Abstract
To monitor employees' work safety and exposure against air contaminants, Trans, trans-muconic acid, Hippuric acid, Methyl hippuric acid, Mandelic acid and Phenylglyoxylic acid can be used as reliable biomarkers of exposure to benzene, toluene, ethylbenzene, and xylene (BTEX) compounds. This study aims to determine the level of urinary metabolites of BTEX compounds using biological monitoring in the employees of a wastewater treatment plant (WWTP) in the south of Iran. The study was performed on 56 employees of the WWTP of one of the southern cities of Iran in 2020. Urine samples (n total = 112) consisting of 60 samples of employees working in the operation section (exposed group) and 52 samples of employees working in the administrative section (control group) in the WWTP were collected before and at the end of their shift. The mean concentration of urinary metabolites of BTEX of both groups ranged from 546.43 (μg/g cr) for trans, trans-muconic acid to 0.006 (μg/g cr) for methyl hippuric acid, which indicates that most of the evaluated metabolites showed a higher concentration than their occupational threshold limit value urine (p < 0.05). Regression analysis results showed a significant correlation (p < 0.05) between age and utilization of flame heaters with changes in the measured BTEX metabolites in the urine. The results of this study illustrate that WWTPs should be considered as one of the workplaces with potential sources of BTEX exposure for employees. Future investigations are recommended to perform itemized appraisals of BTEX intake sources, particularly in employees of the operational sections of WWTP.
Similar content being viewed by others
Introduction
Benzene, toluene, ethylbenzene, and isomers of xylene (BTEX) are classified among the most important volatile compounds (VOCs) released into the environment from natural and manufactured resources1. Although traffic is a critical source of atmospheric BTEX emissions in cities2, workers in various occupations are exposed to BTEX compounds3,4. VOC emissions in wastewater treatment plants (WWTPs) depends on several agents, including the size of the wastewater treatment plant, the amount and characteristics of influent, the wastewater treatment processes and units, and the technologies applied to control or remove VOCs5. Like all of the VOCs, BTEX compounds are organic molecules with high vapor pressure and low boiling points6. The emission of these compounds may be because of the concentration gradient between the air and the water phase in WWTPs processes and turbulence in the aeration processes7.
The concentration of BTEX and the duration of exposure to them are among the most important factors affecting the health condition of people who are exposed to these compounds8. Although inhalation is a standard exposure method to BTEX compounds, dermal absorption should also be considered9. BTEX compounds have a wide range of neurological side effects, including weakness, loss of appetite, fatigue, confusion and nausea, respiratory complications, and hematologic health consequences, including cancer10. According to the IARC, benzene has long been considered a carcinogen11, and the ACGIH standard, the permissible threshold for 8-h exposure is 0.5 ppm12. Toluene, xylene, and ethylbenzene also have toxicological effects on the nervous system and are considered carcinogenic for humans13,14,15.
Occupational exposure to chemicals can be evaluated by determining environmental concentrations in different workplaces or byby determining environmental concentrations in various workplaces by calculating occupational exposures according to workers' work patterns (e.g., time spent in each workplace). Biomonitoring is another method, unlike environmental assessment of pollutants and chemicals, which assesses exposure from all ways and sources to determine the various contaminants16. Urine metabolites may act as indicators of occupational or environmental exposure, especially for water-soluble chemicalssuch as benzene, which includes phenol, hydroquinone, tt-MA acid, urinary hippuric acid (uHA), and S-phenylmercuric acid17,18,19. Among them, urinary tt-MA is recognized as a reliable and relatively facile biomarker20 and represents about 1% of the absorbed amount of benzene21. The range of metabolic conversion of benzene to tt-MA is about 2–25% and dependent on the benzene exposure level, simultaneous exposure to toluene, and probably also to genetic factors22. Recent studies have shown that trans,trans-muconic acid, a minor benzene metabolite, can be determined using HPLC with UV detection23.
Furthermore, tt-MA is known to be involved in bone marrow leukemogenesis, its applications in biological monitoring could thus be important in risk assessment24. Despite the low metabolic conversion of benzene to tt-MA, ACGIH recommends it as a suitable biomarker for occupational exposure assessment to benzene (higher than 0.25 ppm) in limited quantities (biological exposure index) of 500 μg/g cr25. Due to the short half-life of BTEX compounds in the human body26, assessing BTEX metabolized compounds in the urine can be more accurate in recognizing the rate of exposure27.
Recent occupational research using biomonitoring to assess workers' exposure, like in gas station workers28, petrochemical plants29,30, composting plant employees31, and traffic police personnel32, have been examined. Despite confirming the presence of BTEX compounds in the air of this wastewater treatment plant (WWTP) and as a result of potential exposure of the WWTPs employees to BTEX (Dehghani et al. 2021b, Dehghani et al. 2018), estimation of the biological levels of BTEX among the employees of WWTP has not been considered so far. We assessed WWTPs employees' exposure to BTEX compounds by measuring their urinary metabolites, including Trans, trans-muconic acid (tt-MA), Hippuric acid (HA), Methyl hippuric acid (MHA), Mandelic acid (MA), and Phenylglyoxylic acid (PGA) as a valuable biomarker to assess the personal exposure to benzene, toluene, ethylbenzene, and m+p-xylene, respectively. A questionnaire will obtain a systematic baseline among the participants, namely the control group (administrative personnel) and the exposed group (operational crew). The level of biomarkers among the participants before and after their shift allows immediate and accurate exposure estimation, and the post-hoc statistical analysis will determine the levels, significance, and importance for human health.
Materials and methods
Subject of study
This descriptive-analytical study was carried out in February 2020. The subjects of this study were 56 employees of the administrative and operational units of the wastewater treatment plant (WWTP) aged 25 to 59 years (Table 2, demographic characteristics). The spot sampling method (36) was selected so that the samples were representative of all employees of the WWTP (operational unit and administrative unit). The study was accompanied by a 20- to 30-min interview using a questionnaire that included demographic, job, and workplace information, condition of traffic near the living area, lifestyle, eating habits, smoking status, secondary exposure to smoke, and exposure history to BTEX. The studied WWTP is located in Iran. The mean discharge of the treatment plant is 930 L/s, and the peak discharge of transmission lines to the treatment plant is 2600 L/s. The length of transmission lines to the treatment plant is 5.5 km.
Collection of biological samples
To carry out the study and biological monitoring of urinary metabolites of BTEX compounds, after obtaining consent and conducting interviews with the participants, 30 samples of operational employees (all the employees in the operational unit) in the WWTP, as the exposed group, and 26 random samples of the administrative employees were chosen, as a control group. In either group, one sample was taken before the start of the work shift (before the exposure), and the second sample was taken after the end of the shift (after the exposure); 112 urine samples were collected using special containers for urine sampling and were immediately wrapped in aluminum foil (to prevent the penetration of sunlight). All samples were transferred to the laboratory using a cool box and kept at − 20 °C until the analysis.
Approval statement
The informed consent form was received from each subject. Besides, Shiraz University of Medical Sciences Ethics Committee approved the study protocol by the code “IR.SUMS.REC.1398.1267”. We confirm that all methods were performed following the relevant guidelines and regulations.
Sample preparation and analysis
To measure the urinary metabolite trans, trans-muconic acid (ttMA), solid-phase extraction (SPE) was first performed using a potent anion exchange cartridge (SAX—60 mg/3 mL) with 3 mL of methanol and 3 mL. 1 mL of urine sample was used, and then the cartridge was washed with 3 mL of 1% aqueous acetic acid. The ttMA elution was performed using 3 mL of 10% aqueous acetic acid. For the chromatographic analysis, 20 μL of the sample was injected into HPLC (KNAUER, Azura,) with PDA UV–Vis Detector, equipped with a reverse-phase C18 column (Eurospher 100-5, 250 × 4 mm—KNAUER). The flow rate was set to 1 mL per minute and detected at 265 nm.
Extraction and analysis of urinary Hippuric acid (HA) and Methyl hippuric acid (MHA) metabolites were performed according to NIOSH 8301 standard method. Briefly, 80 μL of 6 N HCl was mixed with 1 mL of a urine sample, and then 0.3 g of NaCl was added. 200 μL of the organic layer of the resulting mixture was transferred to a microtube, evaporated, and dried under a stream of nitrogen gas. Then it was redissolved in 200 μl of the utilized mobile phase of HPLC. HPLC analysis was implemented using a C18 column with water/acetonitrile/glacial acetic acid (84:16:0.025% (v/v)) as the mobile phase with a 1 ml per minute flow rate. The column temperature was adjusted to 37 °C, and the detection wavelength was set at 254 nm.
Determination of Mandelic acid (MA) and Phenylglyoxylic acid (PGA) was also accomplished by liquid–liquid extraction based on the method previously reported in ethyl acetate34. Then HPLC analysis was performed using a reverse-phase C18 column (Eurospher 100-5, 250 × 4 mm—KNAUER) with a constant temperature of 25 °C. Water: Methanol 90:10 solution (v/v) containing 0.5% acetic acid was used as the mobile phase in isocratic mode at a flow rate of 1 mL per minute. The injection volume was 20 μL and the UV detector was set at 254 nm.
Urine creatinine levels were measured to normalize the contaminant concentration to present the chemical compounds of samples in terms of urinary metabolites per creatinine (µg/g cr).
Quality control
The limit of detection (LOD) and limit of quantification (LOQ), determined as the concentrations equivalent to three and ten times the noise of the quantifier ion for a blank sample, were considered the quality control method. The LOD and LOQ for urinary metabolites of BTEX are shown in Table 1.
Statistical approach
Descriptive analysis was conducted using frequency, frequency percentage, mean and standard deviation. Before any statistical test, the data normality test was performed using the Kolmogorov–Smirnov test to determine the type of statistical test. Due to the Non-normal distribution of data, a one-sample sign test was used to compare the mean concentration of urinary metabolites with the threshold limit value (TLV). The concentration of urinary biomarkers in the control and case groups and the concentration comparison before and after the shift was performed using the Mann–Whitney U test. A significant level in these analyses was less than 0.05 (p < 0.05). Finally, the logistic regression method was used for regression modeling. After examining all demographic and other variables in the exposed and control group, including smoking status, proximity to possible sources emissions of BTEX, heating devices, etc., the variables were entered into the model with a significant level of 0.25. Data analysis was performed using SPSS software version 21 (SPSS Inc. Chicago, IL).
Ethics approval
Shiraz University of Medical Sciences Ethics Committee approved the study protocol by the code of “IR.SUMS.REC.1398.1267”.
Consent to participate
The informed consent form was received from each subject.
Results
Personal characteristics of the participants
The results related to demographic characteristics and other general parameters of the workers in the case and control groups are shown in Table 2. In the present study, 112 urine samples were collected from WWTP employees. Twenty-six workers were in the administrative, and 30 were in the operational parts of the WWTP. All participants except three of the administrative employees (control group) were male with a mean age of 41.15 ± 0.28 years and a body mass index (BMI) of 23.99 ± 9.57. Fifteen workers in the case group and 11 in the control group were smokers.
There was no significant difference between the exposed and control groups for age, weight, height, and BMI (p > 0.05). As indicated by the data given by the participants, three workers in the control group and five in the exposed group were exposed to cigarette and tobacco smoke in the last 48 h. Four workers in the control group kept solvents such as kerosene, gasoline, thinner, or other solvents at home during the previous 24 h. The control group spent 14.4 ± 16.16 h at home and 7.80 ± 2.81 h at work. The exposed group spent an average of 13.57 ± 3.98 h at home and 9.69 ± 3.48 h at work over the past 24 h.
Urinary concentrations of BTEX in exposed and control groups
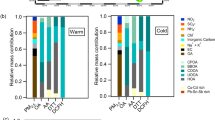
Statistical results related to BTEX metabolites in the urine concentrations of the exposed and control groups are presented in Table 3. The mean concentration of urinary BTEX metabolites ranged from 546.43 (μg/g cr) for tt-MA to 0.006 (μg/g cr) for MHA acid. The mean and SD of concentration of urinary BTEX metabolites in the exposed and control groups are shown in Fig. 1.
The results of the statistical differences test comparing the metabolites of BTEX compounds in the exposed and control groups before and after work shifts are shown in Table 3. These results indicate a significant difference in the concentrations of tt-MA, HA, MHA, and MA and PGA after the end of the shift in the exposed and control groups (p < 0.05). The difference between the concentration of HA and MHA before the start of the shift in the exposed and control groups was statistically significant (p < 0.05). For comparison reasons, there was no significant difference in creatinine concentration before and after the work shift in the control group (p > 0.05).
The difference between the concentrations of urinary BTEX metabolites in each study group is presented in Table 4. This study showed a significant difference between tt-MA acid, Hippuric, Mandelic, and PGA before and after the end of the shift in the exposed group (p < 0.05). In addition, tt-MA, and creatinine before and after work shifts in the case group showed a significant difference (p < 0.05).
We are assessing the concentration of urinary metabolites of BTEX in the exposed and control groups before and after the work shift with the TLV in urine, as shown in Table 5 and Fig. 1. Most of the studied compounds showed a higher statistically significant concentration than the standard values.
Results of the correlation between urinary BTEX metabolites in the exposed and control groups (before and after work shift) showed only a significant relationship between measured tt-MA (p = 0.04, r = 0.37) and MA and PGA (p = 0.01, r = 0.43) before the shift in the group exposed to HA measured before the shift in the same group.
Logistic regression analysis
For regression modeling, all variables were examined (including age, cigarette and tobacco smoke, proximity to emission sources, creatinine, the heating device used by participants, and traffic situation), considering the significance level of 0.25. Individual variables for each exposure group and control group constitute age, cigarette and tobacco smoke, a heating device (including flammable and without flame), and creatinine concentration entered into backward logistic regression models: conditional with p-values of 0.02, 0.08, 0.001, and 0.02, respectively.
After adjusting the age, the absolute values of the concentration of metabolites in the control group were 14% higher than in the exposed group. Adjusting for the difference in creatinine concentration before and after the work shift, metabolites’ concentration was 0.03% more in the exposed group than the control group. Finally, the heating device affected the concentration of pollutants in the exposed groups compared to the control 23.85 times. Univariate and multivariate logistic regression of exposed and control groups with all existing variables has been shown in Table 6 (by the detail of crude and adjusted OR).
Discussion
Volatile Organic Compounds (VOCs) are plentiful in industrial and municipal wastewater7. The main sources of VOCs in wastewater are water supply, industrial and commercial processes, household and consumer goods, surface runoff, and chemical and biological reactions during water and wastewater treatment35. In WWTPs, VOCs are mainly delivered to the air through dispersion and volatilization35. Most research on VOCs in WWTPs has been focused on processes that control or remove VOCs from wastewater36,37. Considering the presence of BTEX and its by-products in WWTPs in the literature38,39, and recognizing benzene and toluene that were released from different treatment units of this WWTP33, the present study aims at assessing the exposure of workers in the WWTP to BTEX compounds (benzene, toluene, ethylbenzene, xylene), as the first biomonitoring campaign using metabolites of BTEX in the urine ever accomplished in the WWTPs of Middle East.
The results of this study demonstrated a high concentration of urinary metabolites of BTEX compounds in both exposed and control groups. Among the studied variables in this research (smoking, using heating devices, age, and creatinine concentration), a significant relationship between age, use of the flame heating device, and creatinine concentration by changing the concentration of benzene, toluene Ethylbenzene, meta, para-xylene metabolites was presented in the urine of study participants.
Based on the results of this study, no significant difference was observed between the exposed and control groups in terms of age, weight, height, and BMI. The current results were consistent with those of the study of Rafiei et al. (2018) and Moridzadeh et al. (2020)40,41.
We report that the detection level of different BTEX metabolites was different due to variations in the absorption, metabolism, and distribution of these compounds42. Our study results showed that the control group was exposed to higher concentrations of BTEX, contrasted with workers in past investigations in various occupational and environmental exposures9,41,43,44.
The highest and lowest urinary metabolites of BTEX compounds were observed in the group exposed, resulting exposed to benzene and ethylbenzene, respectively. These results are in agreement with the study by Oh et al., in which the highest concentration of urinary metabolites among smokers and non-smokers housekeepers in North Korea with a geometric mean and standard error (SE) for all studied participants was 46.80 ± 2.48 (μg/g cr) belonging to tt-MA43. A range of benzene levels from the least of 0.006 ppm to the most elevated of 0.25 ppm was correlated with the average tt-MA metabolite as 360 ± 340 µg/g Cr, representing 83.6% of benzene exposure45. The fluctuation in urinary tt-MA levels between workers could be clarified by various competitive paths of benzene metabolism for muconic acid and other metabolites requiring GSTT (glutathione/sulfotransferase) activity. In addition, the major metabolic stage, CYP2E1 (cytochrome P4502E1), may be reduced to ttMA metabolites by alcohol intake46.
More than half of the measured metabolites were more than the TLV of these compounds in the urine. Even though the control group's exposure level in the workplace was not very high, in some cases close to zero, high levels of tt-MA were detected in all biological samples. It confirms that benzene can be considered one of the widespread environmental pollutants47 and that there are many sources for urinary metabolites of benzene47,48.
Intergroup study of urinary metabolite levels after shift work in control and exposed groups showed a significant difference for all metabolites (p < 0.05). In the present study, the average urinary level of tt-MA measured in the samples of workers after the work shift was statistically different from the samples before the shift. Still, the average level of these metabolites in the group of office workers (control) before and after work shift only showed a significant difference for urinary tt-MA level. In contrast, no significant difference was observed in the other three groups of urinary metabolites in the control group (p > 0.05). A significant amount of tt-MA in the morning sample of workers might be due to the internal distribution time of benzene with tissues (e.g., adipose tissue). It indicates that a large amount of benzene may be accumulated depending on the worker's living conditions. Then redistribution, metabolism, and excretion can occur in the form of tt-MA. Transdermal exposure to benzene can be another important source of delay in the presence of tt-MA in urine47.
Biomonitoring of occupational benzene exposure tends to be distorted by factors affecting tt-MA level, including consuming food of sorbic acid, exposure to the external environment, individual susceptibility, and work behavior49. However, something that should be taken into account is that nowadays, foods containing preservatives are an essential part of the nutrition and diet of most people in society and will have a notable effect on urine tt-MA. Therefore, tt-MA cannot be a specific and highly sensitive biomarker for illustrating benzene exposure. However, the levels of other urinary metabolites in the exposed group were increased in concentration/gr of creatinine after their work shift.
Contrary to the results of previous investigations, the urinary level of BTEX metabolites of WWTP workers in the analysis of logistic regression was not significantly associated with smoking. Benzene and other BTEX compounds are components of ambient tobacco smoke, and in the results of a study by Jalai et al. 2017 the urinary tt-MA level in the morning and evening for smokers and non-smokers of police officers was reported to be 571 ± 120, 820 ± 187, 321 ± 145, and 544 ± 160 (mg/g creatinine), respectively47. This concentration for urban residents was 425 ± 198, 501 ± 161, 224 ± 55, and 275 ± 57 (mg/g cr), respectively47, which is consistent with the results of our study. The tt-MA levels in the urine of chemical company workers were measured at 1824 ± 147 (mg/g cr) and 4616 ± 225 (mg/g cr) in the morning and evening of smokers and 987 ± 111 (mg/g cr) and 2271 ± 780 (mg/g cr) in the morning and evening of non-smokers47 which is more than twice the measured tt-MA concentration in the urine samples of WWTP employees in our study. However, the results of rural residents' tt-MA were relatively lower than the concentrations of tt-MA showed in our study47. Contrary to our study, the urinary tt-MA concentrations in all these population groups were almost twice higher in the smokers than the non-smokers.
The heating device use and urinary creatinine levels showed a positive correlation in the exposed group, whereas age caused a decrease in the concentration of these compounds in this group. The results of the study by Wheeler et al.50 showed that benzene concentration predictors in indoor places were considered frequent smoking at home, the property's garage, remodeling activities in the past 90 days, opening of windows in the previous week to let fresh air into the house, and utilization of candles during the last week. The predictors of ethylbenzene, m-, p-xylenes: the existence of a garage on the property, renovated last month, utilization of perfume on the previous day, and utilization of paint remover the week before. In addition, an additional predictor for m-, p-xylenes was the habitude of household smoking. The toluene predictive indicators were home garages, newer homes, painting or using paint remover in the last week, and frequent home smoking. The type of heating source in the house was not a predictor for BTEX concentration in the Wheeler et al. study50. Canada's long-term residential indoor air quality health guidelines for toluene51 (2300 μg/m3) are within the range of values established by other recognized international organizations (300 to 5000 μg/m3) 52,53,54,55.
The strength of our study is assessing the exposure to BTEX, using metabolized biomarkers in the WWTP workplace for the first time in the Middle Eastern. Some studies have evaluated the amount of BTEX emissions by directly measuring the concentration of internal BTEX in other occupations56,57,58. In all studies, despite the adjustment for many essential factors such as BMI, age, etc., there is a possibility of complications in unknown factors and possible sources. However, the biological monitoring method integrates all exposure pathways and can include random and unexpected exposures in the exposure measurement results59. Biological monitoring of BTEX urinary metabolites in populations exposed to these volatile pollutants in the air provides valuable information to health authorities about its environmental exposure limit reference and, like an easy-to-access biomatrix, provides an accurate assessment of exposure to these compounds through the air60,61.
However, the limitations of our investigation center around the relatively small sample size in some participant groups (smokers or exposed to environmental emissions). However, this was the most significant number of samples possibly accessible at the studied center (WWTP). In addition, the limitations of the method concern sampling; since spotted urine samples represent only recent exposures, the results of this study cannot be used to interpret long-term BTEX exposure in workers, and these results should be interpreted carefully to be confirmed by other studies.
Finally, the lack of specific questions about personal protective equipment, indoor ventilation, or seasonal and daily changes was a limitation of our investigation. Other epidemiological and experimental studies have shown that continuous ventilation reduces the degree of internal toxins62,63. Safety measures may be considered an essential determinant of BTEX occupational exposure40,64,65. Besides, several studies have suggested that tt-MA may not be a reliable biomarker for exposure to low levels of benzene. However, it is still considered a useful biomarker for assessing benzene metabolites in urine with cost-effective analytical screening purposes47,66. However, smoking and alcohol intake should be controlled as confounding factors in selecting specific metabolites as benzene exposure biomarkers. Eating of preserved food sources containing sorbic acid could be additionally limited for the use of specific tt-MA in biological monitoring of exposure assessment to benzene in workplaces, according to the previous suggestion. Last but not least, this study examined the estimation of the potential exposure through the skin, food, or inhalation contact only through a questionnaire. Therefore, future studies can investigate the emission sources of BTEX for WWTP workers.
Conclusions
This investigation attempted to evaluate the exposure of WWTP workers to BTEX compounds. Our findings showed that WWTP workers were exposed to BTEX compounds in some cases more than the permitted reference values, especially for benzene. Therefore, WWTPs should be considered as a source of BTEX, and employees working in such facilities may potentially be exposed to these volatile compounds.
The results of the present study indicated that age, use of flame heaters, and creatinine concentration are significant predictors of increasing concentrations of urinary BTEX metabolites. Therefore, it tends to be proposed that protective methodologies, like functional chemical masks, e.g., VOC absorbing masks, can be utilized to decrease worker exposures. It is also suggested that it is important to implement periodic health checks for WWTP workers.
To the best of the authors’ knowledge, the present study is the first study with biological monitoring approach to assess the WWTP workers' exposure to BTEX, aiming to create fitting programs to promote occupational health. As the monitoring of BTEX compounds in ambient air was beyond our investigation's scope, future research is suggested to assess the relative contribution of various BTEX exposure pathways in these environments.
Data availability
The data that support the findings of this study are available from the corresponding author, [M.A], upon reasonable request.
References
Sheikholeslami, Z., Kebria, D. Y. & Qaderi, F. Nanoparticle for degradation of BTEX in produced water; an experimental procedure. J. Mol. Liq. 264, 476–482 (2018).
Dehghani, M. et al. Characteristics and health effects of BTEX in a hot spot for urban pollution. Ecotoxicol. Environ. Saf. 155, 133–143 (2018).
Nazarparvar-Noshadi, M., Yadegari, M., Mohammadian, Y. & Fakhri, Y. The exposure to BTEX/Styrene and their health risk in the tire manufacturing. Toxin Rev. 41, 43–445 (2021).
Davil, M. F., Naddafi, K., Rostami, R., Zarei, A. & Feizizadeh, M. A mathematical model for predicting 24-h variations of BTEX concentrations in ambient air of Tehran. Int. J. Environ. Health Eng. 2, 4 (2013).
Zhang, Y., Wei, C. & Yan, B. Emission characteristics and associated health risk assessment of volatile organic compounds from a typical coking wastewater treatment plant. Sci. Total Environ. 693, 133417 (2019).
Sun, S., Hussain, T., Zhang, W. & Karton, A. Blue phosphorene monolayers as potential nano sensors for volatile organic compounds under point defects. Appl. Surf. Sci. 486, 52–57 (2019).
Cheng, W.-H., Hsu, S.-K. & Chou, M.-S. Volatile organic compound emissions from wastewater treatment plants in Taiwan: Legal regulations and costs of control. J. Environ. Manag. 88, 1485–1494 (2008).
Golkhorshidi, F. et al. On the nature and health impacts of BTEX in a populated middle eastern city: Tehran, Iran. Atmos. Pollut. Res. 10, 921–930 (2019).
Davidson, C. J., Hannigan, J. H. & Bowen, S. E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environ. Toxicol. Pharmacol. 81, 103518 (2020).
Chiu, C. H. et al. Use of urinary hippuric acid and o-/p-/m-methyl hippuric acid to evaluate surgical smoke exposure in operating room healthcare personnel. Ecotoxicol. Environ. Saf. 217, 112231 (2021).
Loh, M. M. et al. Intake fraction distributions for benzene from vehicles in the Helsinki metropolitan area. Atmos. Environ. 43, 301–310 (2009).
Ross, D. The role of metabolism and specific metabolites in benzene-induced toxicity: evidence and issues. J. Toxicol. Environ. Heal. Part A 61, 357–372 (2000).
Dehghani, F., Omidi, F., Heravizadeh, O. & Yousefinejad, S. Solidified floating organic droplet microextraction coupled with HPLC for rapid determination of trans, trans muconic acid in benzene biomonitoring. Sci. Rep. 11, 1–11 (2021).
Chen, W.-H., Chen, Z.-B., Yuan, C.-S., Hung, C.-H. & Ning, S.-K. Investigating the differences between receptor and dispersion modeling for concentration prediction and health risk assessment of volatile organic compounds from petrochemical industrial complexes. J. Environ. Manag. 166, 440–449 (2016).
Thetkathuek, A., Jaidee, W., Saowakhontha, S. & Ekburanawat, W. Neuropsychological symptoms among workers exposed to toluene and xylene in two paint manufacturing factories in eastern Thailand. Adv. Prev. Med. 2015, 1–10 (2015).
Dehghani, S. et al. Prenatal exposure to polycyclic aromatic hydrocarbons and effects on neonatal anthropometric indices and thyroid-stimulating hormone in a Middle Eastern population. Chemosphere 286, 131605 (2022).
Zhang, L., Ye, F., Chen, T., Mei, Y. & Song, S. Trans, trans-muconic acid as a biomarker of occupational exposure to high-level benzene in China. J. Occup. Environ. Med. 53, 1194–1198 (2011).
Ataboho, E. E., Nsemi, J. R. N. & Moukassa, D. Atmospheric benzene and determination of trans, trans-muconic acid of workers at oil sites in pointe-noire. Curr. J. Appl. Sci. Technol. https://doi.org/10.9734/cjast/2021/v40i1631427 (2021).
Oginawati, K. et al. Urinary hippuric acid level as a biological indicator of toluene exposure on batik workers. Heliyon 7, e07775 (2021).
Gomes, R. D. P., Sanson, A. L., Lobo, F. A., Afonso, R. & Coutrim, M. X. Method for the determination of benzene metabolite t,t-muconic acid in urine by HPLC-UV with an Ion exclusion column. Separations 3, 1–14 (2016).
Wang, X. et al. A novel switchable solvent liquid-phase microextraction technique based on the solidification of floating organic droplets: HPLC-FLD analysis of polycyclic aromatic hydrocarbon monohydroxy metabolites in urine samples. New J. Chem. 44, 3038–3044 (2020).
Scherer, G., Renner, T. & Meger, M. Analysis and evaluation of trans, trans-muconic acid as a biomarker for benzene exposure. J. Chromatogr. B Biomed. Sci. Appl. 717, 179–199 (1998).
Ong, C. N. et al. Biomarkers of exposure to low concentrations of benzene: a field assessment. Occup. Environ. Med. 53, 328–333 (1996).
Ong, C. N. et al. Evaluation of biomarkers for occupational exposure to benzene. Occup. Environ. Med. 52, 528–533 (1995).
McNally, K., Sams, C., Loizou, G. D. & Jones, K. Evidence for non-linear metabolism at low benzene exposures? A reanalysis of data. Chem. Biol. Interact. 278, 256–268 (2017).
Tunsaringkarn, T., Siriwong, W., Rungsiyothin, A. & Nopparatbundit, S. Occupational exposure of gasoline station workers to BTEX compounds in Bangkok, Thailand. Int J Occup Env. Med (The IJOEM) 3, 117–125 (2012).
Basilicata, P. et al. Application of the standard addition approach for the quantification of urinary benzene. J. Chromatogr. B 818, 293–299 (2005).
Geraldino, B. R. et al. Evaluation of exposure to toluene and xylene in gasoline station workers. Adv. Prev. Med. 2021, 1–10 (2021).
Mihajlović, V. et al. Assessment of occupational exposure to BTEX in a petrochemical plant via urinary biomarkers. Sustainability 13, 7178 (2021).
Heibati, B. et al. Biomonitoring-based exposure assessment of benzene, toluene, ethylbenzene and xylene among workers at petroleum distribution facilities. Ecotoxicol. Environ. Saf. 149, 19–25 (2018).
Rafiee, A., Delgado-Saborit, J. M., Sly, P. D., Amiri, H. & Hoseini, M. Lifestyle and occupational factors affecting exposure to BTEX in municipal solid waste composting facility workers. Sci. Total Environ. 656, 540–546 (2019).
Mukherjee, A. K. et al. Work-exposure to PM10 and aromatic volatile organic compounds, excretion of urinary biomarkers and effect on the pulmonary function and heme-metabolism: A study of petrol pump workers and traffic police personnel in Kolkata City, India. J. Environ. Sci. Heal. Part A 51, 135–149 (2016).
Dehghani, M. et al. Health risks of inhalation exposure to BTEX in a municipal wastewater treatment plant in Middle East city: Shiraz, Iran. Environ. Res. 204, 112155 (2021).
Chua, S. C., Lee, B. L., Liau, L. S. & Ong, C. N. Determination of mandelic acid and phenylglyoxylic acid in the urine and its use in monitoring of styrene exposure. J. Anal. Toxicol. 17, 129–132 (1993).
Chen, W.-H. et al. Comparing volatile organic compound emissions during equalization in wastewater treatment between the flux-chamber and mass-transfer methods. Process Saf. Environ. Prot. 109, 410–419 (2017).
Anjum, H. et al. A review on adsorptive removal of oil pollutants (BTEX) from wastewater using carbon nanotubes. J. Mol. Liq. 277, 1005–1025 (2019).
Asadi, M. & McPhedran, K. Estimation of greenhouse gas and odour emissions from a cold region municipal biological nutrient removal wastewater treatment plant. J. Environ. Manag. 281, 111864 (2021).
Bina, B., Amin, M. M., Rashidi, A. & Pourzamani, H. Water and wastewater treatment from BTEX by carbon nanotubes and Nano-Fe. Water Resour. 41, 719–727 (2014).
Fayemiwo, O. M., Daramola, M. O. & Moothi, K. BTEX compounds in water–future trends and directions for water treatment. Water Sa 43, 602–613 (2017).
Moridzadeh, M. et al. Assessing BTEX exposure among workers of the second largest natural gas reserve in the world: a biomonitoring approach. Environ. Sci. Pollut. Res. 27, 44519–44527 (2020).
Rafiee, A. et al. Use of urinary biomarkers to characterize occupational exposure to BTEX in healthcare waste autoclave operators. Sci. Total Environ. 631, 857–865 (2018).
Miri, M. et al. Investigation of outdoor BTEX: Concentration, variations, sources, spatial distribution, and risk assessment. Chemosphere 163, 601–609 (2016).
Oh, H. H. et al. The relationship between urinary BTEX metabolites and residence setting among Korean homemakers: The first Korea National Environmental Health Survey (2009–2011). Ann. Occup. Environ. Med. 29, 38 (2017).
Rafiee, A., Delgado-Saborit, J. M., Sly, P. D., Amiri, H. & Hoseini, M. Exploring urinary biomarkers to assess oxidative DNA damage resulting from BTEX exposure in street children. Environ. Res. 203, 111725 (2022).
Melikian, A. A. et al. Personal exposure to different levels of benzene and its relationships to the urinary metabolites S-phenylmercapturic acid and trans, trans-muconic acid. J. Chromatogr. B 778, 211–221 (2002).
Kim, S. et al. Genetic polymorphisms and benzene metabolism in humans exposed to a wide range of air concentrations. Pharmacogenet. Genomics 17, 789–801 (2007).
Jalai, A., Ramezani, Z. & Ebrahim, K. Urinary trans, trans-muconic acid is not a reliable biomarker for low-level environmental and occupational benzene exposures. Saf. Health Work 8, 220–225 (2017).
Lucialli, P. et al. Indoor and outdoor concentrations of benzene, toluene, ethylbenzene and xylene in some Italian schools evaluation of areas with different air pollution. Atmos. Pollut. Res. 11, 1998–2010 (2020).
Dougherty, D., Garte, S., Barchowsky, A., Zmuda, J. & Taioli, E. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 polymorphisms and biological effects of benzene exposure—A literature review. Toxicol. Lett. 182, 7–17 (2008).
Wheeler, A. J., Wong, S. L., Khoury, C. & Zhu, J. Predictors of indoor BTEX concentrations in Canadian residences. Heal. reports 24, 11–17 (2013).
Canada, H. Residential Indoor Air Quality Guideline Science Assessment Document: Toluene. (2011).
Kotzias, D. et al. The INDEX project: Critical appraisal of the setting and implementation of indoor exposure limits in the EU. Final Report, EUR 21590 (2005).
ATSDR, A. Toxicological profile for toluene. USDoHaH Serv. Ed. Atlanta US Public Heal. Serv. 1–357 (2000).
Flowers, L. et al. Toxicological review of toluene (CAS No. 108-88-3). US Environ. Prot. Agency, Washington, DC (2005).
Alexeeff, G. et al. Determination of noncancer chronic reference exposure levels. Off. Environ. Heal. Hazard Assessment, Calif. Environ. Prot. Agency, Sacramento, CA, USA (2000).
Kang, J., Yun, S., Cho, Y. S. & Jeong, Y. J. Post-intensive care unit depression among critical care survivors: A nationwide population-based study. Japan J. Nurs. Sci. https://doi.org/10.1111/jjns.12299 (2019).
Park, S.-E., Kim, H.-W., Sim, S.-H., Lee, S.-H. & Koo, J.-W. Concentrations of VOCs and formaldehyde in newly constructed apartment buildings before and after residence. J. Environ. Heal. Sci. 33, 98–103 (2007).
Shim, H. S. & Choi, Y. J. Evaluation of the indoor air quality in occupied apartment units after remodeling. J. Arch. Inst. Korea 24, 303–312 (2008).
Crinnion, W. J. The CDC fourth national report on human exposure to environmental chemicals: What it tells us about our toxic burden and how it assists environmental medicine physicians. Altern. Med. Rev. 15, 101–108 (2010).
Langrish, J. P. et al. Reducing personal exposure to particulate air pollution improves cardiovascular health in patients with coronary heart disease. Environ. Health Perspect. 120, 367–372 (2012).
Esteban, M. & Castaño, A. Non-invasive matrices in human biomonitoring: A review. Environ. Int. 35, 438–449 (2009).
Choi, H. et al. International studies of prenatal exposure to polycyclic aromatic hydrocarbons and fetal growth. Environ. Health Perspect. 114, 1744–1750 (2006).
Deng, Q., Yang, X. & Zhang, J. S. Key factor analysis of VOC sorption and its impact on indoor concentrations: the role of ventilation. Build. Environ. 47, 182–187 (2012).
Hoque, R. R., Khillare, P. S., Agarwal, T., Shridhar, V. & Balachandran, S. Spatial and temporal variation of BTEX in the urban atmosphere of Delhi, Inida. Sci. Total Environ. 392, 30–40 (2008).
Ho, K. F., Lee, S. C., Guo, H. & Tsai, W. Y. Seasonal and diurnal variations of volatile organic compounds (VOCs) in the atmosphere of Hong Kong. Sci. Total Environ. 322, 155–166 (2004).
Jäger, T., Bäcker, S., Oberlinner, C. & Bader, M. 339 Human biomonitoring for exposure assessment of benzene during short-term maintenance work (2018).
Acknowledgements
The authors would like to appreciate all the subjects who participated in the study.
Funding
This work was supported by Shiraz University of Medical Sciences [Grant No. 98.01.04.20181].
Author information
Authors and Affiliations
Contributions
M.D.: Conceptualization, Supervision, Validation, Methodology. A.A.: Methodology, Investigation. Z.T.: Writing—Reviewing and Editing. S.D.: Software, Writing and Editing, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dehghani, M., Abbasi, A., Taherzadeh, Z. et al. Exposure assessment of wastewater treatment plant employees to BTEX: a biological monitoring approach. Sci Rep 12, 21433 (2022). https://doi.org/10.1038/s41598-022-25876-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25876-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.