Abstract
Despite increasing knowledge about the factors involved in the progression of diabetic complications, diabetic kidney disease (DKD) continues to be a major health burden. Current therapies only slow but do not prevent the progression of DKD. Thus, there is an urgent need to develop novel therapy to halt the progression of DKD and improve disease prognosis. In our preclinical study where we administered a histone deacetylase (HDAC) inhibitor, valproic acid, to streptozotocin-induced diabetic mice, albuminuria and glomerulosclerosis were attenuated. Furthermore, we discovered that valproic acid attenuated diabetes-induced upregulation of complement C5a receptors, with a concomitant reduction in markers of cellular senescence and senescence-associated secretory phenotype. Interestingly, further examination of mice lacking the C5a receptor 1 (C5aR1) gene revealed that cellular senescence was attenuated in diabetes. Similar results were observed in diabetic mice treated with a C5aR1 inhibitor, PMX53. RNA-sequencing analyses showed that PMX53 significantly regulated genes associated with cell cycle pathways leading to cellular senescence. Collectively, these results for the first time demonstrated that complement C5a mediates cellular senescence in diabetic kidney disease. Cellular senescence has been implicated in the pathogenesis of diabetic kidney disease, thus therapies to inhibit cellular senescence such as complement inhibitors present as a novel therapeutic option to treat diabetic kidney disease.
Similar content being viewed by others
Introduction
The International Diabetes Federation estimated that 537 million adults, or 1 in 10 people, are living with diabetes in 20211. Chronic kidney disease associated with diabetes, also known as diabetic kidney disease (DKD), is the leading cause of end stage renal disease (ESRD) in many developed countries2. It is estimated that 30% of patients with type 1 diabetes mellitus and 40% of patients with type 2 diabetes mellitus will develop DKD3. Current treatments for DKD focuses on anti-hypertensive therapies including angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptors blockers (ARBs), strict glycemic control with anti-diabetic agents such a metformin and more recently, sodium-glucose cotransporter 2 (SGLT2) inhibitors4,5. Nonetheless, the majority of these therapies delay, but do not reduce the risk of progression to DKD3. Therefore, there remains a critical need to identify new therapeutic targets to treat and prevent the development of DKD.
In the search for novel therapies for diabetic kidney disease, there is also interest in drug repurposing opportunities, including the study of histone deacetylase (HDAC) inhibitors6. One of the HDAC inhibitors, valproic acid has shown promising renoprotective effects in preclinical studies of diabetic kidney disease7,8. Valproic acid has been shown to induce histone acetylation and modulate DNA and histone methylation status, thus altering the expression of transcription factors leading to regulation of gene expression9.More recently, an in vitro study has shown that valproic acid can attenuate hyperglycemia-induced activation of complement cascade genes in HepG2 human hepatocytes10. However, whether valproic acid attenuates complement cascade genes in an in vivo setting is unknown. This study aims to investigate if valproic acid mediates its renoprotective effects via the inhibition of the complement cascade in streptozotocin-induced diabetic mice.
Complement is an integral part of the innate immune system. Our group has recently identified complement C5a acting via its receptor, C5aR1, is a key pathogenic driver of DKD11. Importantly, this pathway is not targeted by conventional therapy for DKD, as individuals with diabetes receiving ARBs still exhibited high levels of the circulating complement endproduct, C5a11. We have recently reported that C5a-C5aR1 signaling induces changes in mitochondrial agility in DKD, specifically through changes in cardiolipin remodeling, mitochondrial metabolite flux, and mitochondrial respiratory function11. While the precise mechanisms of how C5a and its receptors mediate renal injury in diabetes are only starting to be unraveled12, early evidence suggests that it is an attractive therapeutic target11,13,14.
Recently, a study demonstrated that C5a, induces tubular senescence and the development of senescence associated secretory phenotype (SASP) in renal tubular epithelial cells (RTEC) after renal ischaemia reperfusion injury15. This is partly due to aberrant DNA methylation induced by C5a in the RTEC, particularly in regions involved in cell cycle control and DNA damage15. There is increasing evidence to support a pathogenic role for senescent renal cells in inducing SASP in DKD16. However, whether C5a is involved in inducing cellular senescence and SASP in DKD is unclear. Thus, we sought to investigate whether C5a plays a role in inducing cellular senescence in our preclinical models of DKD.
Results
Valproic acid improves glycemic control in type 1 diabetes
As expected, streptozotocin-induced diabetic mice displayed a significant increase in blood glucose (Fig. 1A) and HbA1c (Fig. 1B). Importantly, valproic acid significantly reduced HbA1c in diabetic mice suggesting an improvement in glycemic control (Fig. 1B). There was a significant decrease in body weight in streptozotocin-induced diabetic mice (Fig. 1C) and a significant increase in kidney to body weight ratio (Fig. 1D) suggesting renal hypertrophy, however these parameters were not affected by valproic acid treatment. Liver weight was increased in diabetes irrespective of treatment (Fig. 1E). There was a reduction in 24-h urine output (Fig. 1F) and water intake (Fig. 1G) in valproic acid-treated diabetic mice when compared to vehicle, which coincided with diabetes status. Valproic acid treatment did not affect food intake in the diabetic mice (Fig. 1H).
VPA improves glycemic control in STZ mice. (A) Blood glucose, (B) HbA1c, (C) body weight, (D) kidney to body weight ratio, (E) liver weight to body weight ratio, (F) urine output, (G) water intake and (H) food intake were measured in diabetic mice treated with VPA. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs Con; #P < 0.05 vs Diab. Data are presented as mean ± SEM, n = 7–9 mice per group.
Valproic acid attenuates diabetes-induced albuminuria and renal injury
Valproic acid has previously been shown to be renoprotective in streptozotocin-induced diabetic rats7,17. In the current study, 10 weeks after streptozotocin treatment, there was a significant increase in albuminuria (Fig. 2A), tubular injury marker, KIM-1 (Fig. 2B), and glomerulosclerosis (Fig. 2C,D). Treatment of diabetic mice with valproic acid for 8 weeks significantly attenuated these renal injury parameters (Fig. 2).
VPA treatment attenuates renal injury in diabetes. Albuminuria (A), tubular injury marker, KIM-1 (B) and glomerulosclerosis marker, GSI (C) were attenuated by 8 weeks of VPA treatment. (D) Representative micrographs of PAS-stained glomeruli. Original magnification × 400, scale bar = 25 µm. *P < 0.05, **P < 0.01, ****P < 0.0001 vs Con; #P < 0.05, ##P < 0.05 vs Diab. Data are presented as mean ± SEM, n = 7–9 mice per group.
Valproic acid reduces complement C5a receptors in streptozotocin-induced type 1 diabetes
There was a reduction in circulating C5a in diabetes and this was restored by valproic acid (Fig. 3A), while no significant changes were observed in urinary C5a levels across all treatment groups (Fig. 3B). Unlike our previous long term diabetes study which showed an upregulation of C3 expression in the renal cortex at 24 weeks of diabetes duration11, C3 was significantly downregulated 10 weeks after streptozotocin treatment (Fig. 3C). Valproic acid treatment did not result in any changes in C3 expression in the diabetic kidney. However, valproic acid inhibited the diabetes-induced upregulation of C5a receptor gene expression (Fig. 3D,E) in the renal cortex, although the reduction in C5ar1 did not reach statistical significance (P = 0.0822). Importantly, a reduction in circulating C5a but an increased in C5a receptors in the kidneys suggest a local kidney activation of this terminal pathway of the complement cascade at 10 weeks post diabetes induction.
Effects of VPA treatment on Complement in diabetes. (A) Plasma and (B) urine C5a were measured using ELISA. Renal cortical C3 (C), C5ar1 (D) and C5ar2 (E) were measured by RT-qPCR. ***P < 0.001, ****P < 0.0001 vs Con; #P < 0.05, ##P < 0.051vs Diab. Data are presented as mean ± SEM, n = 7–9 mice per group.
Valproic acid inhibits tubular senescence in early DKD
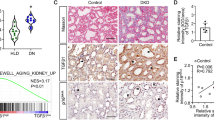
Interestingly, we found that valproic acid had an effect on diabetes-induced cellular senescence. p21waf1/cip1 or p21 is a cyclin-dependent kinase inhibitor that mediates p53-dependent cell cycle arrest in senescent cells18. Treatment of valproic acid for 8 weeks significantly attenuated diabetes-induced expression of p21 (Fig. 4A) and the SASP markers Il-6, TNF-α, EGFR and Fn (Fig. 4A). However, Klotho expression was not altered in the mice after 10 weeks of diabetes (Fig. 4A). Furthermore, we found the levels of p21 to be significantly increased in tubular and interstitial cells in the renal cortex of diabetic mice when compared to nondiabetic controls (Fig. 4B) and this was attenuated by valproic acid (Fig. 4C). Collectively, these results indicate that valproic acid attenuated diabetes-induced cellular senescence.
VPA attenuates diabetes-induced cellular senescence in the kidney cortex. (A) Senescence markers and SASP factors were measured by RT-qPCR. (B) p21 was measured by immunohistochemistry. (C) Representative micrographs of p21 immunostaining. Original magnification × 200, scale bar = 100 µm. ***P < 0.001, ****P < 0.0001 vs Con; #P < 0.05, ##P < 0.01 vs Diab. Data are presented as mean ± SEM, n = 7–9 mice per group.
Inhibition of C5aR1 attenuates senescence and SASP in the kidney
In order to investigate if the effects of valproic acid on cellular senescence are via activation of complement receptors, we examined cellular senescence markers in a preclinical model of DKD where C5aR1 was either genetically deleted or inhibited with a pharmacological antagonist. We have previously shown that inhibition of C5aR1 with a pharmacological inhibitor, PMX53, or genetic deletion of the C5ar1 gene attenuated the development of albuminuria, renal injury and inflammation in chemical-induced diabetes (streptozotocin-induced diabetic mice) after 24 weeks of diabetes11. PMX53 is a highly specific, orally active cyclic peptide antagonist of C5aR1 and does not bind to or inhibit the other C5a receptor, C5aR219. Here, we studied whether inhibition of C5aR1 affected cellular senescence in DKD. SASP marker, IL-6, was increased in renal cortex of mice with diabetes (Fig. 5A,F) and its gene expression was significantly decreased by the genetic deletion of C5aR1, in C5aR1 knockout (KO) mice (Fig. 5A,F). We found that renal cortical Klotho expression is significantly downregulated in diabetes and its expression was restored by the deletion of C5aR1 (Fig. 5B,G). Protein expression of p21 in the renal cortex was attenuated by PMX53 (Fig. 5C,E), and, in the absence of C5ar1 expression (Fig. 5H,J). Collectively, these results suggest a role for C5aR1 in mediating tubular senescence in DKD. Importantly, the levels of p21 were positively correlated with albuminuria in these mice (Fig. 5D,I), further supporting the notion that diabetes-induced senescence may play a role in mediating renal injury in DKD.
Inhibition of C5aR1 attenuates cellular senescence and SASP in DKD. In C5ar1−/− mice, SASP cytokine IL-6 (A) was reduced and diabetes-induced loss of Klotho was restored (B). Senescence marker, p21 immunostaining was increased in renal tubular and tubulointerstitial cells (arrows) in diabetes and attenuated in C5ar1−/− mice in the kidney cortex (C,E). Levels of p21 in these mice were correlated with albuminuria (D). Similarly, inhibition of C5aR1 by PMX53 in diabetic mice attenuated IL-6 (F) and loss of Klotho (G) in the kidney cortex. Immunostaining of p21 (H,J) was increased in diabetes and reduced by PMX53 and was significantly correlated with albuminuria (I). Original magnification × 200, scale bar = 100 µm. **P < 0.01, ***P < 0.001, ****P < 0.0001 vs Con; #P < 0.05, ##P < 0.01, ####P < 0.0001 vs Diab. Data are presented as mean ± SEM, n = 4–12 mice per group.
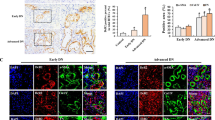
Transcriptomic pathway analysis reveals changes in cell cycle and senescence pathways
We performed whole kidney cortex transcriptomic analysis using RNA-sequencing in diabetic mice treated with PMX53 after 24 weeks of diabetes. Reactome gene set analysis identified that cell cycle and senescence networks were modified by PMX53 treatment (Fig. 6A). Specifically, pathways associated with TP53-regulated transcription of cell cycle genes were upregulated in diabetes and downregulated by PMX53 (Fig. 6A). TP53 encodes for p53 which is a transcription factor integral in regulating genes involved in metabolism, autophagy, DNA damage repair, cell cycle arrest, senescence, and apoptosis20. One of the most important roles for p53 is the induction of cell cycle arrest following DNA damage through p21, leading to cellular senescence21. Rank-rank density plot analysis also showed differential genes associated with cell cycle checkpoint (Fig. 6B), TP53 regulates transcription of G2 cell cycle arrest genes (Fig. 6C) and senescence-associated secretory phenotype (Fig. 6D). These analyses revealed that a significant proportion of genes associated with these pathways were upregulated in diabetes and downregulated by PMX53, further supporting the involvement of C5aR1 in mediating diabetes-induced cell cycle arrest and senescence.
RNA sequencing analysis reveal a role for C5aR1 in modulating cellular senescence in the diabetic kidney. (A) Heatmap of the most significant differentially expressed genes in cell cycle and senescence pathways. Rank-rank plots of gene expression changes in (B) cell cycle checkpoints, (C) TP53 regulates transcription of G2 cell cycle arret genes and (D) senescence-associated secretory phenotype. Both heatmap and rank-rank plots are produced by Mitch in R (https://bioconductor.org/packages/mitch/).
Discussion
In this study, we have shown that valproic acid is renoprotective in a short-term mouse model of streptozotocin-induced diabetes. Furthermore, valproic acid attenuated diabetes-induced upregulation of C5ar1 and C5ar2 expression, concomitant with a downregulation of cellular senescence markers. To investigate if C5a is involved in mediating cellular senescence, we looked at cellular senescence markers in our previous long term diabetes models and we found that inhibition of C5aR1 attenuated diabetes-induced cellular senescence in DKD. These studies thus show for the first time that C5a plays a role in mediating cellular senescence in DKD and further validate complement as a novel therapeutic target for DKD.
Valproic acid is the most frequently prescribed antiepileptic drug and has been successfully used for other indications including bipolar disorder, migraine headache, and diabetic neuropathy-related pain. Although first prescribed almost 60 years ago as an epilepsy treatment, its mechanism of action is still unclear. The most commonly proposed mechanisms are by acting on γ aminobutyric acid (GABA) levels in the central nervous system, blocking voltage-gated ion channels, and inhibiting HDAC22. It is through its inhibitory action on HDAC that valproic acid was discovered to be antidiabetic23,24 and renoprotective in DKD7,8. Valproic acid inhibits HDACs categorized as class Ia (HDAC1 and HDAC2), class Ib (HDAC3), class Ic (HDAC8), and class IIa (HDAC4, HDAC5, and HDAC7), leading to an increase in the acetylation of histones H2, H3, and H4, which modulates the expression of associated genes25. More recently, we have shown valproic acid was able to attenuate hyperglycemia-induced activation of complement genes in hepatocytes10. In this study, we found that valproic acid also attenuated diabetes-induced upregulation of complement genes, such as C5aR1 and C5aR2, in the diabetic kidney, suggesting a new pathway regulated by valproic acid. Interestingly, in this short-term diabetes study, the majority of complement genes were downregulated as opposed to what we observed in a longer-term diabetes study of 24 weeks after STZ11. Nonetheless, expression of the receptor C5ar2 was upregulated. Furthermore, valproic acid treatment attenuated diabetes-induced upregulation of C5ar2. While we have previously demonstrated a pathological role for C5aR1 in DKD, the role of C5aR2 is currently unknown. Thus, whether the inhibition of C5aR2 by valproic acid contributes to a functional phenotype requires further investigation.
Valproic acid is thought to exhibit potent antitumor properties by modulating multiple pathways including cell cycle arrest, apoptosis and senescence26. In our study, we have shown that valproic acid downregulates markers of cellular senescence and SASP. However, these effects are likely cell type specific and may depend on the level of differentiation and the underlying genetic alterations26. Indeed, valproic acid has been shown to exhibit both anti-senescence27 and pro-senescence properties28,29. Nonetheless, we have shown that valproic acid exerts its renoprotective effects in our short-term diabetes study possibly by modulating complement genes and attenuating cellular senescence.
Diabetes is associated with senescence in a “malignant” positive feedback where metabolic dysfunction in prediabetes leads to cellular senescence and the accumulation of senescent cells contributes to further metabolic dysfunction, inflammation and tissue injury30. Accumulating evidence suggests that premature aging of the kidney occurs in DKD. Verzola and colleagues demonstrated that biopsies from patients with DKD showed marked increase of senescent cell markers, p16INK4A and SA-β-Gal, mostly in the tubule cells and to a lesser extent, podocytes compared with age-matched controls, indicating that an age effect could not be responsible for the findings31. Furthermore, accelerated senescence was already observed in proteinuric patients with normal/subnormal eGFR, suggesting that cellular senescence is an early occurrence in disease progression. In our study, senescent marker, p21, is increased in the kidney cortex 10 weeks after diabetes induction, where mice only exhibit mild albuminuria and glomerulosclerosis. Furthermore, previous animal studies have shown the appearance of senescent cells as early as 10 days in rats and 4 weeks in C57BL/6J mice after streptozotocin treatment32,33. Collectively, these findings suggest that intervention targeting senescent cells prior to the development of DKD might be preventative.
A state of chronic, sterile, low grade inflammation, termed inflammaging has been associated with chronic metabolic diseases, such as type 2 diabetes (T2D)34. It is thought that cellular senescence and SASP are central players in inflammaging in diabetes and its complications35. The link between inflammaging and the complement system has recently been explored in the context of acute kidney injury and chronic graft damage36. We and others have previously demonstrated a link between complement-induced inflammation and the pathogenesis and progression of DKD37,38. However, the role of the complement system in inflammaging and senescence in DKD is less well known. Previous studies have reported a role for C5a in cellular senescence15,39. Castellano and colleagues first reported that C5a induced cellular senescence through epigenetic modifications in renal tubular epithelial cells (RTEC)15. Hypomethylation of regions involved in aging pathways such as Wnt/βcatenin led to an upregulation of senescence marker SA-β Gal and cell cycle arrest markers, p53 and p2115. In this study, we showed for the first time that complement C5a is able to induce cellular senescence in DKD. Inhibition of C5aR1 led to an attenuation of senescence markers p21 and SASP cytokine IL-6 in the kidney cortex of diabetic mice. Furthermore, using RNA-Seq transcriptomic analyses, we demonstrated that inhibition of C5aR1 in diabetes rewired the cellular senescence network dampening signals associated with cell cycle checkpoints, TP53-regulated cell cycle and cell death pathway and SASP. In addition, there was a clear positive correlation between p21 in the kidney cortex and albuminuria, suggesting a functional role for C5a-induced senescence in DKD.
Klotho is an anti-aging gene that is expressed primarily in the distal collecting duct and proximal tubules of the kidney40. The decline and/or loss of klotho has been associated with human chronic kidney disease, with the levels of klotho positively correlated with eGFR41. Furthermore, decreased plasma klotho is a predictive marker for the progression of type 2 diabetic kidney disease42. Klotho is a pleiotropic protein that exhibits multifaceted functions related to kidney diseases. Most notably, it inhibits cellular senescence by suppressing Wnt signaling, which is a prominent inducer of cellular senescence43. Although research on the functions of klotho in chronic kidney diseases have gained considerable ground in the last decades, much is still unknown of the regulation of its expression, release, and metabolism. More recently, complement, specifically C5a, is thought to regulate klotho in renal IRI44. Stimulation of renal proximal tubular epithelial cells with C5a induced a marked reduction in Klotho through an NFκB-dependent mechanism44. In our study, loss of Klotho in the diabetic setting was restored in mice lacking C5aR1 expression or function in our long-term diabetes model of 24 weeks. This restoration of Klotho expression was associated with an inhibition of cellular senescence and improvement of kidney function in diabetic mice, suggesting a role of complement in the regulation of klotho in chronic kidney disease, similar to what is observed in acute kidney injury. However, it should be noted that Klotho expression was not changed in our short-term diabetes model of 10 weeks, suggesting that the loss of Klotho occurs later in DKD disease progression.
Limitations of the present study include not including a valproic acid-treated non-diabetic control group. However, our previous study has shown that valproic acid does not cause adverse effects in hepatocytes cultured in normal glucose and valproic acid did not alter the expression of complement genes in the control group10. Furthermore, previous studies have shown no adverse renal effects of valproic acid in nondiabetic rats and mice8,17.
In conclusion, we have shown for the first time that complement C5a modulates cellular senescence in DKD via its receptor C5aR1. Although the precise mechanisms are yet to be determined, C5a regulation of the anti-senescence gene, Klotho appear to be of significance. This area of research is of clinical significance since the current preferred treatments for DKD including renin-angiotensin system inhibitors and SGLT2 inhibitors, have been shown not to target the complement cascade11 or cellular senescence45. We have shown that the HDAC inhibitor, valproic acid, modulates complement cascade genes which partly contributes to its renoprotective effects in DKD. However, mechanisms of valproic acid is complex and is associated with many side effects including gastrointestinal discomfort, weight gain, tremor, hair loss, thrombocytopenia, infertility, and teratogenicity24. Although these side effects can be managed on a patient-to-patient basis, therapy to directly inhibit complement activation may present as a more attractive option. Importantly, C5aR1 inhibitors such as PMX53 have been proven safe and well-tolerated in Phase I clinical trials24. Increasing evidence indicates a pivotal role for complement in the pathogenesis of DKD. With the more recent discovery of the epigenetic role for C5a in cellular senescence and its role in mediating kidney injury, inhibition of C5a has once again proven to be an important therapeutic target for DKD.
Methods
Animal experiments
All animal experiments were approved by the Alfred Research Alliance Animal Ethics Committee (Ethics approval numbers: E/0926/2010/B and E/1870/2018/M) and performed in accordance with guidelines from the National Health and Medical Research Council of Australia. All rodents were housed in a temperature-controlled environment, with a 12-h light/12-h dark cycle, and had access to chow (Specialty Feeds, Perth, Western Australia, Australia) and water ad libitum. C57BL/6J and C5ar1−/− mice backcrossed onto a C57BL/6J background (gift from Professor Rick Wetsel, University of Texas) were bred at Alfred Research Alliance Animal Services. Diabetes was induced in 6-week-old mice by five daily intraperitoneal injections of low-dose streptozotocin (STZ; 55 mg/kg) (Sigma-Aldrich, St. Louis, MO). Mice in the nondiabetic group were given 0.5 M sodium citrate. For the knockout study, diabetic and nondiabetic WT mice (n = 7–10) and C5ar1−/− mice (n = 4–12) were followed for 24 weeks after the onset of diabetes. For the PMX53 study, diabetic and nondiabetic mice were randomized to receive either 1) the C5aR1 peptide inhibitor PMX53 (Ac-Phe-[Orn-ProdCha-Trp-Arg]); synthesized as previously described46 at 2 mg/kg body weight, administered in the drinking water, or 2) drinking water alone (n = 6–12 mice). These mice were followed for 24 weeks. For the VPA study, diabetic mice were randomized to receive VPA at 150 mg/kg body weight daily via oral gavage (n = 7) two weeks after STZ treatment, and the vehicle group was given water (n = 8). Nondiabetic controls were given water via oral gavage daily (n = 9). These mice were followed for 8 weeks. At the end of the study, plasma and kidneys were collected for analysis. The study is reported in accordance with ARRIVE guidelines.
Assessment of renal function and metabolic parameters
Mice were housed singly in metabolic cages a week before the end of the study to collect 24-h urine and food and water intake recorded. Renal function and metabolic parameters for C5ar1−/− and PMX53 studies have been previously published11. For the VPA study, plasma glucose was measured using a glucose colorimetric assay kit (Cayman Chemical). Glycated hemoglobin was measured using a Cobas Integra 400 Autoanalyzer (Roche Diagnostics Corp.). Urinary albumin was measured using a mouse albumin ELISA kit (Bethyl Laboratories, Montgomery, TX). Urinary and plasma C5a was measured using a mouse Complement Component C5a ELISA kit (RayBiotech) and urinary KIM-1 was measured using a mouse KIM-1 ELISA kit (Cloud-clone Corp).
Renal histology
Paraffin-embedded kidney sections (3 μm thick) were stained with periodic acid Schiff (PAS) and scored for glomerulosclerotic index (GSI) as previously described11.
Immunohistochemistry
Paraffin sections of mouse kidney (4 mm thick) were immunostained for p21 (Abcam). Briefly, endogenous peroxidases were blocked with 3% hydrogen peroxide for 15 min and incubated in 0.5% skim milk/Tris-buffered saline for 1 h at room temperature. The primary antibody was left on overnight at 4 °C. This was followed by incubation with biotinylated secondary antibody at room temperature for 10 min. Sections were then incubated with Vectastain ABC reagent (Vector Laboratories). Peroxidase activity was identified by reaction with 3,39-diaminobenzidine tetrahydrochloride (Sigma-Aldrich Pty. Ltd.). Sections were counterstained with hematoxylin. All sections were examined under an Olympus BX-50 light microscope (Olympus Optical) and digitized with a high-resolution camera. All digital quantitation (Image-Pro Plus software version 6.0) and assessments were performed in a blinded manner.
Quantitative real-time RT-PCR
RNA from renal cortex was extracted using TRIzol Reagent, and cDNA was synthesized as described previously47. Gene expression was determined using a 7500 Fast Real-Time PCR System (Applied Biosystems, Victoria, Australia). Gene expression was normalized relative to 18S rRNA, and the relative fold difference in expression was calculated using the comparative 2-ΔΔCt method.
RNA sequencing and analysis
Previously described kidney RNA-Seq data from our laboratory (Tan et al.11), was downloaded from the NCBI GEO database under accession number GSE118089 (September 2021). Two contrasts were examined (i) control vehicle-treated compared to diabetic vehicle-treated and (ii) diabetic vehicle-treated compared to diabetic PMX53-treated. All sample groups contained n = 6 replicates. Genes with fewer than 10 reads per gene were omitted from downstream analysis. Differential expression analysis was conducted with DESeq2 (v1.32.0)48. Integrative pathway analysis was conducted using the Mitch package (v1.4.1; https://bioconductor.org/packages/mitch/)49 with default settings and gene sets downloaded from REACTOME on the 22nd September50. To focus on senescence-related pathways, we selected gene sets relevant to cell cycle, senescence and performed mitch once again.
Statistical analysis
The data are expressed as mean ± SEM. Statistical analyses were performed using GraphPad Prism software version 8.0 (GraphPad Software). All data were analyzed with one-way ANOVA with the Tukey post hoc test, unless otherwise stated. A P value < 0.05 was considered statistically significant. All figure panels were made in GraphPad Prisms software version 8.0.
Data availability
Sequence data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus with accession number GSE118089.
References
International Diabetes Federation. IDF Diabetes Atlas, 10th edn, https://www.diabetesatlas.org (2021).
Koye, D. N., Magliano, D. J., Nelson, R. G. & Pavkov, M. E. The global epidemiology of diabetes and kidney disease. Adv. Chronic Kidney Dis. 25, 121–132. https://doi.org/10.1053/j.ackd.2017.10.011 (2018).
Alicic, R. Z., Rooney, M. T. & Tuttle, K. R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. CJASN 12, 2032–2045. https://doi.org/10.2215/cjn.11491116 (2017).
DeFronzo, R. A., Reeves, W. B. & Awad, A. S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 17, 319–334. https://doi.org/10.1038/s41581-021-00393-8 (2021).
Giorgino, F., Vora, J., Fenici, P. & Solini, A. Renoprotection with SGLT2 inhibitors in type 2 diabetes over a spectrum of cardiovascular and renal risk. Cardiovasc. Diabetol. 19, 196. https://doi.org/10.1186/s12933-020-01163-9 (2020).
Hadden, M. J. & Advani, A. Histone deacetylase inhibitors and diabetic kidney disease. Int. J. Mol. Sci. 19, 2630. https://doi.org/10.3390/ijms19092630 (2018).
Khan, S., Jena, G. & Tikoo, K. Sodium valproate ameliorates diabetes-induced fibrosis and renal damage by the inhibition of histone deacetylases in diabetic rat. Exp. Mol. Pathol. 98, 230–239. https://doi.org/10.1016/j.yexmp.2015.01.003 (2015).
Sun, X. Y. et al. Valproate attenuates diabetic nephropathy through inhibition of endoplasmic reticulum stress-induced apoptosis. Mol. Med. Rep. 13, 661–668. https://doi.org/10.3892/mmr.2015.4580 (2016).
Mello, M. L. S. Sodium valproate-induced chromatin remodeling. Front. Cell Dev. Biol. https://doi.org/10.3389/fcell.2021.645518 (2021).
Felisbino, M. B. et al. Valproic acid influences the expression of genes implicated with hyperglycaemia-induced complement and coagulation pathways. Sci. Rep. 11, 2163 (2021).
Tan, S. M. et al. Complement C5a induces renal injury in diabetic kidney disease by disrupting mitochondrial metabolic agility. Diabetes 69, 83–98. https://doi.org/10.2337/db19-0043 (2020).
Tan, S. M., Snelson, M., Østergaard, J. A. & Coughlan, M. T. The complement pathway: New insights into immunometabolic signalling in diabetic kidney disease. Antioxid. Redox Signal. https://doi.org/10.1089/ars.2021.0125 (2021).
Li, L. et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism 64, 597–610. https://doi.org/10.1016/j.metabol.2015.01.014 (2015).
Yiu, W. H. et al. Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol. Dial. Transplant. 33, 1323–1332. https://doi.org/10.1093/ndt/gfx336 (2018).
Castellano, G. et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury. Aging (Albany NY) 11, 4382–4406 (2019).
Wiley, C. D. Role of senescent renal cells in pathophysiology of diabetic kidney disease. Curr. Diab.Rep. 20, 33. https://doi.org/10.1007/s11892-020-01314-y (2020).
Khan, S., Jena, G., Tikoo, K. & Kumar, V. Valproate attenuates the proteinuria, podocyte and renal injury by facilitating autophagy and inactivation of NF-κB/iNOS signaling in diabetic rat. Biochimie 110, 1–16. https://doi.org/10.1016/j.biochi.2014.12.015 (2015).
Shtutman, M., Chang, B. D., Schools, G. P. & Broude, E. V. Cellular model of p21-induced senescence. Methods Mol. Biol. 1534, 31–39. https://doi.org/10.1007/978-1-4939-6670-7_3 (2017).
Manthey, H. D., Woodruff, T. M., Taylor, S. M. & Monk, P. N. Complement component 5a (C5a). Int. J. Biochem. Cell Biol. 41, 2114–2117. https://doi.org/10.1016/j.biocel.2009.04.005 (2009).
Mijit, M., Caracciolo, V., Melillo, A., Amicarelli, F. & Giordano, A. Role of p53 in the regulation of cellular senescence. Biomolecules https://doi.org/10.3390/biom10030420 (2020).
Rufini, A., Tucci, P., Celardo, I. & Melino, G. Senescence and aging: The critical roles of p53. Oncogene 32, 5129–5143. https://doi.org/10.1038/onc.2012.640 (2013).
Rahman, M. & Nguyen, H. In StatPearls (StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., 2022).
Khan, S. & Jena, G. Valproic acid improves glucose homeostasis by increasing beta-cell proliferation, function, and reducing its apoptosis through HDAC inhibition in juvenile diabetic rat. J. Biochem. Mol. Toxicol. 30, 438–446. https://doi.org/10.1002/jbt.21807 (2016).
Rakitin, A. Does valproic acid have potential in the treatment of diabetes mellitus?. Front. Endocrinol. 8, 147. https://doi.org/10.3389/fendo.2017.00147 (2017).
Soria-Castro, R. et al. Exploring the drug repurposing versatility of valproic acid as a multifunctional regulator of innate and adaptive immune cells. J. Immunol. Res. 2019, 9678098. https://doi.org/10.1155/2019/9678098 (2019).
Duenas-Gonzalez, A. et al. Valproic acid as epigenetic cancer drug: Preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat. Rev. 34, 206–222. https://doi.org/10.1016/j.ctrv.2007.11.003 (2008).
Zhai, Y. et al. Histone deacetylase inhibitor valproic acid promotes the induction of pluripotency in mouse fibroblasts by suppressing reprogramming-induced senescence stress. Exp. Cell Res. 337, 61–67. https://doi.org/10.1016/j.yexcr.2015.06.003 (2015).
Li, X. N. et al. Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol. Cancer Ther. 4, 1912–1922. https://doi.org/10.1158/1535-7163.mct-05-0184 (2005).
Elknerova, K. et al. Epigenetic modulation of gene expression of human leukemia cell lines: Induction of cell death and senescence. Neoplasma 58, 35–44. https://doi.org/10.4149/neo_2011_01_35 (2011).
Palmer, A. K. et al. Cellular senescence in type 2 diabetes: A therapeutic opportunity. Diabetes 64, 2289–2298. https://doi.org/10.2337/db14-1820 (2015).
Verzola, D. et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 295, F1563-1573. https://doi.org/10.1152/ajprenal.90302.2008 (2008).
Kitada, K. et al. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J. Diabetes Complic. 28, 604–611. https://doi.org/10.1016/j.jdiacomp.2014.05.010 (2014).
Satriano, J. et al. Transition of kidney tubule cells to a senescent phenotype in early experimental diabetes. Am. J. Physiol. Cell Physiol. 299, C374–C380. https://doi.org/10.1152/ajpcell.00096.2010 (2010).
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C. & Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14, 576–590. https://doi.org/10.1038/s41574-018-0059-4 (2018).
Prattichizzo, F. et al. “Inflammaging” as a druggable target: A senescence-associated secretory phenotype—centered view of type 2 diabetes. Oxid. Med. Cell. Longev. 2016, 1810327. https://doi.org/10.1155/2016/1810327 (2016).
Franzin, R. et al. Inflammaging and complement system: A link between acute kidney injury and chronic graft damage. Front. Immunol. https://doi.org/10.3389/fimmu.2020.00734 (2020).
Tan, S. M., Snelson, M., Østergaard, J. A. & Coughlan, M. T. The complement pathway: New insights into immunometabolic signaling in diabetic kidney disease. Antioxid. Redox Signal. https://doi.org/10.1089/ars.2021.0125 (2022).
Tang, S. C. W. & Yiu, W. H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 16, 206–222. https://doi.org/10.1038/s41581-019-0234-4 (2020).
Wen, Y., Shen, F. & Wu, H. Role of C5a and C5aR in doxorubicin-induced cardiomyocyte senescence. Exp. Ther. Med. 22, 1114. https://doi.org/10.3892/etm.2021.10548 (2021).
Hu, M. C. et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 24, 3438–3450. https://doi.org/10.1096/fj.10-154765 (2010).
Neyra, J. A., Hu, M. C. & Moe, O. W. Klotho in clinical nephrology: Diagnostic and therapeutic implications. Clin. J. Am. Soc. Nephrol. CJASN 16, 162–176. https://doi.org/10.2215/CJN.02840320 (2020).
Kim, S. S. et al. Decreased plasma α-Klotho predict progression of nephropathy with type 2 diabetic patients. J. Diabetes Complic. 30, 887–892. https://doi.org/10.1016/j.jdiacomp.2016.03.006 (2016).
Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 389, 233–241. https://doi.org/10.1515/bc.2008.028 (2008).
Castellano, G. et al. Complement modulation of anti-aging factor klotho in ischemia/reperfusion injury and delayed graft function. Am. J. Transplant. 16, 325–333. https://doi.org/10.1111/ajt.13415 (2016).
Al-Dabet, M. D. M. et al. Reversal of the renal hyperglycemic memory in diabetic kidney disease by targeting sustained tubular p21 expression. Nat. Commun. 13, 5062. https://doi.org/10.1038/s41467-022-32477-9 (2022).
Li, X. X. et al. Pharmacological characterisation of small molecule C5aR1 inhibitors in human cells reveals biased activities for signalling and function. Biochem. Pharmacol. 180, 114156. https://doi.org/10.1016/j.bcp.2020.114156 (2020).
Tan, S. M. et al. The modified selenenyl amide, M-hydroxy ebselen, attenuates diabetic nephropathy and diabetes-associated atherosclerosis in ApoE/GPx1 double knockout mice. PLoS ONE 8, e69193. https://doi.org/10.1371/journal.pone.0069193 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. https://doi.org/10.1186/s13059-014-0550-8 (2014).
Kaspi, A. & Ziemann, M. mitch: Multi-contrast pathway enrichment for multi-omics and single-cell profiling data. BMC Genom. 21, 447. https://doi.org/10.1186/s12864-020-06856-9 (2020).
Jassal, B. et al. The reactome pathway knowledgebase. Nucleic Acids Res. 48, D498–D503. https://doi.org/10.1093/nar/gkz1031 (2019).
Acknowledgements
The authors thank the following people for providing technical assistance: Matthew Snelson, Megan Lyttle, Dilan Karagoz and Maryann Arnstein. The authors acknowledge the use of facilities at Monash Histology Platform. MTC was supported by a Career Development Award from JDRF Australia, the recipient of the Australian Research Council Special Research Initiative in Type 1 Juvenile Diabetes. SMT was supported by a JDRF Advanced Postdoctoral Fellowship.
Author information
Authors and Affiliations
Contributions
M.T.C. designed the study, interpreted the data and revised the manuscript. M.Z. conceptualized the study; acquired, analyzed, and interpreted the data and revised the manuscript; A.L. acquired and analyzed the data. T.M.W. interpreted the data and revised the manuscript. S.M.T. conceptualized and designed the study; acquired, analyzed and interpreted the data; drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coughlan, M.T., Ziemann, M., Laskowski, A. et al. Valproic acid attenuates cellular senescence in diabetic kidney disease through the inhibition of complement C5a receptors. Sci Rep 12, 20278 (2022). https://doi.org/10.1038/s41598-022-24851-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24851-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.