Abstract
Currently, evidence for effective physiotherapy interventions in patients with cervicogenic headache (CeH) is inconsistent. Although inter-individual variability in pain response is predictive for successful physiotherapy interventions, it was never explored in patients with CeH. Therefore the objective of the current study was to explore inter-individual variability in mechanical pain sensation, and its association with biopsychosocial-lifestyle (BPSL) characteristics in patients with CeH. A cross-sectional explorative analysis of inter-individual variability in mechanical pain sensation in 18 participants with CeH (29–51 years) was conducted. Inter-individual variability in mechanical pain sensation (standard deviations (SDs), F-statistics, Measurement System Analysis) was deducted from bilateral pressure pain thresholds of the suboccipitals, erector spine, tibialis anterior. BPSL-characteristics depression, anxiety, stress (Depression Anxiety Stress Scale-21), quality of life (Headache Impact Test-6), sleep-quality (Pittsburgh Sleep Quality Index), and sedentary time (hours/week) were questioned. Inter-individual variability in mechanical pain sensation explained 69.2% (suboccipital left), 86.8% (suboccipital right), 94.6% (erector spine left), 93.2% (erector spine right), 91.7% (tibialis anterior left), and 82% (tibialis anterior right) of the total variability in patients with CeH. The significant p-values and large F-statistic values indicate inter-individual differences in SDs. Significant associations between (1) lower quality of life and lower SDs of the suboccipital left PPT (p .005), and (2) longer sedentary time and higher SDs of the suboccipital left PPT (p .001) were observed. Results from our explorative study could suggest inter-individual variability in mechanical pain sensation at the left suboccipitals which associates with quality of life and sedentary time. These novel findings should be considered when phenotyping patients and ‘individually’ match interventions.
Similar content being viewed by others
Introduction
There is consensus among clinicians and researchers on the cervical origin of cervicogenic headache (CeH)1,2. Interventions directed at the cervical spine are therefore recommended3,4,5,6. The cervical anatomical and neurophysiological basis of CeH is supported by the fundamental mechanism of convergence7. Potential pain generators related to CeH are innervated by C1–C3 afferent spinal nerves and include the suboccipital muscles, atlanto-occipital, atlanto-axial and C2-3 zygapophysial joints, C2-3 intervertebral disc, cervical dura mater, and vertebral arteries. Although there is no direct connection between the cervical spine and the trigeminal nerve, nociceptive information from the trigeminal and cervical regions activate neurons in the trigeminal nucleus caudalis, which extends to the C2 spinal segment and lateral cervical nucleus in the dorsolateral cervical area. This overlap between the trigeminal and cervical nerves is known as a convergence mechanism which could be responsible for pain in the face and head region1,8. Such mechanism also explains recurrent headaches, caused by repeated activation of cervico-occipital structures9.
Although the pathophysiological mechanism of CeH is well defined, non-responsiveness, low clinically relevant effect sizes5, and low patient-reported subjective benefit (28% reporting ≥ 50% decrease in headache frequency) of localized physiotherapy interventions targeting the cervical spine are reported10. It already was previously stated that not all patients with CeH were equally responsive to localized physiotherapy interventions11,12, and tentative attempts have been made to identify predictors of responsiveness13. The patient’s history and cervical physical impairments (e.g. range of motion, cranio-cervical flexion test, posture) for instance are not predicting non-responsiveness to localized physiotherapy interventions13. According to physiotherapists, predictors of such non-responsiveness include: previous severe trauma, neural sensitivity, immunological comorbidities, and latency of response to treatment14.
Pain is no longer considered to be a simple transmission of nociception, but rather an output subsequent to complex interactions between physiological, cognitive, emotional, lifestyle, and social individual inputs on neurophysiological mechanisms15,16. Both the subjective and personal character of experiencing pain remain major challenges for clinicians and researchers. Whereas the subjective character of pain prevents direct measurements, its personal character results from complex interactions between factors unique to that person17,18. Both characteristics contribute to inter- and intra-individual differences in pain reporting16. Inter-individual differences are unveiled by the large pain variability between individuals as a response to a standardized pain-provoking experimental stimulus19,20. Such differences are also observed using objective measures such as the analysis of brain morphology, cerebral activity, and neurophysiological processes during experimental pain provocation21,22,23. Thus, while the cervical origin of pain is a shared feature in patients with CeH, before defining such predictors one should realise that, variation in its experience is common19,24.
Individual differences in pain experience claim for an initial more patient-centred approach within study designs, rather than Randomized Controlled Trials (RCT) to evaluate the efficacy of an intervention. Although RCTs have been proven to be the gold standard to determine efficacy of interventions, they might at the beginning (i.e. understand interactions, fine-tune individual care) not be the optimal design within a heterogeneous human sample24. Inter-individual variability in the response to analgesic therapy has led to recommendations to design patient-centred interventions based on sound clinical reasoning2,17. Indeed, low success rates for the localized physiotherapeutic management of patients with CeH could relate to inter-individual variability in experiencing pain. Understanding such variability, its predictors, and unravelling its potential interactions with biopsychosocial-lifestyle (BPSL)-characteristics (e.g. gender, age, race, socio-economic status, perceived stress, trait negative affect, social support, lifestyle) seems thus crucial to explore16,25,26.
Therefore, as a first step we explored if in patients with CeH inter-individual differences (i.e. variability) in mechanical pain sensation can be identified, and could be linked to BPSL-characteristics. Identifying such differences and profiling the BPSL-interaction would stress the need for a more individualised patient-centred management approach. Such an intervention should include multiple modalities targeting modifiable factors that shape the individual pain experience. It is hypothesised that patients with CeH show variability in mechanical pain sensation, and that such variability is associated with BPSL-characteristics based on the BPS-model of pain16,25. We opted to explore variability in mechanical pain sensation since pain is often associated with mechanical hyperalgesia, i.e. decreased pressure pain threshold (PPT)27.
Methods
Design
Cross-sectional observational design to explorative variability in mechanical pain sensation within a contemporary BPSL-framework in patients with CeH.
Sample size
A priori sample size calculation based on the Coefficient of Variation (CV) of suboccipital PPTs in patients with CeH resulted in a required sample size of 18 participants (power 80%, α = 0.05)28. Sample size was estimated based on the work of Van Belle et al.29:
where CV is the coefficient of variation (CV = σ0/μ0 = σ1/μ1).
Participants and ethics
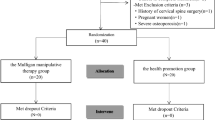
Participants were recruited between January 2018 to August 2019 (Appendix A, Fig. A.1). The neurological staff at the headache departments of the AZ Vesalius hospitals (Tongeren and Bilzen, Belgium) identified and referred participants meeting the study’s inclusion criteria for CeH based on the international Classification for Headache Disorders, 3rd edition (ICHD)30. Additionally, a general call was launched at the Hasselt University, Zuyd Hogeschool, and private practice of the main researcher. Each potential participant had to be declared eligible by a neurologist (external member of the research team). The neurologist was involved in determining the inclusion and exclusion criteria, confirmed the diagnosis of CeH based on the ICHD-3 criteria30, and referred eligible participants with CeH to the main researcher (manual therapist with a PhD in Rehabilitation Sciences and Physiotherapy, > 10 years of clinical experience).
Inclusion criteria were: Dutch-speaking participants between 18 and 55 years, body mass index (BMI) between 18.5 and 24.9 kg/m231, diagnosed with CeH according to the ICHD-330 by a neurologist, normal cognitive capacity (Mini Mental State Examination test score of 30). Exclusion criteria were: pregnancy, physiotherapy for head- or neck-related disorders in the past month before the start of the study, serious pathology (musculoskeletal, neurological, endocrine, cardiovascular, psychiatric), medication overuse (intake of NSAID’s, opioids, acetylsalicylic acid, triptans, simple analgesics for > 10 days/month > 3 months), smoking, history of neck/head trauma, orthodontics. Nineteen participants were originally recruited and selected to compose the CeH-group. These participants were given a four-week headache-diary.
The current study was part of phase 1 of a larger project which is registered as an observational study at ClinicalTrials.gov (NCT02887638). The Medisch Ethische ToetsingsCommissie of Zuyderland and Zuyd Hogeschool (NL. 55720.09615) and the Comité Medische Ethiek of the Ziekenhuis Oost-Limburg (B371201423025) granted approval to execute the experimental protocol. Eligible participants had to read and sign the informed consent before officially being enrolled. Protection of personal data is legally determined by the Belgian law of December 8th 1992. All test procedures involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki Declaration and later amendments. The manuscript is prepared in accordance to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist.
Outcomes, measurements, and instruments
Primary outcome: Variability in mechanical pain sensation (expressed via standard deviations (SDs), CV) was deducted from the PPTs (kPa/cm2/sec) of the bilateral suboccipitals (cephalic), erector spine at L1, and tibialis anterior (extra-cephalic). PPTs were measured with an electronic pressure algometer (Somedic AB, Stockholm, Sweden)32,33,34,35,36. PPT is defined as the minimal amount of pressure that elicits pain. Hypersensitivity over remote, extra-cephalic sites is considered a sign of central sensitization. Intrarater reliability of cervical PPT-measurements are good to excellent (ICC 0.82–0.99) in patients with headache33,37. Intrarater reliability of erector spine PPT-measurements are excellent (ICC 0.91 ± 0.07) in healthy participants38. Intrarater reliability of tibialis anterior PPT-measurements are excellent patients with neck pain (ICC 0.97)33. SDs were deducted from the PPT-measurements as an index for variability in mechanical pain sensation39.
Secondary outcome: Headache-intensity was measured using the 11-point Numeric Pain Rating Scale (NPRS) which ranges from 0 (no pain) to 10 (worst pain imaginable). Scores ≤ 3 correspond to mild, 4–6 to moderate, and ≥ 7 to severe pain. The meaningful clinically important change amounts to 2.5. Psychometric properties of the NPRS are solid in patients with CeH40.
Secondary outcomes: BPSL-characteristics depression, anxiety, and stress (Depression Anxiety Stress Scale 21), quality of life (Headache Impact Test 6), sleep quality (Pittsburgh Sleep Quality Index), and sedentary time (mean hours/week) were questioned through standardized Dutch questionnaires. Psychometric characteristics (e.g. validity, internal consistency, reliability) of the questionnaires used to estimate the BPSL-characteristics, and results on the completed questionnaires are described in our previous work and the Appendices (Appendix B and Appendix C, respectively)41.
Test procedure
Participants were asked not to take analgesics, muscle relaxants, or caffeine-containing beverages 24 h prior to the measurements. Prophylactic treatment(s) remained unchanged1. Measurements (M) were performed twice (at M1 and M2) in a set-up with constant room temperature of 25 °C at the motion laboratory of Zuyd Hogeschool (Heerlen, The Netherlands). A condition to be measured was a score of 0 on the 11-point NPRS for headache intensity on the test day42,43.
Questionnaires to estimate the BPSL-characteristics were completed first, followed by PPT- measurements of the bilateral suboccipitals, erector spine at L1 (neutral prone position), and tibialis anterior (seated with 80° knee-flexion)38,44. Pressure was perpendicular applied directly on the muscle belly (probe 1 cm2), starting at 0 to maximal 1000 kPa with a slope of 30 kPa/sec44,45. Participants were instructed to push the stop-button when the sensation of pressure first changed into pain. An exercise trial was performed once on the right thigh before actually measuring. PPT-measurements were executed twice (ICC 0.86–0.99) with a five-minute interval in a standardised sequential order: suboccipital left, erector spine at L1 left, tibialis anterior left, suboccipital right, erector spine at L1 right, tibialis anterior right35,45,46,47.
The entire test procedure (45 min) described above was executed and guided by the main researcher (manual therapist with a PhD in Rehabilitation Sciences and Physiotherapy, > 10 years of clinical experience).
Statistics
Analysis was completed via JMP Pro 14 and IBM SPSS Statistics 25. Two-tailed tests at 5% level of significance were reported.
Group characteristics. Continuous outcomes (mean, SD) and proportions (number, %) were used to described characteristics on age, BMI, marital and socioeconomic state, and CeH.
Mechanical pain sensation. 1. PPTs. Continuous outcomes (mean, SD) for each muscle at M1 and M2 were compared via paired t-tests. 2. Intraclass correlation coefficients (ICCs) were calculated to estimate intrarater reliability of the PPT-measurements. A two-way mixed-effects absolute agreement model was composed48. ICCs were interpreted as: values < 0.50 are indicative of poor, values between 0.50 < 0.75 of moderate, values between 0.75 < 0.90 of good, and values > 0.90 indicate excellent reliability49. Underlying criteria for ICC calculations (normality, homogeneity of variance) were met. 3. Variability in mechanical pain sensation. Measurements System Analysis (% variability) and F-statistics (σ2Between/σ2Within) were used to estimate inter-individual variability. 4. Coefficients of Variation (CV) for the PPT-measurements were calculated to express variation referred to its mean [(SD/Mean)*100].
Associations between independent variables age, BMI (continuous), gender, socioeconomic status, measurement (at M1 or M2) (categorical), and (1) PPTs and (2) SDs (dependent continuous) were evaluated via stepwise multiple linear regression to obtain the best model fit (i.e. smallest mean square of the error, variance inflation factor ≤ 4) (Appendix D).
Associations between independent BPSL-variables depression, anxiety, stress, (categorical) quality of life, sleep quality, and sedentary time (continuous), and SDs of the PPTs (dependent continuous) were evaluated via stepwise multiple linear regression to obtain the best model fit (i.e. smallest mean square of the error, variance inflation factor ≤ 4) (Appendix E). Conditions to apply linear models had to be met. SDs, to express variability of PPTs, were deducted for each participant (= i) from the PPTi,M1 and PPTi,M2, and used to build the model.
Results
Group characteristics
Eighteen participants with CeH met the inclusion criteria. One participant had to be excluded because of technical artefacts. Group characteristics are presented in Table 1. Appendix F provides more detailed information on the headache characteristics of the participants (Appendix F, Table F.1).
Mechanical pain sensation
Age, BMI, gender, level of education, employment, and the measurement (at M1 or M2) did not significantly associate with PPT-measurements, or to their SDs (Appendix D).
-
a.
Pressure Pain Thresholds (PPT) (Table 2)
Comparison of absolute PPTs between M1 and M2 revealed no significant differences for each PPT-measurement, i.e. at the bilateral suboccipitals, erector spine, and tibialis anterior muscles.
-
b.
Intraclass Correlation Coefficient (ICC) (Table 3)
ICCs ranged from moderate (suboccipital left ICC 0.69) and good (suboccipital right ICC 0.87; tibialis anterior right ICC 0.82) to excellent (erector spine left and right ICC 0.94, 0.93, respectively; tibialis anterior left ICC 0.92).
-
c.
Variability in mechanical pain sensation (Table 4, Fig. 1)
Inter-individual variability explained 69.2% (suboccipital left), 86.8% (suboccipital right), 94.6% (erector spine left), 93.2% (erector spine right), 91.7% (tibialis anterior left), and 82% (tibialis anterior right) of the total variability in patients with CeH. The significant p values and large F-statistic values indicate inter-individual differences in standard deviations.
Averaging PPT-measurements as such at M1 and M2 smoothed out this variability. CVs ranging between 34.9 and 55.2% additionally indicate that the data have a greater level of dispersion around the mean.
-
d.
Associations between BPSL-characteristics and variability in mechanical pain sensation (Table 5, Appendix E)
Significant associations between (1) lower quality of life and smaller SDs of the suboccipital left PPT, and (2) longer sedentary time and larger SDs of the suboccipital left PPT were observed.
No significant associations were seen between BPSL-characteristics and SDs of the remaining PPTs.
Averaging data might mask underlying associations between BPSL-characteristics and SDs of PPTs. Individual plots of associations between BSPL-characteristics and SDs reveal inter-individual differences (Fig. 2).
Discussion
Although inter-individual variability in pain response is predictive for successful physiotherapy interventions, it was never explored in patients with CeH. Therefore the aim of this explorative study was to examine inter-individual variability in mechanical pain sensation, and its potential association with BPSL-characteristics in patients with CeH. Inter-individual variability in mechanical pain sensation in patients with CeH could be hypothesized based on our novel findings.
Mechanical pain sensation varies between patients with CeH
This cross-sectional observational study is the first to explore variability in mechanical pain sensation within a contemporary BPSL-framework in patients with CeH.
Jones and O’Shaughnessy15 previously reported that the source of greatest variation in PPTs (ICC) in healthy participants was the inter-individual variance (70.1% of the total variance). Measurements System Analysis and F-statistics in our work demonstrate in a similar way that most variability of the PPT-measurements was due to inter-individual variability. Such variability is sometimes derogatorily referred to as error variance, i.e. a statistical term referring to sources of variance other than those of most interest to the investigator48,49. However, elucidating inter-individual pain variability (i.e. mechanisms and factors influencing pain experience) is of scientific and clinical significance since an enhanced understanding will provide important information to design a patient-centred model of care50,51. Identifying predictors influencing such variability might be of great interest since it reflects clinically realistic situations. Previous attempts to identify possible sources of variability in PPT-measurements in healthy adults revealed that male participants had 25% higher PPTs compared to female participants, anxiety levels were negatively associated with PPTs, and testing experience (i.e. higher level of self-efficacy) could contribute to greater pain tolerance53,54,55. It was further advised to reflect on the influence of psychosocial characteristics on PPT-measurements52.
Different ICCs were observed concerning the left (0.69) and right (0.89) suboccipitals. Regarding the left suboccipitals, eight participants endure higher pressure during M2, and regarding the right suboccipitals, five participants tolerated higher pressure during M2 (Fig. 1). This difference between left and right is also observed when comparing M1 and M2 averages (Table 2). It could be hypothesized that, at individual level, the non-provocative side for CeH (i.e. left suboccipital region) endures higher pressure due to a learning effect during M2. Maybe patients were anxious during the first measurement (PPTs at the left suboccipitals were measured before PPTs at the right suboccipitals) because the measurement took place at their ‘vulnerable’ upper-cervical spine. More pressure might have been tolerated at the left suboccipital region since this pressure could not provoke headache in 17 patients (Appendix F, Table F.1). At the provocative side for CeH (i.e. right suboccipital region), there is more consistency in the measurements, which might be explained by the peripheral provocative source being located there (17/18 patients, Appendix F, Table F.1).
Further, exploring variability in (extra)cephalic left- and right-sided PPT-measurements places previous findings, namely that patients with CeH may suffer from central sensitization, in perspective9,41. Although ICCs were moderate to excellent, we cannot assume that each patient with CeH encounters such central sensitization (Fig. 2). Though patients respond quite comparable to M1 and M2, PPTs fluctuate between patients. Averaging these data might give the false idea that each patient with CeH suffers from central sensitization. Consequently, therapy might be off-target.
The CVs, which ranged between 34.9 and 55.2%, indicate that our data have a greater level of dispersion around the mean. Such findings stress the need to be careful when interpreting averages39.
Biopsychosocial-lifestyle characteristics associate with inter-individual variability in mechanical pain sensation
In our study we noticed that BPSL-characteristics were associated with variability in mechanical pain sensation in patients with CeH. Significant associations between (1) lower quality of life and smaller SDs of the suboccipital left PPT, and (2) longer sedentary time and larger SDs of the suboccipital left PPT were observed in patients with CeH (Table 5).
Psychosocial and lifestyle characteristics have been identified as modifiable drivers of pain and disability in other musculoskeletal pain disorders (e.g. low back pain, knee osteoarthritis)56,57,58,59. Therefore, we hypothesise that some patients with CeH might share a common risk profile for disability and pain, and that a shift of focus to the individual is required as suggested by Caneiro et al.59 for musculoskeletal pain. The variable interaction making-up a pain experience should therefore not be considered as ‘noise’, but rather as an opportunity to develop patient-centred interventions15,38,51. For instance, individual differences in reporting pain at baseline were recognized as important predictors for clinical trial outcomes in patients with fibromyalgia60. Demographic characteristics in our study did not significantly associate with variability in mechanical pain sensation. However, it should be kept in mind that strict eligibility criteria (e.g. restricting age, BMI) were used.
Although it is generally accepted to adopt a BPSL-approach in the management of pain, a recent Delphi-study on managing patients with headache by physiotherapists proposed active mobilisation exercises, upper cervical spine mobilisations, work-related ergonomic training, and active and passive mobilisations with movement as useful in the treatment of CeH. Lifestyle advice, pain education, and cognitive therapy were not considered to be relevant61,62. Though, based on our new approach, and previous findings42,43,63, further research is needed to determine which patient is likely to benefit from which type of intervention(s) based on the patient’s BPSL-profile61.
Limitations and suggestions
In the current study several statistical analyses have been used for which reflection is required. Caution is needed to generalize and interpret the results concerning the analyses of associations between BPSL-characteristics and variability in mechanical pain sensation. Several variables were selected based on a priori hypotheses and entered in the regression model, leading to many hypotheses being tested. The backward stepwise approach already downsized the model, and Bonferroni corrections were applied whenever needed64. The variance inflation factor (< 4) was used in case two independent variables were associated with the dependent variable. The rather small sample size (n = 18) might overestimate an effect. Post hoc power calculations for the most relevant outcomes ranged between 96 and 100%. A statistical correct interpretation (i.e. erroneously rejecting the null hypothesis) is difficult since the current study was only performed once, and no probability can be assigned to a singular, observed result. Thus, we currently have no method for deciding whether this was a false-negative or a true-negative finding and advice further research65. The results of this study must be interpreted in this context.
Our study investigated variability in mechanical pain sensation in adults with CeH, which of course limits generalisability of the results. Comprehensive (predictive) phenotypic profiling, including quantitative sensory testing, conditioned pain modulation, psychosocial, and lifestyle variables, is needed to evolve towards an individualized pain management program17 since unidimensional scales to assess pain undervalue its complexity66. Seventeen out of 18 patients (94.4%) reported a dominant right-sided CeH. Further analyses are needed to explore if the dominant headache-side (i.e. nociceptive information from the dominant C1-C3 level) influences these measurements. We also advice to explore if the location of PPT-measurements (i.e. close or at remote distance from the provocative source and head) associates with the consistency of such measurements.
An interesting finding in the current study was the association between quality of life and sedentary-time, and the SDs of the suboccipital left PPTs. Such association might be influenced by the ICCs of the left suboccipitals scoring the lowest. ICCs can be influenced by a number of factors including the experience of raters, learning effects, and remembering measurements from one testing occasion to the next67. We tried to anticipate these factors by using one experienced researcher, and a standardised protocol to measure PPTs. It could however be hypothesised that some kind of learning effect was present in the participants given that the suboccipital left PPTs were the first being measured. It could be interesting to explore to which degree the level of ICCs (i.e. poor, moderate, good, excellent) might influence associations between variability in mechanical pain sensation and BPSL-characteristics.
A condition to be measured was a score of 0 on the 11-point NPRS for headache intensity on the test day. Suffering from pain at the test day would have complicated the interpretation of the PPT-measurements, given its variation within and between individuals51,68. Measuring PPTs bilaterally, and at remote sites of the painful source in a pain-free period, might uncover hidden potential features of pain modulation of the individual, which are not necessarily related to a current CeH episode. We propose a prospective study design to explore if less efficient pain inhibition is secondary to the presence of pain, or if less efficient pain inhibition is primary to the clinical pain. Such studies are primordial to develop a targeted intervention for CeH.
In the current study participants were asked not to take analgesics, muscle relaxants, or caffeine-containing beverages up until 24 h prior to the measurements. Future studies, involving muscle relaxants, should apply a larger time-frame depending on the relaxant (e.g. type, dose) given the potential longer half-life of such medication.
Further, intensive longitudinal interventional designs are needed to gain more insight in inter- and intra-individual pain variability39 and the effect of an intervention. In particular, there is a need to evaluate both efficacy and effectiveness69 of an approach that assesses modifiable BPSL-factors contributing to pain using appropriate designs (e.g. single case experimental design as precursors for an RCT approach).
Conclusion
Currently, evidence for success of physiotherapeutic managing patients with CeH is inconsistent. And, although inter-individual variability in pain response is predictive for the effect of an intervention, it was never explored in patients with CeH. Results from our explorative study could suggest inter-individual variability in mechanical pain sensation at the left suboccipitals which associates with quality of life and sedentary time.
Future studies should evaluate the efficacy of phenotyping patients in a more individualised multidimensional patient-centred intervention for CeH.
Data availability
Raw data generated during this study are included in the supplementary file (Appendix G).
Abbreviations
- BMI:
-
Body mass index
- BPS(L):
-
Biopsychosocial lifestyle
- CeH:
-
Cervicogenic headache
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
- DASS-21:
-
Depression anxiety stress scale
- ES:
-
Effect size
- HIT-6:
-
Headache impact test 6
- ICC:
-
Intraclass correlation coefficient
- ICHD:
-
International classification of headache disorders
- IQR:
-
Inter quartile range
- L:
-
Left
- M:
-
Measurement
- n:
-
Number of participants
- NPRS:
-
Numeric pain rating scale
- PPT(s):
-
Pressure pain threshold(s)
- PSQI:
-
Pittsburgh sleep quality index
- R:
-
Right
- RCT:
-
Randomized controlled trial
- SD(s):
-
Standard deviation(s)
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- VAS:
-
Visual analogue scale
References
Bogduk, N. & Govind, J. Cervicogenic headache: an assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 8, 959–968 (2009).
Fernández-de-Las-Peñas, C., Florencio, L. L., Plaza-Manzano, G. & Arias-Buría, J. L. Clinical reasoning behind non-pharmacological interventions for the management of headaches: A narrative literature review. Int. J. Environ. Res. Public Health 17, 4126 (2020).
Racicki, S., Gerwin, S., Diclaudio, S., Reinmann, S. & Donaldson, M. Conservative physical therapy management for the treatment of cervicogenic headache: A systematic review. J. Man. Manip. Ther. 21, 113–124 (2013).
Gross, A. R. et al. Exercises for mechanical neck disorders: A Cochrane review update. Man Ther. 24, 25–45 (2016).
Luedtke, K., Allers, A., Schulte, L. H. & May, A. Efficacy of interventions used by physiotherapists for patients with headache and migraine-systematic review and meta-analysis. Cephalalgia 36, 474–492 (2016).
Moore, C. S., Sibbritt, D. W. & Adams, J. A critical review of manual therapy use for headache disorders: Prevalence, profiles, motivations, communication and self-reported effectiveness. BMC Neurol. 17, 61 (2017).
Bogduk, N. Cervicogenic headache: Anatomic basis and pathophysiologic mechanisms. Curr. Pain Headache Rep. 5, 382–386 (2001).
Castien, R. & De Hertogh, W. A neuroscience perspective of physical treatment of headache and neck pain. Front. Neurol. 10, 276 (2019).
Chua, N. H., van Suijlekom, H. A., Vissers, K. C., Arendt-Nielsen, L. & Wilder-Smith, O. H. Differences in sensory processing between chronic cervical zygapophysial joint pain patients with and without cervicogenic headache. Cephalalgia 31, 953–963 (2011).
Knackstedt, H. et al. Cervicogenic headache in the general population: The Akershus study of chronic headache. Cephalalgia 30, 1468–1476 (2010).
Fernández-de-las-Peñas, C. & Cuadrado, M. L. Therapeutic options for cervicogenic headache. Expert Rev. Neurother. 14, 39–49 (2014).
Jull, G. et al. A randomized controlled trial of exercise and manipulative therapy for cervicogenic headache. Spine 27, 1835–1843 (2002).
Jull, G. & Stanton, W. R. Predictors of responsiveness to physiotherapy management of cervicogenic headache. Cephalalgia 25, 101–108 (2005).
Liebert, A., Rebbeck, T., Elias, S., Hawkins, D. & Adams, R. Musculoskeletal physiotherapists’ perceptions of non-responsiveness to treatment for cervicogenic headache. Physiother. Theory Pract. 29, 616–629 (2013).
Jones, L. E. & O’Shaughnessy, D. F. The pain and movement reasoning model: Introduction to a simple tool for integrated pain assessment. Man. Ther. 19, 270–276 (2014).
Fillingim, R. B. Individual differences in pain: Understanding the mosaic that makes pain personal. Pain 158, S11–S18 (2017).
Edwards, R. R. et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 157, 1851–1871 (2016).
International Association for the Study of Pain. IASP Terminology. https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698 (2021).
Chapman, W. P. & Jones, C. M. Variations in cutaneous and visceral pain sensitivity in normal subjects. J. Clin. Invest. 23, 81–91 (1944).
Yarnitsky, D., Granot, M. & Granovsky, Y. Pain modulation profile and pain therapy: Between pro- and antinociception. Pain 155, 663–665 (2014).
Coghill, R. C., McHaffie, J. G. & Yen, Y. F. Neural correlates of interindividual differences in the subjective experience of pain. Proc. Natl. Acad. Sci. U. S. A. 100, 8538–8542 (2003).
Emerson, N. M. et al. Pain sensitivity is inversely related to regional grey matter density in the brain. Pain 155, 566–573 (2014).
Khan, H. S. & Stroman, P. W. Inter-individual differences in pain processing investigated by functional magnetic resonance imaging of the brainstem and spinal cord. Neuroscience 307, 231–241 (2015).
Avijgan, M., Thomas, L. C., Osmotherly, P. G. & Bolton, P. S. A systematic review of the diagnostic criteria used to select participants in randomised controlled trials of interventions used to treat cervicogenic headache. Headache 60, 15–27 (2020).
Tracy, L. M. Psychosocial factors and their influence on the experience of pain. Pain Rep. 2, e602 (2017).
Nijs, J. et al. Lifestyle and chronic pain across the lifespan: An inconvenient truth?. PM R. 12, 410–419 (2020).
Graven-Nielsen, T. & Arendt-Nielsen, L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat. Rev. Rheumatol. 6, 599–606 (2010).
Zito, G., Jull, G. & Story, I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man. Ther. 11, 118–129 (2006).
Van Belle, G. & Martin, D. C. Sample size as a function of coefficient of variation and ratio of means. Am. Stat. 3, 165–167 (1993).
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia 38, 1–211 (2018).
Tashani, O. A., Astita, R., Sharp, D. & Johnson, M. I. Body mass index and distribution of body fat can influence sensory detection and pain sensitivity. Eur. J. Pain 21, 1186–1196 (2017).
Ylinen, J., Nykänen, M., Kautiainen, H. & Häkkinen, A. Evaluation of repeatability of pressure algometry on the neck muscles for clinical use. Man. Ther. 12, 192–197 (2007).
Walton, D. M. et al. Reliability, standard error, and minimum detectable change of clinical pressure pain threshold testing in people with and without acute neck pain. J. Orthop. Sports Phys. Ther. 41, 644–650 (2011).
Koppenhaver, S. L. et al. Changes in lumbar multifidus muscle function and nociceptive sensitivity in low back pain patient responders versus non-responders after dry needling treatment. Man. Ther. 20, 769–776 (2015).
Balaguier, R., Madeleine, P. & Vuillerme, N. Is one trial sufficient to obtain excellent pressure pain threshold reliability in the low back of asymptomatic individuals? A test-retest study. PLoS ONE 11, e0160866 (2016).
Castien, R. F., van der Wouden, J. C. & De Hertogh, W. Pressure pain thresholds over the cranio-cervical region in headache: A systematic review and meta-analysis. J. Headache Pain. 19, 9 (2018).
Martínez-Segura, R., De-la-Llave-Rincón, A. I., Ortega-Santiago, R., Cleland, J. A. & Fernández-de-Las-Peñas, C. Immediate changes in widespread pressure pain sensitivity, neck pain, and cervical range of motion after cervical or thoracic thrust manipulation in patients with bilateral chronic mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 42, 806–814 (2012).
Binderup, A. T., Arendt-Nielsen, L. & Madeleine, P. Pressure pain sensitivity maps of the neck-shoulder and the low back regions in men and women. BMC Musculoskelet. Disord. 11, 234 (2010).
Mun, C. J. et al. Investigating intraindividual pain variability: Methods, applications, issues, and directions. Pain 160, 2415–2429 (2019).
Young, I. A., Dunning, J., Butts, R., Cleland, J. A. & Fernández-de-Las-Peñas, C. Psychometric properties of the Numeric Pain Rating Scale and Neck Disability Index in patients with cervicogenic headache. Cephalalgia 39, 44–51 (2019).
Mingels, S., Dankaerts, W., van Etten, L., Bruckers, L. & Granitzer, M. Exploring multidimensional characteristics in cervicogenic headache: Relations between pain processing, lifestyle, and psychosocial factors. Brain Behav. 11, e2339 (2021).
Mingels, S., Dankaerts, W., van Etten, L., Bruckers, L. & Granitzer, M. Lower spinal postural variability during laptop-work in subjects with cervicogenic headache compared to healthy controls. Sci. Rep. 11, 5159 (2021).
Hodges, P. W. & Tucker, K. Moving differently in pain: A new theory to explain the adaptation to pain. Pain 152, S90–S98 (2011).
Alburquerque-Sendín, F., Madeleine, P., Fernández-de-Las-Peñas, C., Camargo, P. R. & Salvini, T. F. Spotlight on topographical pressure pain sensitivity maps: A review. J. Pain Res. 11, 215–225 (2018).
O’Sullivan, P. et al. Sensory characteristics of chronic non-specific low back pain: A subgroup investigation. Man. Ther. 19, 311–318 (2014).
Prushansky, T., Dvir, Z. & Defrin-Assa, R. Reproducibility indices applied to cervical pressure pain threshold measurements in healthy subjects. Clin. J. Pain. 20, 341–347 (2004).
Finocchietti, S., Nielsen, M., Mørch, C. D., Arendt-Nielsen, L. & Graven-Nielsen, T. Pressure-induced muscle pain and tissue biomechanics: A computational and experimental study. Eur. J. Pain. 15, 36–44 (2011).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163 (2016).
Portney, L. G. & Watkins, M. P. Foundations of Clinical Research: Applications to Practice (F.A. Davis Company, 2015).
Fillingim, R. B. Individual differences in pain responses. Curr. Rheumatol. Rep. 7, 342–347 (2005).
Madden, V. J. et al. Variability in experimental pain studies: Nuisance or opportunity?. Br. J. Anaesth. 126, e61–e64 (2021).
Liew, B. et al. A novel metric of reliability in pressure pain threshold measurement. Sci. Rep. 11, 6944 (2021).
Melia, M. et al. Pressure pain thresholds: Subject factors and the meaning of peak pressures. Eur. J. Pain 23, 167–182 (2019).
Melia, M. et al. Measuring mechanical pain: The refinement and standardization of pressure pain threshold measurements. Behav. Res. Methods. 47, 216–227 (2015).
Bandura, A., O’Leary, A., Taylor, C. B., Gauthier, J. & Gossard, D. Perceived self-efficacy and pain control: Opioid and nonopioid mechanisms. J. Pers. Soc. Psychol. 53, 563–571 (1987).
Synnott, A. et al. Physiotherapists report improved understanding of and attitude toward the cognitive, psychological and social dimensions of chronic low back pain after Cognitive Functional Therapy training: A qualitative study. J. Physiother. 62, 215–221 (2016).
MacKay, C., Hawker, G. A. & Jaglal, S. B. Qualitative study exploring the factors influencing physical therapy management of early knee osteoarthritis in Canada. BMJ Open 8, e023457 (2018).
Lin, I. et al. What does best practice care for musculoskeletal pain look like? Eleven consistent recommendations from high-quality clinical practice guidelines: Systematic review. Br. J. Sports Med. 54, 79–86 (2020).
Caneiro, J. P. et al. It is time to move beyond “body region silos” to manage musculoskeletal pain: Five actions to change clinical practice. Br. J. Sports Med. 54, 438–439 (2020).
Harris, R. E. et al. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum. 52, 3670–3674 (2005).
Bishop, M. D. et al. What effect can manual therapy have on a patient’s pain experience?. Pain Manag. 5, 455–464 (2015).
De Pauw, R. et al. Consensus among musculoskeletal experts for the management of patients with headache by physiotherapists? A delphi study. Musculoskelet. Sci. Pract. 52, 102325 (2021).
Mingels, S., Dankaerts, W., van Etten, L., Bruckers, L. & Granitzer, M. Spinal postural variability relates to biopsychosocial variables in patients with cervicogenic headache. Sci. Rep. 11, 13783 (2021).
Andrade, C. Author’s response to ‘Multiple testing and protection against type I error using P value correction: Application in cross-sectional study designs’. Indian J. Psychol. Med. 41, 198 (2019).
Levine, M. & Ensom, M. Post hoc power analysis: An idea whose time has passed?. Pharmacotherapy 21, 405–409 (2001).
Gordon, D. B. Acute pain assessment tools: Let us move beyond simple pain ratings. Curr. Opin. Anaesthesiol. 28, 565–569 (2015).
Dudley, L. A. et al. Interrater and intrarater reliability of the tuck jump assessment by health professionals of varied educational backgrounds. J. Sports Med. 2013, 483503 (2013).
Graeff, P., Itter, A., Wach, K. & Ruscheweyh, R. Inter-individual differences explain more variance in conditioned pain modulation than age, sex and conditioning stimulus intensity combined. Brain Sci. 11, 1186 (2021).
Cook, C., Donaldson, M. & Lonnemann, E. ‘Next steps’ for researching orthopedic manual therapy. J. Man. Manip. Ther. 29, 333–336 (2021).
Acknowledgements
The authors want to acknowledge dr. van Etten L to provide a lab set-up.
Funding
SM was supported by the Special Research Fund (BOF) of the Hasselt University (BOF19OWB28).
Author information
Authors and Affiliations
Contributions
S.M., W.D., and M.G. conceived and planned the experiments. S.M. carried out the experiments and took the lead in writing the manuscript. W.D. and M.G. supervised and provided feedback throughout the entire research and writing phase. L.B. supported the data-analysis and provided statistical support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mingels, S., Dankaerts, W., Bruckers, L. et al. Inter-individual variability in mechanical pain sensation in patients with cervicogenic headache: an explorative study. Sci Rep 12, 20635 (2022). https://doi.org/10.1038/s41598-022-25326-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25326-8
This article is cited by
-
Therapeutic Patient Education as Part of the Physiotherapy Management of Adults with Headache: A Scoping Review
Current Pain and Headache Reports (2024)
-
A systematic review of neurophysiological sensing for the assessment of acute pain
npj Digital Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.