Abstract
The aim of this placebo-controlled randomized in situ study was to evaluate the effect of different surface polishing protocols on enamel roughness, bacterial adhesion and caries-protective effect of a resin infiltrant. Seventy-five bovine enamel samples having artificial caries lesions were treated with a resinous infiltrant and afterwards randomly dividided into five polishing protocols: aluminum oxide flexible disks (Al2O3-Disks), silicon carbide tips (SIC-Tips), silicon carbide brush (SIC-Brush), silicon carbide polyester strips (SIC-Strips) or no polishing [negative control (NC)]. Average surface roughness (Ra) was assessed by profilometry. Samples were mounted in palatal appliances under a mesh for biofilm accumulation. Fifteen volunteers wore the intraoral appliances (14-days) and cariogenic challenge was triggered by sucrose solutions. Biofilm formed was collected for microbiological analysis of caries-related bacteria (Streptococcus mutans, Lactobacillus acidophilus) and demineralization was assessed by cross-sectional microhardness. Mean Knoop hardness numbers (Kg/mm2) were plotted over lesion depth (µm) and area under the lesion curve was subtracted from sound enamel to determine demineralization (ΔS, Kg/mm2xµm). Data were analyzed by ANOVA and post-hoc comparisons (α = 0.05). NC resulted in significantly higher Ra means than Al2O3-Disks and SIC-Strips. Bacterial counts were not significantly different between the groups (p > 0.05). Regards ΔS means, however none of the groups were significantly different to NC (6983.3 kg/mm2xµm /CI 4246.1–9720.5, p > 0.05). Conclusions: Polishing protocols (Al2O3-Disks, SIC-Strips) significantly decreseased roughness of infiltrated-enamel, however none of the polishing protocols could signicantly decrease bacterial counts nor resulted in significant less demineralization.
Similar content being viewed by others
Introduction
Currently a more biological approach to caries management is at least scientifically widely accepted. The understanding of caries as a multifactorial disease driven by a dysbiosis in the biofilm, the so called ecological plaque hypothesis, supports more holistic approaches for disease management including effective mechanical plaque control, diet modification and modulation of the remineralization1. Regarding the management of approximal non-cavitated caries lesions, the most actual consensus statements, systematic reviews and meta-analyses recommend both non- and micro-invasive treatment options2,3,4. The improvement of biofilm control via oral hygiene instructions and professional fluoride application (i.e., varnish) is a main component of the non-invasive approach. For patients at low caries risk, these measures should be enough to control the disease, yet, for patients at high caries risk, a higher probability and velocity of lesion progression might be expected, and in these cases the micro-invasive treatment options, like caries sealing and caries infiltration, have been recommended2.
However up to now, not much discussion has been provided on the susceptibility of infiltrated lesions to secondary caries or lesion progression due to increased plaque stagnation. Concurrently the control of biofilm formation or the predisposition of material surfaces, such as restorations, sealants and infiltrants to accumulate biofilm is known to play a significant role in caries control. Specially as surface irregularities in materials not only predispose for higher biofilm accumulation5,6, but also act like niches where biofilm may grow undisturbed from mechanical actions of occlusal function, friction from cheeks, tongue, and oral hygiene measures7.
A recent systematic review also confirms that considering composite resins polishing techniques may decrease bacterial adhesion through decrease of surface roughness8. Besides surface roughness, several surface properties can influence bacterial adhesion to restorative or sealing/infiltrant materials surfaces, i.e. surface free energy (surface wettability), charge, polarity, and morphology6. However, surface roughness seems to be the most important and most studied one, especially as regards the accumulation and composition of the biofilm7,9. Moreover, some years ago, a threshold value for average surface roughness (Ra) of 0.2 µm has been proposed, indicating that dental material surfaces with roughness means below this value should have no impact on bacterial adhesion10. However, this concept has been contested recently, since the influence of roughness on bacterial adhesion seems to be more related to roughness range rather than a threshold11. In addition factors like type of material and study design may also influence bacterial adhesion, with studies including formation of salivary pellicle, in situ or in vivo, having a clear advantage of better simulating the clinical conditions, where early colonizers first attach to salivary pellicle and the latter may also modify the surface of properties of the materials6,12.
Incipient/white-spot carious lesions are known to show higher roughness than sound enamel, due to increased episodes of enamel dissolution13 and resin infiltration of these lesions indeed decreases their surface roughness. Nevertheless, infiltrated caries lesions still have significantly higher roughness than non-treated sound enamel14. After the hydrochloric acid etching, opening of the lesion micropores and the lesion body for the infiltration of low-viscosity monomers, an increased lesion surface roughness is present15. The resin infiltration afterwards has been shown to effectively fill up this micro-porosities16, however without recovering of the initial roughness values15,17. As increased surface roughness among other factors play an important role in dental plaque accumulation and biofilm formation5,6,12, which in turn can favour the occurrence of caries adjacent to restorations and sealants (CARS)3, this factor might be of clinical relevance for the long-term treatment survival rate. This leads to the question of, whether surface polishing procedures, a common clinical step in restorative treatment, may be also indispensable for the long-term success of caries infiltration and prevention of caries around the infiltrated lesions as well as a more holistic caries control.
Currently, only one study have investigated the influence of polishing on the recovery of enamel surface roughness after resin infiltration of incipient carious lesions15. The polishing of infiltrated lesions, solely with one type of polishing strip showed no significant benefit regarding surface roughness and as compared to the non-polished infiltrated lesion15; even if the effect of the increased lesion surface roughness on bacterial adhesion was not tested. In fact, bacterial adhesion has also been tested only in one study including resin infiltration of incipient carious lesion, but without any polishing procedure after resin infiltration18.
In summary there is clear evidence that resin infiltration prevents caries progression clinically, but also that it increases lesion surface roughness, which (at least in theory) favours higher plaque accumulation. Until now no clinical recommendation has been made in the current clinical guidelines19, meta-analyses2 and consensus reports3 as regards how infiltrated approximal and buccal non-cavitated caries lesions should be polished in order to decrease surface roughness, plaque accumulation and the consequent increased risk of developing both gingivitis and secondary caries. Therefore, it is still unclear if certain polishing protocols after carious lesion infiltration with low-monomers infiltrant, could further considerably decrease enamel surface roughness as compared to non-polished infiltrated lesions and additionally decrease bacterial adhesion. Thus, the aim of this placebo-controlled randomized in situ study was to evaluate the effect of different surface polishing protocols on enamel surface roughness, bacterial adhesion and caries-protective effect of an infiltrant. It was thus hypothesized that polishing of resin-infiltrated incipient carious lesions would cause for all protocols a decrease in average surface roughness, adhesion of caries-associated bacteria and less in situ demineralization than the non-polished negative control.
Materials and methods
This study was conducted according to the principles of the declaration of Helsinki and all experimental protocols were approved by the local Research and Ethical Committee of the Christus University Center (Protocol # 2758611). All participants signed a written informed consent before the start of the study.
Study design and subjects
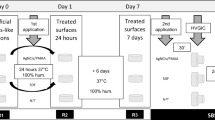
This study was a single-center, placebo-controlled, double-blinded (volunteer and examiner) randomized in situ study composed of one single- factor (polishing types), at 5 levels (polishing protocols). Randomization and blinding were performed by the supervisor of the study using the random sequences function of the Software Excel. The total duration of the intraoral phase was 14 days (Fig. 1) with a 1-week wash-in period preceding it, as previously described20,21,22.
Study Design—Palatal appliance worn by the volunteers containing 5 bovine enamel specimens (4 × 4 × 2 mm3) with artificial caries lesions infiltrated with a resin infiltrant. Specimens were rinsed with sucrose 8 times/day for 14 days. (A) Mesh net placed 1 mm over the specimens for allowing plaque accumulation. (B) Specimens removed from the appliances after 14 days. (C) Biofilm collection from one specimen/appliance (n = 15/group) and determination of CFU/mg of total bacteria, S. mutans and Lactobacillus acidophilius. (D) Specimens sectioned longitudinally trough the center of the caries lesions (n = 1 specimen/group/appliance and n = 15 specimens/group). (E) Cross-sectional Knoop microhardness analysis. Two lines of indentations 100 µm apart from each other and up to a depth of 180 µm. Indentation interval was 10 µm up to 100 µm depth and 20 µm from 100 to 180 µm, so that 14 indentations were performed per line. (Drawing of Ms. Bernadette Rawyler).
The volunteers were additionally informed to avoid consuming high fluoride-containing food, such as fluoridated salt, green/black tea and fish. At the beginning of each phase, the volunteers were also extensively trained to follow the study instructions. The intraoral appliances were worn during the whole day except for meals and oral hygiene. Through the whole study and additionally in the wash-in phase, individual oral hygiene was performed with a fluoride toothpaste (NaF, 1.450 ppm F−, Colgate Total 12, São Paulo, Brazil), without the appliances in situ. After meals, 20 min were elapsed before mouth appliance reinsertion.
Sample size
The sample size was calculated based on the results of a previous study evaluating the inhibition of caries progression after resin infiltration in situ23. According to that, mean enamel mineral loss after the intraoral phase of 6261 (SD = 2009) vol% x µm for the negative control and 2559 (SD = 1178) vol%x µm for the infiltrated group would be expected. The tooth was considered as experimental unit and to obtain a power of 80%, a sample size of fourteen enamel blocks per treatment group was calculated (two-sided, α = 0.05). In order to account for a possible dropout, 15 specimens/groups were then included in the experiments.
Fifteen young adult volunteers (9 women, 6 men, aged 18–22 years), were recruited for the study. All subjects lived in Fortaleza, Brazil (fluoride concentration in tap water of approximately 0.8 mg/l) and met the following inclusion criteria: the ability to wear an intraoral palatal device for 24 h per day, except at eating and brushing times; no evidence of active caries, or periodontal disease; no signs of decreased salivary flow rate and the ability to comply with the study protocol. Exclusion criteria were any medical condition potentially interfering with the subject’s health; use of orthodontic appliance; pregnancy or breastfeeding and absence of compliance to the study protocol.
Specimens
Seventy-five enamel blocks of 4 × 4 × 2 mm, disinfected as previously described24,25 were cut (Isomet, Buehler, Illinois, USA) from labial central area of bovine incisor crowns (teeth donated from a slaughterhouse, Fortaleza, Brazil). The enamel surface was serially polished using silicon carbide papers (grits #800, #1200, and #4000, Buehler, USA) under water cooling and polishing cloths with a 1-µm diamond suspension (Buehler, USA). After each polishing steps, the specimens were washed in ultrasonication bath containing deionized water for 3 min. The surface microhardness of all enamel blocks was determined with a Knoop indenter (6 indentations in the center of the specimens: 25 g load for 15 s), as previously described by Zero et al.26. Seventy-five enamel specimens were selected (average knoop hardness number (KHN): 252.69 ± 50.53 KHN), after discarding 6 blocks that presented outlier values. All specimens were examined under a stereo microscope to ensure the absence of hypomineralized areas or surface defects27 and were stored at 100% of humidity in a box containing deionized water, throughout the study, except for the time they were in situ.
Artificial caries lesions
All selected specimens were all were laterally protected with acid resistant varnish (Colorama-CEIL, São Paulo, SP, Brazil), leaving only the labial enamel surface of the specimens exposed. Afterwards they were submitted to a well-stablished pH-cycling model for 8 days, in order to create artificial white-spot lesions, as previously described28. Shortly, each cycle consisted of 4 h immersion in the demineralization solution followed by 20 h in the remineralization solution at 37 °C. The demineralization solution was composed of (50 mmol/lactate buffer pH 5.0, containing 1.28 mmol/L Ca, 0.74 mmol/L Pi and 0.03 μg F/ml prepared to form the Ca(NO3) salt 2.4 H2O, KH2PO4 and NaF) (100 ml/16 mm2 of enamel. And the chemical composition of remineralization solution was 1.5 mmol/Ca, 0.9 mmol/P, 150 mmol/KCl and 0.05 μgF/mL in 0.01 mmol/L Tris buffer (pH 7.0 / 37° C) (2.5 ml solution/mm2 enamel surface)28. Twice a day (before and after immersion in the demineralization solution), the blocks were washed with deionized water for 5 min and under agitation, and on the fourth day, the demin- and remineralization solutions were renewed with fresh ones. After the end of the pH-cycling, the blocks were kept immersed in remineralization solution for 24 h28.
Resin infiltration of caries lesions
After lesion formation all specimens were washed in running water and dried with sterile gauze. Subsequently micro-invasively treatment by the application of resinous infiltrating agent (ICON, Dental Milestones Guaranteed DMG, Hamburg, Germany) was carried out, according to the manufacturer’s protocol and as previously described29.
Surface polishing protocols (treatments)
After that, the specimens were randomly divided, using an Excel-Sheet with random sequence of numbers, into five groups receiving the following polishing treatments:
-
No polishing, as a negative control group (NC)
-
polishing with aluminium oxide flexible disks (Al2O3-Disks), Sof-Lex Pop On
-
polishing with silicon carbide tips (SIC-Tips), Astropol
-
polishing with silicon carbide brush (SIC-Brush), Optishine
-
polishing silicon carbide polyester strips (SIC-Strips), Epitex
All polishing protocols were performed by only one operator, for the same amount of time, in a pre-tested manner as well as following the manufacturers’ instructions. Further details are described in Table 1.
Surface roughness
In order to evaluate the effect of the different polishing systems on the surface characteristics of infiltrated enamel all specimens were subjected to a surface roughness analysis (Fig. 2) using a contact profilometer (Hommel-Etamic W10, Schwenningem, Germany). The device has an accuracy of 0.01 µm, and the diamond stylus has a radius of 5 µm. Measurements were done in the center of the specimens at a constant speed of 0.15 mm/s and under a force of 0.8 mN. The average roughness values (Ra) were obtained after three successive scannings, located at 100 μm distance to each other, as previously described30,31. An arithmetic mean of the values measured was considered the value (Ra) in μm for each specimen.
Palatal appliances
After hard and soft tissue examination intraoral palatal devices of acrylic resin were prepared for each volunteer. In each palatal device, 5 niches were created, where the specimens containing the infiltrated caries lesions were fixed (Fig. 2). A free space of 1.0 mm over the specimens and the plastic mesh covering then was preserved, in order to allow for biofilm accumulation. The mesh net also protected the biofilm against mechanical disturbances21,24.
Intraoral phase
To trigger a cariogenic challenge, the volunteers dripped 10% sucrose solution over each specimen 8 times a day, for 14 days (Fig. 2). In total the volunteers wore the intraoral appliances for 5 phases of 14 days each, with continuous in situ exposure except during meals and oral hygiene, meaning a total wearing time of 22–23 h/day.
Cross-sectional microhardness analysis
After the in situ cariogenic challenge and biofilm collection, a longitudinal section was performed in the center of each specimen. One of the halves of each specimen was embedded in acrylic resin, the cut surface being exposed. The cut surfaces were subsequently serially polished as described in the specimen preparation section and the cross-sectional microhardness was measured. For this, two lines of fourteen Knoop indentations were done at 25 g load for 5 s20,32,33. The two lines of indentations were located 100 μm apart from each other34. Within each sequence, the first ten indentations were spaced 10 µm from the previous one and the last ones were spaced at 20 μm distance (10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, 180 μm distance from the enamel surface)35. The mean Knoop hardness number (Kg/mm2) of the two rows at each distance from the surface were then averaged and plotted over the lesion depth (µm). The data set of each artificial caries lesion was curve-fitted33, and the area under the lesion tracing was calculated and subtracted from the area under the curve of sound enamel mean values to give the parameter (ΔS, Kg/mm2 x µm)32. As a linear relationship between cross-sectional microhardness and mineral content of enamel measured by transversal microradiography methods has been demonstrated33.
Microbiological analysis
Microbiological analysis of the biofilm formed on each specimen was performed, in order to evaluate the effect of polishing of the resin infiltrants on the adhesion of caries-associated bacteria species36. A biofilm sample from each specimen was collected. The obtained suspension (addition of 0.9% NaCl solution (1 ml/mg of biofilm) was diluted in decimal series and inoculated in triplicate in blood agar culture media to determine total microorganisms (TM), and MSKB (90 g mitis salivarius dehydrated agar; 1 ml 1% potassium tellurite; 20% w/v sorbitol; 1ug/ml kanamycin monosulfate; 0.1 U/ml bacitracin) for counting Streptococcus mutans and Man Rogosa Sharpe agar for counting Lactobacillus acidophilus (kept for 48 h in an oven at 37 °C with a partial pressure of 5% CO2) for 48 h, the ratio of colony forming units (CFU) per milligram of biofilm was established (CFU/mg).
Scanning Electron Microscope (SEM) analysis
After the in situ phase, representative blocks of the treatment groups were subjected to SEM analysis. The specimens were cleaned in a ultrasonic bath, rinsed with ethanol (96%), dried and subsequently mounted on metallic stubs. After gold sputtering-coat they were examined under a SEM (Quanta 450 FEG, FEI Company®, Oregon, EUA) at 1000 ×, 5000 × and 10,000 × magnification. The images were obtained using an Everhardt-Thornley detector, the accelerating voltage was 20 kV and the working distance 13, 2 mm.
Statistical analysis
Normal distribution of all collected data was assessed using the Shapiro-Wilks statistical test as 5% significance level. Since the average surface roughness data were not normally distributed, they were analysed using a Kruskal–Wallis test and post hoc comparisons. Data obtained from the cross-sectional microhardness and ΔS analyses were analysed by means of a one-way ANOVA and Tukey’s post hoc comparisons to detect differences between the treatment groups. For the microbiological analysis, the variables of colony forming units (CFU) of each group were logarithmically transformed and then analysed by means of ANOVA and Tukey-test for post hoc comparisons. All tests were conducted at a significance level of 5% using the Statistical Package for the Social Sciences (SPSS 22.0) for Windows.
Results
Surface roughness
The results of the surface roughness analysis after each polishing protocol are shown in Fig. 3. In general, there was a significant effect of the type of polishing protocol on enamel surface roughness (p = 0.01). Statistically significant difference was found between NC and Al2O3-disks (p < 0.01) and between NC and SIC-strips (p = 0.04). Among the test groups, meaning the different polishing protocols, no significant difference was observed (p > 0.05).
Cross-sectional microhardness analysis
Regarding protection from demineralization under the in situ cariogenic challenge, the lowest ΔS mean (Kg/mm2Xµm) was observed for Al2O3-Disk (5931.9/CI 1769.3–10,094.6), however none of the groups were significantly different to NC (6983.3/CI 4246.1–9720.5, p > 0.05) (Table 2, Fig. 4).
Microbiological analysis
The logarithms of the microbiological counts after polishing in the in situ microbiological assay can be found in Fig. 5. In all the bacterial counts investigated, namely Streptococcus mutans, Lactobacillus acidophilus and total microorganisms there was no statistically significant difference between the groups (p = 0.89, p = 0.37, p = 0.58 respectively).
Scanning Electron Microscope (SEM) analysis
The SEM analysis of the enamel surfaces after the different polishing protocols and after 14 days in situ showed a wide range of characteristic modifications (Fig. 6). In general, most of the groups still showed some debris of the polishing procedures, while only groups SIC-brush and Al2O3-disks presented groves, valleys and scratches caused by the polishing procedures and which were still present even after the intraoral caries challenge. Moreover, the group polished with Al2O3-disks showed the least amount of biofilm still present over enamel surface, which relates well to the previous results in which this group also showed the least amount of total bacterial counts, the lowest surface roughness, and the lowest demineralization. It is interesting to note that, although all samples were previously cleaned in an ultrasonic bath, a mixture of polishing debris and bacterial biofilm was still shown for the negative control as well as for SIC-tips and SIC-strips polishing approaches.
Representative scanning electron microscopy images of the enamel surfaces after the different polishing protocols and after 14 days in situ (Magnifications: 1.000 X, 5.000 X, 10.000 X). White arrows indicate groves, valleys and scratches on the enamel surfaces caused by the polishing procedures, which were still present after the intraoral caries challenge. Moreover, the group polished with Al2O3-disks showed the least amount of biofilm still present over enamel surface, which relates well to the previous results in which this group also showed the least amount of total bacterial counts, the lowest surface roughness, and the lowest demineralization. Although all samples were previously cleaned in an ultrasonic bath a mixture of polishing debris and bacterial biofilm was still shown for the negative control as well as for SIC-tips and SIC-strips polishing approaches. (a) SIC-tips, (b) SIC-brush, (c) SIC-strips, (d) Al2O3-disks, (e) NC.
Discussion
The present study evaluated for the first time the efficacy of polishing protocols after resin infiltration using an in situ model. The results showed that infiltrated and subsequently polished enamel lesions do not demineralize significantly less compared to unpolished lesions. Moreover, bacterial adhesion of caries-associated bacteria, like Streptococcus mutans and Lactobacillus acidophilus, but also of total bacterial was not significantly reduced, partially rejecting the formulated study hypothesis. However, average surface roughness of resin-infiltrated enamel caries lesions was significantly reduced after polishing with Al2O3-Disks and SIC-Strips confirming what was initially hypothesized; at least for these two protocols.
Currently, there are little evidence from in vitro and only limited indirect evidence from clinical studies as regards the effect of solely resin infiltration or infiltration followed by several polishing protocols on surface roughness and bacterial adhesion of infiltrated carious lesions. The significantly reduced surface roughness observed after polishing with Al2O3-Disks and SIC-Strips are not totally in agreement with the only one in vitro study investigating polishing of resin-infiltrated lesions. In the study from Mueller, et al.15 polishing the incipient lesions with only one type of polishing system, namely polishing strips (coarse, medium, fine and superfine, each: 15 s, total time:60 s), after light curing of the resin infiltrant resulted in average surface roughness means not significantly different to the non-polished groups. The differences in the results may be related to the fact that the caries lesions in the mentioned study15 were much deeper than in our study, implicating in the presence of much larger surface pores, which may have resulted in much higher initial surface roughness and a more difficult baseline situation for achieving a high degree of polishing. Furthermore, the study design may have been also an influence factor, as the present study the cariogenic challenge was performed in situ, including the influence of the salivary pellicle, and in the previous study only in vitro simulations were performed.
As regards the bacterial adhesion to the best of our knowledge this was the first study investigating bacterial adhesion of two caries-associated bacteria (S. mutans and L. acidophilus) in situ and after polishing of resin-infiltrated lesions. In fact, the only previous study investigating bacterial adhesion after a resin infiltration procedure, did not included any polishing afterwards, while investigating only one bacteria species, namely Streptococcus mutans18. In this way the approaches are not directly comparable, but anyway also without polishing there was a slightly reduced adhesion of S. mutans, which was not significantly different to the non-treated control18. This indicates a tendency towards absence of an impact of resin infiltration on biofilm formation. Nevertheless, a better understanding of the influence of resin infiltration on bacterial adhesion is still needed and must be further investigated. Moreover, the present results must be interpreted with caution as other cariogenic species play a key role in a cariogenic biofilm or in the transition to dysbiosis of the microbiome1.
Another aspect interest to observe is that even in the groups causing a significant decrease in surface roughness, like Al2O3-Disks and SIC-Strips, this decrease was not accompanied by a decrease in the bacterial adhesion over these more polished surfaces. This was somehow unexpected, but at the same time shows that surface roughness is not the solely topography parameter impacting bacterial adhesion. Indeed, also surface free energy, charge, polarity and morphology has been shown to substantially influence bacterial adhesion and growth6,7. Moreover, the means of average surface roughness (Ra) in the present study varied from 0.3 to 0.6 µm, slightly above a limit of 0.2 µm, over which surfaces were supposed to favour higher bacterial adhesion10. This is also in agreement with a recent systematic review contesting this threshold value for bacterial adhesion over dental material surfaces11. In addition factors like type of material and study design may also influence bacterial adhesion, with studies including formation of salivary pellicle, in situ or in vivo, having a clear advantage of better simulating the clinical conditions, where early colonizers first attach to salivary pellicle and the latter may also modify the surface of properties of the materials6,12.
It can be speculated that one of the reasons for the absence of influence of surface roughness on bacterial adhesion in the present study may be related to the presence of a naturally formed salivary pellicle over the enamel surfaces. Since there is evidence that the presence of the pellicle may level out grooves and valleys of a rough enamel surface6,7. However, bacterial adhesion to dental and material surfaces is a complex multi-stage process, which is up to now not completely understood. There is only a clear understanding that surface topography may influence the near-surface micro-environment and therefore plays a important role in bacterial adhesion7,11. Therefore, future investigations could systematically evaluate also other topographic parameters for improving the understanding of the biofilm-surface interactions.
For sure the use of bovine enamel samples provides similar but not equal demineralization responses as human enamel and thus represent a limitation of the results shown. However, at the same time, the study design allowed for the first time an analysis of the influence of polishing of resin-infiltrated incipient caries lesions on surface roughness, bacterial adhesion, and the caries protective effect within the oral cavity and under cariogenic conditions. This represents an important advantage of the model as, among other reasons, it is well known that bacterial growth over enamel or restoration surfaces in highly influenced by the presence or absence of a naturally formed salivary pellicle6. Thus, a better clinical simulation of the biofilm film formed clinically was achieved. Nonetheless, the clinical efficacy of polishing resin-infiltrated caries lesions must still be investigated under a more severe cariogenic model, including longer sucrose challenges and/or longer periods in situ.
Furthermore, the comparative effectiveness of the resin infiltrate against newly introduced treatments for arresting or remineralizing carious white-spot lesions, like the biomimetic approaches using hydroxyapatite37,38 and self-assembling peptide P11-439 therapies should be further investigated in the future. Both in relation to their remineralizing and their effects on enamel surface properties as well as bacterial colonization.
As regards the methods used, it is important to notice that cross-sectional microhardness is also a wide-used and valid method of measuring changes in enamel mineral content (i.e. demineralization). The reason for that is because a linear correlation between values of cross-sectional microhardness and volume mineral loss (%vol x µm) has been demonstrated both in vitro33 as in situ40. Transversal microradiography is the current gold standard technique, and this clear correlation indicates that cross-sectional microhardness is a sensitive and reliable method for assessing mineral loss of incipient carious lesions. As regards the microbial counts through cultivation methods and counting of colony forming units, it is reasonable to say that although currently more modern molecular biological methods are available, for several research questions the classic cultivation methods still provide very useful information24,36. As both microorganisms tested here grow well in the cultivation plates and the microbiological analyses were just an ancillary method in the current work, it surely fulfilled the nees. For future studies it might be interesting though to include also other analyses like fluorescence microscopy or investigations of the 16S rRNA gene.
Although the present results showed that polishing neither influenced the caries-protective effect of resin infiltration nor the bacterial adhesion, from a clinical point of view and specially for labial white-spot lesions infiltrated for masking the whitish appearance, it is still probably more reasonable to polish then anyway. Data from previous clinical studies on this application showed that labial lesions may suffer from long-term staining, when not polished41,42,43. Moreover, contrary to the clinical studies on approximal lesions, which all did not report polishing procedures29,44,45,46,47, for labial lesions most of the clinical studies have been performed including polishing steps after light curing of the infiltrant41. In the study from Kobbe et al. 2019 for example polishing with disks (Sof-Lex) and a polishing brush (Okklubrush) were included and resulted in non-staining in the short-term follow-up.
It was shown for the first time in a pre-clinical intraoral model that some polishing protocols influenced surface roughness, while not significantly affecting neither bacterial adhesion nor enamel demineralization, under the conditions of this study. In this way, not contradicting the clinical recommendation of only removing surplus infiltrant material before light curing. However, one must bear in mind that this question of plaque stagnation over resin-infiltrated should be further investigated either in vivo or with in situ models including longer and more aggressive cariogenic challenges as well as including other polishing protocols before a clinical recommendation as regards the polishing need after infiltration of both buccal as well as approximal caries lesions can be made.
Thus, in conlcusion polishing protocols including Al2O3-Disks and SIC-strips significantly decreased average roughness of resin-infiltrated caries lesions in enamel. However, none of the polishing protocols could significantly decrease the bacterial counts nor resulted in significantly less demineralization than the non-polished control. From a clinical point of view and specially for labial white-spot lesions infiltrated for masking the whitish appearance, it is still probably more reasonable to polish then anyway.
Data availability
The data collected and analysed in this paper is available from the corresponding author upon reasonable request.
References
Marsh, P. D. In sickness and in health-what does the oral microbiome mean to us? An ecological perspective. Adv. Dent. Res. 29, 60–65. https://doi.org/10.1177/0022034517735295 (2018).
Splieth, C. H., Kanzow, P., Wiegand, A., Schmoeckel, J. & Jablonski-Momeni, A. How to intervene in the caries process: Proximal caries in adolescents and adults-a systematic review and meta-analysis. Clin. Oral Invest. 24, 1623–1636. https://doi.org/10.1007/s00784-020-03201-y (2020).
Schwendicke, F. et al. How to intervene in the caries process in adults: Proximal and secondary caries? An EFCD-ORCA-DGZ expert Delphi consensus statement. Clin. Oral Invest. 24, 3315–3321. https://doi.org/10.1007/s00784-020-03431-0 (2020).
Dorri, M., Dunne, S. M., Walsh, T. & Schwendicke, F. Micro-invasive interventions for managing proximal dental decay in primary and permanent teeth. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD010431.pub2 (2015).
Hannig, C. & Hannig, M. The oral cavity–a key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Invest. 13, 123–139. https://doi.org/10.1007/s00784-008-0243-3 (2009).
Sterzenbach, T., Helbig, R., Hannig, C. & Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Invest. 24, 4237–4260. https://doi.org/10.1007/s00784-020-03646-1 (2020).
Cheng, Y., Feng, G. & Moraru, C. I. Micro- and nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 10, 191. https://doi.org/10.3389/fmicb.2019.00191 (2019).
Nedeljkovic, I., Teughels, W., De Munck, J., Van Meerbeek, B. & Van Landuyt, K. L. Is secondary caries with composites a material-based problem?. Dental Mater. 31, e247-277. https://doi.org/10.1016/j.dental.2015.09.001 (2015).
Quirynen, M. & Bollen, C. M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 22, 1–14. https://doi.org/10.1111/j.1600-051x.1995.tb01765.x (1995).
Bollen, C. M., Lambrechts, P. & Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dental Mater. 13, 258–269. https://doi.org/10.1016/s0109-5641(97)80038-3 (1997).
Dutra, D., Pereira, G., Kantorski, K. Z., Valandro, L. F. & Zanatta, F. B. Does finishing and polishing of restorative materials affect bacterial adhesion and biofilm formation? A systematic review. Oper. Dent. 43, E37–E52. https://doi.org/10.2341/17-073-L (2018).
Song, F., Koo, H. & Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 94, 1027–1034. https://doi.org/10.1177/0022034515587690 (2015).
Fejerskov O, N. B., Kidd EAM in Dental Caries: The Disease and Its Clinical Management. (ed Kidd EAM (eds) Fejerskov O) Ch. Chapter 3, 20--48 (Blackwell Munksgaard, 2008).
Yazkan, B. & Ermis, R. B. Effect of resin infiltration and microabrasion on the microhardness, surface roughness and morphology of incipient carious lesions. Acta Odontol. Scand. 76, 473–481. https://doi.org/10.1080/00016357.2018.1437217 (2018).
Mueller, J., Yang, F., Neumann, K. & Kielbassa, A. M. Surface tridimensional topography analysis of materials and finishing procedures after resinous infiltration of subsurface bovine enamel lesions. Quintessence Int. 42, 135–147 (2011).
Meyer-Lueckel, H. & Paris, S. Infiltration of natural caries lesions with experimental resins differing in penetration coefficients and ethanol addition. Caries Res. 44, 408–414. https://doi.org/10.1159/000318223 (2010).
Ulrich, I., Mueller, J., Wolgin, M., Frank, W. & Kielbassa, A. M. Tridimensional surface roughness analysis after resin infiltration of (deproteinized) natural subsurface carious lesions. Clin. Oral Invest. 19, 1473–1483. https://doi.org/10.1007/s00784-014-1372-5 (2015).
Arslan, S. et al. Effect of resin infiltration on enamel surface properties and Streptococcus mutans adhesion to artificial enamel lesions. Dent. Mater. J. 34, 25–30. https://doi.org/10.4012/dmj.2014-078 (2015).
Giacaman, R. Evidence-based Approaches for the Management of the Proximal Lesion in Adults. Summary from the ORCA/EFCD/DGZ Consensus. Web Summaries of the European Organisation for Caries Research (ORCA). https://www.orca-caries-research.org/sites/default/files/inline-files/Giacaman%20-%20How%20to%20Manage%20Caries%20in%20Adults.pdf (2022). Accessed 10 October 2022.
Featherstone, J. D. & Zero, D. T. An in situ model for simultaneous assessment of inhibition of demineralization and enhancement of remineralization. J. Dent. Res. 7, 804–810 (1992).
Zero, D. T. In situ caries models. Adv. Dent. Res. 9, 214–230 (1995).
Lima, J. P. et al. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. Eur. J. Oral Sci. 117, 568–574. https://doi.org/10.1111/j.1600-0722.2009.00662.x (2009).
Paris, S. & Meyer-Lueckel, H. Inhibition of caries progression by resin infiltration in situ. Caries Res. 44, 47–54. https://doi.org/10.1159/000275917 (2010).
Hara, A. T., Queiroz, C. S., Paes Leme, A. F., Serra, M. C. & Cury, J. A. Caries progression and inhibition in human and bovine root dentine in situ. Caries Res. 37, 339–344. https://doi.org/10.1159/000072165CRE2003037005339 (2003).
Souza, J. G. et al. Calcium prerinse before fluoride rinse reduces enamel demineralization: An in situ caries study. Caries Res 50, 372–377. https://doi.org/10.1159/000446407 (2016).
Zero, D. T. et al. An improved intra-oral enamel demineralization test model for the study of dental caries. J. Dent. Res. 71, 871–878 (1992).
Ramalho, K. M. et al. Randomized in situ study on the efficacy of CO2 laser irradiation in increasing enamel erosion resistance. Clin. Oral Invest. 23, 2103–2112. https://doi.org/10.1007/s00784-018-2648-y (2019).
Molina, G. F. et al. Lead deposition in bovine enamel during a pH-cycling regimen simulating the caries process. Caries Res. 45, 469–474. https://doi.org/10.1159/000330602 (2011).
Meyer-Lueckel, H. et al. Proximal caries infiltration—Pragmatic RCT with 4 years of follow-up. J. Dent. 111, 103733. https://doi.org/10.1016/j.jdent.2021.103733 (2021).
Esteves-Oliveira, M. et al. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J. Dent. 39, 414–421. https://doi.org/10.1016/j.jdent.2011.03.006 (2011).
Esteves-Oliveira, M. et al. A new laser-processing strategy for improving enamel erosion resistance. J. Dent. Res. 96, 1168–1175. https://doi.org/10.1177/0022034517718532 (2017).
Cury, J. A., do Amaral, R. C., Tenuta, L. M., Del Bel Cury, A. A. & Tabchoury, C. P. Low-fluoride toothpaste and deciduous enamel demineralization under biofilm accumulation and sucrose exposure. Eur. J. Oral Sci. 118, 370–375. https://doi.org/10.1111/j.1600-0722.2010.00745.x (2010).
Featherstone, J. D., ten Cate, J. M., Shariati, M. & Arends, J. Comparison of artificial caries-like lesions by quantitative microradiography and microhardness profiles. Caries Res. 17, 385–391. https://doi.org/10.1159/000260692 (1983).
Melo, M. A. S., Morais, W. A., Passos, V. F., Lima, J. P. M. & Rodrigues, L. K. A. Fluoride releasing and enamel demineralization around orthodontic brackets by fluoride-releasing composite containing nanoparticles. Clin. Oral Invest. 18, 1343–1350. https://doi.org/10.1007/s00784-013-1073-5 (2014).
Calvo, A. F. et al. Effect of acidulated phosphate fluoride gel application time on enamel demineralization of deciduous and permanent teeth. Caries Res. 46, 31–37. https://doi.org/10.1159/000335125 (2012).
Henne, K. et al. Analysis of bacterial activity in sound and cariogenic biofilm: A pilot in vivo study. Caries Res. 50, 480–488. https://doi.org/10.1159/000448485 (2016).
Esteves-Oliveira, M., Santos, N. M., Meyer-Lueckel, H., Wierichs, R. J. & Rodrigues, J. A. Caries-preventive effect of anti-erosive and nano-hydroxyapatite-containing toothpastes in vitro. Clin. Oral Invest. 21, 291–300. https://doi.org/10.1007/s00784-016-1789-0 (2017).
Scribante, A. et al. In vitro re-hardening of bleached enamel using mineralizing pastes: Toward preventing bacterial colonization. Materials (Basel) https://doi.org/10.3390/ma13040818 (2020).
Wierichs, R. J., Kogel, J., Lausch, J., Esteves-Oliveira, M. & Meyer-Lueckel, H. Effects of self-assembling peptide P11–4, fluorides, and caries infiltration on artificial enamel caries lesions in vitro. Caries Res. 51, 451–459. https://doi.org/10.1159/000477215 (2017).
Kielbassa, A. M., Wrbas, K. T., Schulte-Monting, J. & Hellwig, E. Correlation of transversal microradiography and microhardness on in situ-induced demineralization in irradiated and nonirradiated human dental enamel. Arch. Oral Biol. 44, 243–251. https://doi.org/10.1016/s0003-9969(98)00123-x (1999).
Kobbe, C., Fritz, U., Wierichs, R. J. & Meyer-Lueckel, H. Evaluation of the value of re-wetting prior to resin infiltration of post-orthodontic caries lesions. J. Dent. 91, 103243. https://doi.org/10.1016/j.jdent.2019.103243 (2019).
Mazur, M. et al. In-vivo colour stability of enamel after ICON(R) treatment at 6 years of follow-up: A prospective single center study. J. Dent. 122, 103943. https://doi.org/10.1016/j.jdent.2021.103943 (2022).
Knaup, I. et al. Correlation of quantitative light-induced fluorescence and qualitative visual rating in infiltrated post-orthodontic white spot lesions. Eur. J. Orthod. https://doi.org/10.1093/ejo/cjac051 (2022).
Martignon, S., Ekstrand, K. R. & Ellwood, R. Efficacy of sealing proximal early active lesions: An 18-month clinical study evaluated by conventional and subtraction radiography. Caries Res. 40, 382–388. https://doi.org/10.1159/000094282 (2006).
Arthur, R. A. et al. Proximal carious lesions infiltration-a 3-year follow-up study of a randomized controlled clinical trial. Clin. Oral Invest. 22, 469–474. https://doi.org/10.1007/s00784-017-2135-x (2018).
Peters, M. C., Hopkins, A. R. Jr., Zhu, L. & Yu, Q. Efficacy of proximal resin infiltration on caries inhibition: Results from a 3-year randomized controlled clinical trial. J. Dent. Res. 98, 1497–1502. https://doi.org/10.1177/0022034519876853 (2019).
Paris, S., Bitter, K., Krois, J. & Meyer-Lueckel, H. Seven-year-efficacy of proximal caries infiltration—Randomized clinical trial. J. Dent. 93, 103277. https://doi.org/10.1016/j.jdent.2020.103277 (2020).
Acknowledgements
We would like to acknowledge the technical support from Ms. Ines Badertscher and Ms. Bernadette Rawyler, co-workers of the Dentistry Faculty (zmk) of the University of Bern, for their assistance in the preparation of the schemas and graphical images of this study. Moreover the authors would like to thank the Central Analítica (UFC/CT-INFRA/MCTI-SISNANO/Pró-Equipamentos CAPES) for the technical support and SEM analyses.
Author information
Authors and Affiliations
Contributions
M.E.O and J.P.M.L. developed the study design and methodology, T.M.A.Z.C.R., A.R.R.F, and C.N.N.T. conducted the experiments and acquired data, G.C., R.J.W., H.M.L., M.E.O., V.F.P., J.S.M. and J.P.M.L. analysed the data, M.E.O., G.C., J.S.M., V.F.P., J.P.M.L. were involved in interpretation of the data, M.E.O and J.P.M.L. wrote the paper and all authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
HML is appointed as inventors US and European patents for an infiltration technique for dental caries lesions, held by Charité Universitätsmedizin Berlin, and receive royalties from DMG. All other authors declare no competing financial and non-financial interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esteves-Oliveira, M., Passos, V.F., Russi, T.M.A.Z.C. et al. Randomized in situ evaluation of surface polishing protocols on the caries-protective effect of resin Infiltrant. Sci Rep 12, 20648 (2022). https://doi.org/10.1038/s41598-022-25091-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-25091-8
This article is cited by
-
Evaluation of experimental resin infiltrant containing nanohydroxyapatite on color stability and microhardness in demineralized enamel
Clinical Oral Investigations (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.