeng. Biotechnol. 8, 431 (2020).
Datta, S. S. et al. 25th anniversary article: Double emulsion templated solid microcapsules: Mechanics and controlled release. Adv. Mater. 26, 2205–2218 (2014).
Kim, B., Jeon, T. Y., Oh, Y.-K. & Kim, S.-H. Microfluidic production of semipermeable microcapsules by polymerization-induced phase separation. Langmuir 31, 6027–6034 (2015).
Nam, C., Yoon, J., Ryu, S. A., Choi, C.-H. & Lee, H. Water and oil insoluble PEGDA-based microcapsule: Biocompatible and multicomponent encapsulation. ACS Appl. Mater. Interfaces 10, 40366–40371 (2018).
Joesaar, A. et al. DNA-based communication in populations of synthetic protocells. Nat. Nanotechnol. 14, 369–378 (2019).
Browning, M. B. & Cosgriff-Hernandez, E. Development of a biostable replacement for PEGDA hydrogels. Biomacromolecules 13, 779–786 (2012).
Leonaviciene, G., Leonavicius, K., Meskys, R. & Mazutis, L. Multi-step processing of single cells using semi-permeable capsules. Lab Chip 55, 159 (2020).
Cole, R. H., Tran, T. M. & Abate, A. R. Double emulsion generation using a polydimethylsiloxane (PDMS) co-axial flow focus device. J. Vis. Exp. 2015, 1–7 (2015).
Loiseau, E. et al. Strong microcapsules with permeable porous shells made through phase separation in double emulsions. Langmuir 33, 2402–2410 (2017).
Huang, X. et al. Interfacial assembly of protein-polymer nano-conjugates into stimulus-responsive biomimetic protocells. Nat. Commun. 4, 2239 (2013).
Rotem, A., Abate, A. R., Utada, A. S., Van Steijn, V. & Weitz, D. A. Drop formation in non-planar microfluidic devices. Lab Chip 12, 4263 (2012).
Niederholtmeyer, H., Chaggan, C. & Devaraj, N. K. Communication and quorum sensing in non-living mimics of eukaryotic cells. Nat. Commun. 9, 1–8 (2018).
Kim, K. T., Angerani, S., Chang, D. & Winssinger, N. Coupling of DNA circuit and templated reactions for quadratic amplification and release of functional molecules. J. Am. Chem. Soc. 141, 16288–16295 (2019).
Steinacher, M., Cont, A., Du, H., Persat, A. & Amstad, E. Monodisperse selectively permeable hydrogel capsules made from single emulsion drops. ACS Appl. Mater. Interfaces 13, 15601–15609 (2021).
Michielin, G. Microfluidics and cell-free synthetic biology in the development of new diagnostic and therapeutic platforms. Ecole Polytech. Féd. de Lausanne 2021, 1–192 (2021).
Acknowledgements
The authors thank Rohan Thakur for his contribution in setting up the syringe pumps and for his contributions in initial platform development. They thank Prof. Esther Amstad, Jui-Chia Chang, Dr. Gianluca Etienne, and the SMaL lab at EPFL, for supplying us with the microfluidic device photomask and for their help with preliminary experiments. GM was supported by the MD-PhD Program of the Swiss National Science Foundation (SNF, 323530\(\_\)171144) and by a SNF NRP (National Research Program) 78 Covid-19 Grant 198412 (to S.J.M.). This work was published as part of EPFL MD-PhD thesis No. 838516.
Author information
Authors and Affiliations
Contributions
G.M. and S.J.M. designed research; G.M. performed research; G.M. and S.J.M. analyzed results and data; G.M. and S.J.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michielin, G., Maerkl, S.J. Direct encapsulation of biomolecules in semi-permeable microcapsules produced with double-emulsions. Sci Rep 12, 21391 (2022). https://doi.org/10.1038/s41598-022-25895-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25895-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.