Abstract
Bipolar disorder (BD) is a mental disorder that leads to abnormal swings in mood, energy, activity level, attention, and the capability to accomplish daily tasks. Several long non-coding RNAs (lncRNAs) are dysregulated in BD patients. We have compared expression levels of five NF-κB-associated lncRNAs, namely ANRIL, CEBPA-DT, H19, NKILA and HNF1A-AS1 in blood samples of BD patients compared with controls. While ANRIL, CEBPA-DT and HNF1-AS1 were significantly under-expressed in BD patients compared with controls, NKILA levels were higher in patients versus controls. Among differentially expressed genes, HFN1A-AS1 exhibited the best diagnostic parameters in the separation of patients from controls (AUC ± SD = 0.86 ± 0.03, sensitivity = 0.82, specificity = 0.82, P value < 0.0001). AUC values for NKILA, ANRIL and CEBPA-DT were 0.71, 0.68 and 0.65, respectively. In accordance with the previously reported participation of NF-ƙB in the pathophysiology of BD, the current study provides evidence for dysregulation of NF-κB-associated lncRNAs in BD.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) is a mental disorder that leads to abnormal swings in mood, energy, activity level, attention, and the capability to accomplish daily tasks1. BD can be classified into two main categories, namely BD type I and type II with severe and persistent mood elevation in the former type but less severe mood elevation in the latter type1. BD, particular type I has been shown to have a strong genetic background2. Based on the results of family studies, it seems that a small number of genes with modest effects establish genetic background of BD2. In addition, a number of linked chromosomal regions as well as candidate genes have been identified2. Moreover, expression assays have shown dysregulation of several genes in the circulation of BD patients or in the postmortem brain samples3,4,5. Ubiquitin cycle, synaptic function3, apoptosis regulators5 and vitamin D related genes6 are among dysregulated genes in BD.
Nuclear factor kappa B (NF-κB) is an important transcription factor which regulates inflammatory signals. Expression of this transcription factor has been dysregulated in BD. Elevation in the spontaneous levels of NF-κB have been reported in several types of immune cells in adolescents with BD. Moreover, BD patients have exhibited greater upsurges in the NF-κB levels in monocytes after induction with TNF-α. Most notably, the latter observation has been associated with the contemporary severity of depressive symptoms7.
Several non-coding RNAs have exhibited functional association with NF-κB8. Association of number of NF-κB-interacting long non-coding RNAs (lncRNAs) with human disorders has been more investigated. LncRNAs represent a group of transcripts with sizes more than 200 nucleotides that contribute in the regulation of gene regulation. These transcripts participate in the pathogenesis of several human disorders through changing expression of genes and influencing activity of signaling pathways9,10. Among these lncRNAs are ANRIL, CEBPA-DT, H19, NKILA and HNF1A-AS1 whose participation in the pathogenesis of Parkinson's disease11 and autism spectrum disorder12 has been assessed. In the current study, we measured expression levels of these lncRNAs in the blood samples of BD patients compared with controls to appraise their possible contribution in this disorder. We hypothesized that expression of these lncRNAs has been changed in the peripheral blood of BD patients due to the abnormalities in gene regulation pathways. Since they are associated with NF-κB signaling, it is possible that they contribute to the pathoetiology of BD. For instance, ANRIL as a constituent of NF-κB pathway can regulate inflammatory response13. H19 has been shown to promote atherosclerosis through regulation of MAPK and NF-κB pathways14. NKILA has also been identified as an inhibitor of NF-κB, since it inhibits phosphorylation of IκBα and suppresses nuclear translocation of p6515. CEBPA-DT is another lncRNA that is transcribed from up-stream of the CEBPA gene regulating its expression in cis16. Notably, expression of CEBPA has also been shown to be regulated by NF-κB p50 17. Besides, CEBPA contributes to the relocation of histone deacetylases from NF-κB p50 homodimers and induction of expression of NF-κB target genes18. Finally, HNF1A-AS1 is an NF-κB-regulated lncRNA that enhances the phosphatase activity of SHP-119. Notably, this phosphatase has a role in the pathogenesis of immune-related disorders20.
Material and methods
Subjects
The Ethical Committee of Shahid Beheshti University of Medical Sciences has confirmed the study protocol (IR.SBMU.MSP.REC.1400.620). All enrolled cases and controls signed the written informed consent. Blood samples of 50 type I BD patients and 50 normal subjects were collected from Imam Hussein hospital during 2016–2019. Cases were recruited consecutively from Imam Hossein hospital during the study period and were examined in this hospital. BD cases were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders-521. Moreover, depressive and mania symptoms and the presence of euthymia were assessed using the Hamilton Depression Rating Scale (HAM-D)22 and Young Mania Rating Scale (YMRS)23. Patients were also assessed using a semi-structured interview which was based on asking questions within a prearranged thematic structure by skilled specialists who were trained through passing relevant courses. Clinical data including disease duration/ onset and drug history were collected. All recruited patients were not responsive to first-line mood stabilizers and took standard dose of Carbamazepine (200 mg, 2 times a day). In fact, most of patients who were not responsive to the first-line mood stabilizers in the mentioned clinic were under treatment with carbamazepine. So, in order to have a homogenized cohort of patients, we just included these patients. With the purpose of decreasing heterogeneity of the patients’ cohort, cases who took other drugs were excluded. History of head trauma, encephalitis or other mental illnesses and systemic disorders were regarded as exclusion criteria. Control subjects were assessed by a specialist to rule out the presence of signs or symptoms related with psychiatric disorders.
Sample collection and RNA extraction
Five ml of peripheral blood was collected from all cases and controls. Total RNA was extracted from blood samples using RNX kit (EX6101, Cinnagen, Tehran, Iran). Extracted RNA was assessed using gel electrophoresis and spectrophotometer.
cDNA production and real-time PCR assay
cDNA was made using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems), based on the Company's comments. Expression levels of ANRIL, CEBPA-DT, H19, NKILA and HNF1A-AS1 were enumerated in comparison with beta 2 microglubolin (B2M) as an internal control using self-designed primers which were similar to our previous study24. qRT-PCR was implemented in the ABI 7500 sequence detection system (Applied Biosystem, Foster City, CA, USA) using BIOFACT™ 2X Real-Time PCR Master Mix. All experiments were conducted in duplicate. Table 1 shows the primer sequences. Primers were synthetized by Macrogen Company (Seoul, Korea). Transcripts were enumerated using the comparative –delta Ct method. The primers were designed to amplify 14 isoforms of ANRIL and 3 isoforms of H19. For other lncRNAs, only one isoform was amplified by designed primers.
Statistical methods
GraphPad Prism version 9.0 (La Jolla, CA, USA) was used for statistical analysis. We compared expression levels of five lncRNA genes, namely ANRIL, CEBPA-DT, H19, NKILA and HNF1A-AS1 in blood samples obtained from BD patients and controls. Normal/Gaussian distribution of the values was evaluated using the Shapiro–wilk test. As data was not normally distributed, we used the non-parametric Mann–Whitney U test to detect differentially expressed transcripts between cases and controls. Two-way ANOVA test and Tukey post hoc test (as a complementary test for ANOVA test) were used for assessment of the effect of disease and gender on expression of genes and their interactions.
Correlations between gene expression levels of lncRNAs were measured with Spearman’s rank correlation coefficient as data was not normally distributed. In addition, correlations between gene expression levels and age, disease duration, sex and age at onset were measured using the Spearman’s rank correlation coefficient.
Receiver operating characteristic (ROC) curves were illustrated to judge the diagnostic power of expression levels of differentially expressed lncRNAs. P value < 0.05 was considered as significant.
Ethical approval and consent to participant
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences. All methods were performed in accordance with the relevant guidelines and regulations.
Results
General information about cases and controls
Table 2 shows age, sex ratio and other available information about enrolled persons. There was no significant difference in age between cases and controls (P value = 0.11).
Expression assays
Since we hypothesized that expression of NF-ƙB-related lncRNAs would be different among BD patients and controls, we assessed their expression in the peripheral blood of both study groups using real time PCR method. Figure 1 shows the corresponding band to the assessed lncRNAs as well as the housekeeping gene after RT-PCR.
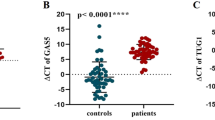
We detected significant difference in expression of ANRIL, CEBPA-DT (ADINR), NKILA and HNF1A-AS1 between BD patients and controls (Fig. 2).
Relative expression levels of five lncRNAs in bipolar disorder (BD) patients (total) and healthy controls (total) as described by –delta Ct values (A–E).—delta Ct values were plotted as box and whisker plots showing median, mean, interquartile range, and minimum and maximum values. Mann–Whitney U test was used to detect differentially expressed genes between cases and controls (**P value < 0.01 and ****P value < 0.0001).
Group (disease) factor had significant effect on expression levels of ANRIL, CEBPA-DT, HNF1-AS1 and NKILA. However, neither gender factor nor interaction of gender and group has significant effects on expression levels of studied gene; therefore, we did not perform post hoc tests for multiple comparisons (Table 3).
While ANRIL, CEBPA-DT and HNF1A-AS1 were significantly under-expressed in BD patients compared with controls, NKILA levels were higher in patients versus controls.
In order to assess possible relation between expressions of mentioned lncRNAs, we performed correlation analysis in each study subgroup. We detected significant pairwise correlation between all lncRNA pairs among BD patients and healthy controls (Table 4).
In order to assess the possibility of using expression levels of mentioned lncRNAs as diagnostic markers in BD, we assessed their diagnostic power using ROC curve analysis. Among differentially expressed genes, HFN1A-AS1 exhibited the best diagnostic parameters in separation of patients from controls (AUC ± SD = 0.86 ± 0.03, sensitivity = 0.82, specificity = 0.82, P value < 0.0001). AUC values for NKILA, ANRIL and CEBPA-DT were 0.71, 0.68 and 0.65, respectively (Fig. 3 and Table 5).
Finally, we checked whether expression of mentioned lncRNAs is different between male and female subgroups or whether their expression is correlated with demographic/clinical parameter. In spite of significant difference in expression of four lncRNAs between cases and controls, expression levels of none of lncRNAs were correlated with clinical/demographic parameters (Table 6).
Discussion
NF-κB pathway has an indispensable part in the development of innate and adaptive immunity25, which are dysregulated in BD26. The impact of NF-κB signals in inflammation, neuroprotection, and apoptosis are particularly obvious in the nervous system27. NF-κB can also modulate neuronal excitability and susceptibility to excitotoxicity28. In addition, pro-inflammatory cytokines which are associated with NF-κB can influence the process of neuroplasticity28,29. An early study in the postmortem tissue samples of BD patients has demonstrated elevation of NF-κB2 in BD samples. Based on the reported impact of viruses and cytokines on expression of NF-κB2, authors have suggested that up-regulation of NF-κB2 is in line with the contribution of possible environmental factors in the pathogenesis of BD30. Subsequently, Rao et al. have demonstrated up-regulation of NF-κB in the BD brain samples consistent with the elevation of various inflammatory cytokines, induction of apoptosis, brain atrophy and cognitive deficits in these patients31.
We have assessed expression levels of NF-κB-related lncRNAs in BD patients and healthy subjects. While ANRIL, CEBPA-DT and HNF1-AS1 were significantly under-expressed in BD patients compared with controls, NKILA levels were higher in patients versus controls. ANRIL has been revealed to regulate inflammatory response as a constituent of NF-κB pathway13. We have recently reported association between rs1333048 variants of ANRIL and risk of BD I in an Iranian cohort of patients. Moreover, rs1333045 and rs1333048 variants of ANRIL have been associated with risk of BD II. Moreover, T A haplotype block (rs1333045 and rs1333048, respectively) has been found to decrease risk of BD I and II, while C C haplotype decreases risk of BD II32. Thus, the present study shows further evidence for participation of ANRIL in the pathoetiology of BD.
We have also previously reported up-regulation of HNF1A-AS1 in patients with schizophrenia compared with controls24. Thus, this lncRNA might influence pathogenesis BD and schizophrenia in different directions.
Among differentially expressed genes, HFN1A-AS1 exhibited the best diagnostic parameters in separation of patients from controls, potentiating this lncRNA as a possible biomarker for BD.
We also reported correlations between expression levels of these lncRNAs in both patient and control groups, providing evidence for our hypothesis regarding association between these lncRNAs and NF-κB. However, expression of none of lncRNAs was associated with clinical and demographic data of BD patients. Our study has limitations regarding sample size, lack of drug-naïve patients, lack of access to HAM-D and YMRS scores, unavailability of history of drugs other than antipsychotic drugs, lack of functional studies and verification of the obtained results with other techniques. Expression of genes might be affected by administration of carbamazepine. The small sample size might affect the significance of obtained data. Finally, functional studies are needed to find the mechanistical points about contribution of mentioned genes to the pathogenesis of BD.
Taken together, this study provides evidence for participation of NF-κB-associated lncRNAs in BD and warrants additional functional studies. Future studies involving patients in different phases (i.e., mania, depression) should be performed in order to evaluate whether the lncRNA changes are independent of the disease phase. Moreover, based on the literature-based approach that was used for selection of mentioned lncRNAs, we just can conclude functional interactions between these lncRNAs and NF-κB signaling, possibly downstream or upstream of this pathway. Further assays should find their exact role on the activity of NF-κB signaling.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Anderson, I. M., Haddad, P. M. & Scott, J. Bipolar disorder. Bmj 345, e8508 (2012).
Escamilla, M. A. & Zavala, J. M. Genetics of bipolar disorder. Dialogues Clin. Neurosci. 10, 141–152. https://doi.org/10.31887/DCNS.2008.10.2/maescamilla (2008).
Ryan, M. et al. Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol. Psychiatry 11, 965–978 (2006).
Chen, H. et al. Gene expression alterations in bipolar disorder postmortem brains. Bipolar Disord. 15, 177–187 (2013).
Sayad, A. et al. Peripheral expression of long non-coding RNAs in bipolar patients. J. Affect. Disord. 249, 169–174 (2019).
Eghtedarian, R. et al. Abnormal pattern of vitamin D receptor-associated genes and lncRNAs in patients with bipolar disorder. BMC Psychiatry 22, 1–10 (2022).
Miklowitz, D. J. et al. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 241, 315–322. https://doi.org/10.1016/j.psychres.2016.04.120 (2016).
Ghafouri-Fard, S. et al. The interaction between miRNAs/lncRNAs and nuclear factor-κB (NF-κB) in human disorders. Biomed. Pharmacother. 138, 111519 (2021).
Zhang, X. et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int. J. Mol. Sci https://doi.org/10.3390/ijms20225573 (2019).
Ghafouri-Fard, S. et al. A comprehensive review on the role of non-coding RNAs in the pathophysiology of bipolar disorder. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22105156 (2021).
Ghafouri-Fard, S. et al. Expression analysis of NF-κB-related lncRNAs in Parkinson’s disease. Front. Immunol. 4243, (2021).
Honarmand Tamizkar, K. et al. Dysregulation of NF-κB-associated LncRNAs in autism spectrum disorder. Front. Mol. Neurosci. 208, (2021).
Zhou, X. et al. Long non-coding RNA ANRIL regulates inflammatory responses as a novel component of NF-κB pathway. RNA Biol. 13, 98–108 (2016).
Pan, J. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 21, 322–328 (2017).
Ke, S., Li, R. C., Meng, F. K. & Fang, M. H. NKILA inhibits NF-κB signaling and suppresses tumor metastasis. Aging 10, 56–71. https://doi.org/10.18632/aging.101359 (2018).
Xiao, T. et al. Long noncoding RNA ADINR regulates adipogenesis by transcriptionally activating C/EBPα. Stem Cell Rep. 16, 1006–1008. https://doi.org/10.1016/j.stemcr.2021.03.024 (2021).
Wang, D., Paz-Priel, I. & Friedman, A. D. NF-kappa B p50 regulates C/EBP alpha expression and inflammatory cytokine-induced neutrophil production. J. Immunol. 182, 5757–5762. https://doi.org/10.4049/jimmunol.0803861 (2009).
Paz-Priel, I., Houng, S., Dooher, J. & Friedman, A. D. C/EBPα and C/EBPα oncoproteins regulate nfkb1 and displace histone deacetylases from NF-κB p50 homodimers to induce NF-κB target genes. Blood 117, 4085–4094. https://doi.org/10.1182/blood-2010-07-294470 (2011).
Ding, C. H. et al. The HNF1α-regulated lncRNA HNF1A-AS1 reverses the malignancy of hepatocellular carcinoma by enhancing the phosphatase activity of SHP-1. Mol. Cancer 17, 63. https://doi.org/10.1186/s12943-018-0813-1 (2018).
Markovics, A., Toth, D. M., Glant, T. T. & Mikecz, K. Regulation of autoimmune arthritis by the SHP-1 tyrosine phosphatase. Arthr. Res. Ther. 22, 160. https://doi.org/10.1186/s13075-020-02250-8 (2020).
in DSM 5 Diagnostic and statistical manual of mental disorders. 947 (2013).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56 (1960).
Young, R. C., Biggs, J. T., Ziegler, V. E. & Meyer, D. A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435 (1978).
Safa, A. et al. Expression of NF-κB associated lncRNAs in schizophrenia. Sci. Rep. 10, 1–9 (2020).
Elhaik, E. & Zandi, P. Dysregulation of the NF-κB pathway as a potential inducer of bipolar disorder. J. Psychiatr. Res. 70, 18–27 (2015).
Rege, S. & Hodgkinson, S. J. Immune dysregulation and autoimmunity in bipolar disorder: Synthesis of the evidence and its clinical application. Aust. N. Z. J. Psychiatry 47, 1136–1151 (2013).
Mattson, M. P. & Camandola, S. NF-κB in neuronal plasticity and neurodegenerative disorders. J. Clin. Investig. 107, 247–254 (2001).
Brietzke, E. & Kapczinski, F. TNF-α as a molecular target in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1355–1361 (2008).
Potvin, S. et al. Inflammatory cytokine alterations in schizophrenia: A systematic quantitative review. Biol. Psychiat. 63, 801–808 (2008).
Sun, Y., Johnston, N. L., Yolken, R. H., Zhang, L. & Fullertorrey, E. Serial analysis of gene expression in the frontal cortex of patients with bipolar disorder. Br. J. Psychiatry 178, s137–s141 (2001).
Rao, J. S., Harry, G. J., Rapoport, S. I. & Kim, H.-W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry 15, 384–392 (2010).
Namvar, A. et al. ANRIL variants are associated with risk of neuropsychiatric conditions. J. Mol. Neurosci. 70, 212–218 (2020).
Acknowledgements
This study was financially supported by Shahid Beheshti University of Medical Sciences (Grant Number 28465).
Author information
Authors and Affiliations
Contributions
S.G.F. wrote the manuscript and revised it. M.T. and A.S. designed and supervised the study. S.A.T., P.S., Z.S. and B.M.H. collected the data and performed the experiment. S.E. analyzed the data. All authors read and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teshnizi, S.A., Shahani, P., Taheri, M. et al. Expression analysis of NF-ƙB-related long non-coding RNAs in bipolar disorder. Sci Rep 12, 20941 (2022). https://doi.org/10.1038/s41598-022-25670-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25670-9
This article is cited by
-
The expression analysis of long noncoding RNAs PCAT-1, PCAT-29, and MER11C in bipolar disorder
BMC Psychiatry (2024)
-
Deregulation of NF-κB associated long non-coding RNAs in bipolar disorder
Metabolic Brain Disease (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.