Abstract
Genetically tractable animal models provide needed strategies to resolve the biological basis of drug addiction. Intravenous self-administration (IVSA) is the gold standard for modeling psychostimulant and opioid addiction in animals, but technical limitations have precluded the widespread use of IVSA in mice. Here, we describe IVSA paradigms for mice that capture the multi-stage nature of the disorder and permit predictive modeling. In these paradigms, C57BL/6J mice with long-standing indwelling jugular catheters engaged in cocaine- or remifentanil-associated lever responding that was fixed ratio-dependent, dose-dependent, extinguished by withholding the drug, and reinstated by the presentation of drug-paired cues. The application of multivariate analysis suggested that drug taking in both paradigms was a function of two latent variables we termed incentive motivation and discriminative control. Machine learning revealed that vulnerability to drug seeking and relapse were predicted by a mouse’s a priori response to novelty, sensitivity to drug-induced locomotion, and drug-taking behavior. The application of these behavioral and statistical-analysis approaches to genetically-engineered mice will facilitate the identification of neural circuits driving addiction susceptibility and relapse and focused therapeutic development.
Similar content being viewed by others
Introduction
Drug overdose is the leading cause of injury-related mortality in the U.S., claiming more than 70,000 lives in 2017 alone1 and costing an estimated $78.5 billion per year in healthcare, lost productivity, and criminal justice involvement2. While synthetic opioids, including fentanyl and its derivatives, are responsible for the spike in overdose deaths in recent years, mortality associated with cocaine and methamphetamine use disorders also tripled between 2012 and 20163. Available therapies for opioid use disorders are inadequate, and there are no Food and Drug Administration (FDA)-approved therapies for the treatment of stimulant use disorders. This paucity of treatments persists despite a medical consensus that opioid and stimulant addictions are brain disorders and, as such, should be amenable to pharmacological interventions4. The lack of effective therapeutics may be due to chemical addictions being a family of disorders whose multiple etiologies remain ill-defined. An understanding of the genetic and molecular determinants of addiction-associated behaviors will facilitate the identification of therapeutic targets and at-risk individuals.
Animal studies permit levels of control and manipulations that are unfeasible with human subjects. Multiple paradigms, with varying levels of complexity, exist for modeling the effects of drug use in animals. Intravenous self-administration (IVSA) with operant conditioning is the “gold standard” for studying the effects of stimulants (e.g., cocaine, methamphetamine) and opioids (e.g., morphine, heroin, fentanyl) in animals. Pioneered in non-human primate and rat studies5,6,7,8,9, this approach has proven to be the most translationally-relevant addiction model, having greater construct validity than locomotor and place preference protocols and demonstrated predictive validity10,11,12.
The vast majority of IVSA studies conducted over the past 50 years have continued to use non-human primates and rats. Far fewer studies have utilized mice. A systematic review of the cocaine and opioid mouse IVSA literature from the last 5 years revealed an average of only 7 studies published per year using some form of this approach (Supplemental Table S1). Compared to other mammalian species, the mouse genome is the most amenable to genetic engineering. As such, many genetic approaches, including global, conditional, inducible, and conditional-inducible transgenic strategies are available in mice. Other techniques that are well-developed in mice, but not other mammals, include optogenetic and chemogenetic approaches; strategies that permit the direct, real-time assessment of specific cell types and neural circuits as they relate to behavior.
The relative paucity of mouse IVSA studies to date can be attributed to the technical challenges of applying this model to mice13. Recent advances in materials, surgical techniques, and operant protocols have, however, led to unprecedented success in mouse self-administration approaches. While studies evaluating cocaine or opioid self-administration in mice are becoming more common, IVSA in mice continues to be limited by small group numbers, low rates of study completion, restricted data collection (e.g., evaluation of a single drug dose on a single delivery schedule), and abbreviated study length14,15,16. Our own review of the literature returned 32 studies in the last 5 years employing cocaine or opioid IVSA in mice (Supplemental Table S114,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47). In the studies examined, fewer than 20% maintained self-administration beyond 30 days and only a single study maintained self-administration beyond 45 days25. Only 40% of the studies used both male and female mice and a single study compared stimulant and opioid self-administration behaviors in parallel paradigms23, albeit in head-restrained mice.
Few studies to date have employed a longitudinal design in which acquisition, maintenance, extinction, and reinstatement of self-administration are assessed over extended periods of time within the same cohort of mice and none have compared cocaine and opioid IVSA behaviors in freely moving mice in analogous longitudinal paradigms. Longitudinal, multi-stage studies afford several advantages over past approaches, including the opportunity to describe the relationship between risk factors (e.g., trait constructs, behavioral signatures in a priori behavioral testing) and the development of addiction-associated behaviors (e.g., drug-taking, drug-seeking). Moreover, because addiction behaviors occur on a chronic scale, with discrete stages associated with distinct neurobiological determinants48, longitudinal studies better approximate the trajectory of clinical illness.
The primary objectives of the present study were two-fold. One goal was to develop multi-stage intravenous (iv) stimulant and opioid self-administration paradigms in mice. A second purpose was to use statistics to evaluate the relationships among observed variables in these paradigms and advance our understanding of their underlying behavioral constructs. To achieve these objectives, we evaluated self-administration of the psychostimulant cocaine and the short-acting opioid remifentanil. Here, we demonstrate that iv cocaine or remifentanil self-administration acquisition, maintenance, extinction, and reinstatement can be studied longitudinally in large cohorts of C57BL/6J mice and provide a detailed description of drug-reinforced behavior in these paradigms. Mice were trained to self-administer cocaine or remifentanil paired with a cue light on fixed ratio (FR) schedules of reinforcement, in which the mouse must press a lever a fixed number of times to receive a drug infusion. This behavior was extinguished by withholding the drug and drug-paired cues, and it was reinstated by the reintroduction of paired cues. Exploratory factor analysis revealed that individual mouse performance during both the cocaine and remifentanil self-administration paradigms was a function of two latent variables that were differentially associated with a priori novelty- and drug-induced behavior in the open field. Application of machine learning approaches allowed us to develop predictive models of drug-seeking and relapse. Critically, our approach to the behavioral and statistical analyses can be readily applied to genetically-engineered mice and adapted for the study of other drugs of abuse under diverse operant protocols.
Results
Jugular catheterization and maintenance of catheter patency
IVSA requires the placement and maintenance of indwelling jugular catheters. In our optimized paradigm, post-surgical survival was high (i.e., 84% on post-surgery day 7; Fig. 1A) and jugular catheter placement was highly effective (i.e., 98% patent on post-surgery day 7; Fig. 1B). Throughout the duration of the study, attrition due to poor health was modest, with more than 70% of animals surviving to study completion or to catheter patency loss (Fig. 1C). The catheters proved to be long-lasting, with a patency half-life of ≥ 100 days (Fig. 1D). These data suggest that, with optimized materials and handling, jugular catheters can be placed reliably and maintained over long periods in mice. The newly established longevity of the animals and the catheters permitted us to adopt a longitudinal design for the study of iv cocaine and remifentanil self-administration acquisition, maintenance intake, extinction, and cue-induced reinstatement in the same mice.
Long-lasting jugular catheters and low attrition permit longitudinal, multi-stage intravenous self-administration in mice. (A) Recovery from jugular catheterization procedure. Percent and number (n) of mice that met survival and health criteria on post-catheterization day 7. (B) Success of jugular catheter placement. Percent and number (n) of all animals that passed a test of catheter patency on post-catheterization day 7. (C) Attrition over the study due to animals meeting designated health endpoints. Animals were removed from the study when they displayed signs of deteriorating health. The median time in the study (i.e., survival) exceeded 100 days. (D) Attrition over the study due to loss of catheter patency. Cather patency was followed throughout the study. The catheters of animals removed from the study for health reasons were considered patent unless they had a documented patency test failure. The median catheter patency life was ≥ 100 days. (E) Overview of study design. Mice with indwelling jugular catheters underwent behavioral testing in the open field and were then trained to self-administer drugs paired with a cue light via lever responding in operant chambers. For the details of the contingent advancement training and testing paradigms, see Figs. S1 and S2.
Acquisition of cocaine and remifentanil self-administration
Following catheter placement and prior to self-administration training, novelty-induced exploratory behaviors and cocaine (20 mg/kg, i.p.)- or remifentanil (0.1, 1 and 10 mg/kg, i.p.)-induced locomotor activities were assessed in the open field (Figs. 1E, S3). Like cocaine (Fig. S3A) and other opioids, remifentanil produced transient dose-dependent increases in locomotor activity, consistent with its short half-life (Fig. S3B). Mean values were within the anticipated ranges for all parameters.
Mice with patent indwelling jugular catheters were trained to self-administer cocaine (0.5 mg/kg/infusion, iv) or remifentanil (0.1 mg/kg/infusion, iv) paired with a cue light by lever responding in operant chambers (Fig. 1E, middle to right). We established contingent advancement protocols (Figs. S1, S2) to address the challenges of behavioral inconsistency and high inter-mouse variability. Our protocols used predominantly short access, 1-h daily sessions to reduce the required chamber time per mouse per day and, thereby, increase the number of animals that could be tested as a cohort. In sequential sessions mice learned to lever press for cocaine, to discriminate an active, drug-delivering lever from an inactive lever, and to press the active lever up to 4 times to receive a single drug infusion.
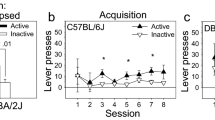
Eighty-four percent and 94% of mice with patent catheters acquired cocaine (0.5 mg/kg/infusion) and remifentanil (0.1 mg/kg/infusion) self-administration, respectively (Fig. 2A,C); this percent did not differ by drug class (Table S2). The mean session number required to meet final acquisition criteria was lower for remifentanil (10.8 ± 0.2 sessions) than for cocaine (13.5 ± 0.6 sessions), although the peaks of the frequency histogram curves were indistinguishable (Fig. 2B,D, Table S2). Notably, variability in this time-to-train measure was significantly lower for remifentanil relative to cocaine [F(36, 32) = 9.077, p < 0.001, F test for variances], suggesting that, at these doses, remifentanil may be the more effective reinforcer.
Acquisition of cocaine and remifentanil self-administration. Mice with indwelling jugular catheters were trained to self-administer iv cocaine (0.5 mg/kg/infusion) or remifentanil (0.1 mg/kg/infusion) by lever responding in operant chambers n FR1, FR2, and FR4 schedules. Group means ± SEM are represented by solid green (cocaine) or purple (remifentanil) lines and individual replicates by dashed lines. Note, solid gray lines represent the vehicle. (A,C) Acquisition success rates. Percent of animals with patent catheters that acquired or failed to acquire iv cocaine (A) or remifentanil (C) self-administration at FR4 using contingent advancement protocols. (B,D) Sessions to self-administration acquisition. Frequency plot of the number of sessions required to meet final cocaine (B) or remifentanil (D) self-administration acquisition criteria at FR4. The minimum session requirements and group means are indicated by dashed vertical lines. (E,F). Lever responses by acquisition session type. Lever responses by mice that acquired cocaine (E) or remifentanil (F) self-administration. Data are presented from the first sessions of each session type, as indicated. Two levers were always available in the chamber. During 2-lever training sessions both levers were active and resulted in a drug delivery. In 1-lever training sessions, one lever was designated active and the other inactive. The non-preferred lever in the final 2 active lever session became the active lever in the first 1-lever training session. The active lever was retained for individual mice for the duration of the study. In FR1 sessions, the mouse had to press an active lever once to receive a drug infusion. In FR2 sessions, the mouse had to press the active lever twice to receive a drug infusion. In FR4 sessions, the mouse had to press the active lever four times to receive a drug infusion. (G,I) Active lever responses. Total active lever presses by mice self-administering cocaine (G) or remifentanil (I) at FR1, FR2, and FR4. Responses include both those that contributed to earned reinforcements and those that occurred during the post-reinforcement time-out period. (H,J) Inactive lever responses. Total inactive lever presses by mice self-administering cocaine (H) or remifentanil (J) at FR1, FR2, and FR4. (K,M) Lever accuracy. Percent of total lever responses that occurred at the designated active lever by mice self-administering cocaine (K) or remifentanil (M) at FR1, FR2, and FR4. (L,N) Reinforcements. Earned reinforcements by mice self-administering cocaine (L) or remifentanil (N) at FR1, FR2, and FR4. For details on statistical comparisons, see Tables S2, S3. For the experimental designs, see Figs. S1, S2.
Because cues themselves can be reinforcing49 and some mice initially display high rates of responding in the absence of a reward50,51, specificity of lever responding for the drug reinforcers was assessed. Like mice in the cocaine and remifentanil reinforcement groups, animals in a vehicle reinforcement group initially engaged in moderate levels of lever responding. However, they failed to develop active versus inactive lever discrimination, and their lever responding was unaffected by increasing the FR schedule (Fig. 2E–F). Together, these data demonstrate that iv cocaine and remifentanil self-administration can be readily and rapidly acquired in short, daily sessions by mice without prior operant training.
Cocaine and remifentanil self-administration are fixed-ratio-dependent
As the response requirement to earn a reinforcement increases, the extent of self-administration decreases. This inverse relationship generalizes across species and drug classes52. As described for rats, non-human primates, and humans, self-administration behaviors in mice changed as the FR schedule was increased. In both drug paradigms, active lever responses increased from FR1 to FR2 and from FR2 to FR4 (Fig. 2G,I). During these transitions, inactive lever responding increased only modestly for cocaine (Fig. 2H) and remained unchanged for remifentanil (Fig. 2J). Consequently, lever discrimination (i.e., accuracy) increased between FR1 and FR4 (Fig. 2K,M). At FR4, 80 ± 2% and 82 ± 2% of lever responses occurred at the active, drug-delivering lever for cocaine and remifentanil, respectively. The increase in active lever responding with the increasing FR schedule was not sufficient to maintain levels of drug intake. The number of self-administered reinforcements of both drugs was reduced at FR4 compared to FR1 and FR2 (Fig. 2L,N). These results demonstrate that the number and percent of active lever responses, and therefore drug intake, is a function of the FR schedule in mice. The number of reinforcements earned per hour and the amount of cocaine or remifentanil intake at FR1 is consistent with published results for mice trained using other paradigms, which have used different acquisition protocols and longer sessions14.
Cocaine and remifentanil self-administration are dose-dependent
Once cocaine or remifentanil self-administration was acquired at FR4 at the training doses, we systematically varied the dose delivered per infusion. We assessed stable lever responding at 5 cocaine doses (0.1, 0.3, 0.5, 1.0, and 3.0 mg/kg/infusion) and 6 remifentanil doses (0.01, 0.03, 0.1, 0.3, 1.0, and 3.0 mg/kg/infusion), permitting the establishment of dose–effect curves. As demonstrated previously in rats and non-human primates9,53,54, there was a clear drug dose-dependency to self-administration responses. For both cocaine and remifentanil, there was a characteristic inverted U-shape relationship52,55 between the earned reinforcements relative to the log drug dose (Fig. 3A,I) and active lever responses relative to log drug dose (Fig. 3B,J). A main effect of sex was not identified in the earned reinforcements dose–response curves for either drug. Nevertheless, a significant dose by sex interaction was identified for remifentanil (Fig. S4). An examination of the male and female remifentanil dose–response curves revealed that this interaction was driven by a selective decrement in self-administration at the lowest 0.01 mg/kg/infusion dose in female mice (Fig. S4B). The cocaine intake versus log cocaine dose data were fit to a sigmoidal curve (Fig. 3D) and the remifentanil intake versus log remifentanil dose data were fit to an exponential growth curve (Fig. 3L). Notably, inactive lever responding for both drugs was also drug dose-dependent, decreasing linearly with the log cocaine or remifentanil dose (Fig. 3C,K). Lever accuracy increased linearly with log drug dose for both drugs (Fig. 3E,M). Two latency measures, latency to initiate lever responding and latency to earn the first reinforcement, are considered indices of urgency or motivation to initiate drug taking56,57. While the latency to the first lever response within a session was unaffected by drug dose (Fig. 3F,N), the latency to the first reward decreased linearly with log drug dose (Fig. 3G,O). The number of lever responses that occurred during the post-reinforcement time-out periods, a parameter that may be related to action impulsivity58, was also drug dose-dependent, as it decreased linearly with the log cocaine and remifentanil dose (Fig. 3H,P). This time-out responding relative to the log drug dose relationship remained significant after normalizing the data to total lever responding, and thus the length of the time-out interval, for cocaine but not for remifentanil (Fig. S5). Total earned reinforcements and active lever responses were higher and the latency to initiate drug taking was lower in the remifentanil paradigm, suggesting that, in these dose ranges, remifentanil may be a stronger reinforcer than cocaine.
Dose–response relationships for cocaine and remifentanil self-administration behaviors. Stable self-administration of cocaine at FR4 was assessed in 60 min sessions at doses of 0.1, 0.3, 0.5, 1.0, and 3.0 mg/kg/infusion. Stable self-administration of remifentanil at FR4 was assessed in 60 min sessions at doses of 0.01, 0.03, 0.1, 0.3, 1.0, and 3.0 mg/kg/infusion. Ranges of axes were selected to emphasize curve fits and differences by drug class. Data are represented as mean ± SEM. (A,I) Reinforcements. Earned reinforcements versus log cocaine (A) or remifentanil (I) dose. Data were fit to a second order polynomial curve. (B,J) Active lever responses. Active lever presses versus log cocaine (B) or remifentanil (J) dose. Responses include both those that contributed to earned reinforcements and those that occurred during the post-reinforcement time-out period. Data were fit to a second order polynomial curve. (C,K) Inactive lever responses. Inactive lever presses versus log cocaine (C) or remifentanil (K) dose. Data were fit to a straight line. (D,L) Drug intake. Cocaine (D) or remifentanil (L) consumed versus log drug dose. Cocaine data were fit to a sigmoidal curve. Remifentanil data were fit to an exponential growth curve. (E,M) Lever accuracy. Percent of total lever responses that occurred at the designated active lever versus log cocaine (E) or remifentanil (M) dose. Data were fit to a straight line. (F,N) Latency to first lever response. Time from the start of the session until the first lever response versus log cocaine (F) or remifentanil (N) dose. Data were fit to a straight line. (G,O) Latency to first earned reinforcement. Time from the start of the session until the first earned reinforcement versus log cocaine (G) or remifentanil (O) dose. Data were fit to a straight line. (H,P) Post-reinforcement time-out responses. Total number of lever responses that occurred during the post-reinforcement time-out periods versus log cocaine (H) or remifentanil (P) dose. Data were fit to a straight line. For information on curve fits, see Table S4. Also see Figs. S4, S5, S6.
To investigate the kinetics of self-administration during the 1-h sessions, we constructed cumulative reinforcement time courses for all drug doses (Fig. S6). These plots were linear for all remifentanil doses and for cocaine up to the 1.0 mg/kg/infusion dose, indicating that self-administration occurred at a constant rate over the course of the sessions. Together, these results demonstrate that mice exhibit dose-dependent self-administration behaviors.
Cocaine- and remifentanil-associated lever responding can be extinguished and reinstated
It has been demonstrated in rats and non-human primates59,60,61, and, more recently, in mice49,62,63,64 that drug-associated lever responding can be extinguished over repeated sessions where no drug is available, drug-associated cues are withheld, and lever responses have no programmed consequences—a process known as extinction65. After mice completed dose–response testing, they were subjected to once daily, 1-h extinction sessions (Figs. S1, S2). In the cocaine paradigm mice were extinguished to a criterion (i.e., ≤ 20% of active lever responses for a 1.0 mg/kg/infusion of cocaine), and in the remifentanil paradigm all mice completed 20 extinction sessions regardless of performance. For both drugs, active lever responding decreased as a function of extinction session number (Fig. 4A,E), with mice requiring 20 ± 1.6 sessions to meet extinction criteria for cocaine. The reduction in total lever responding over the first 20 extinction sessions can be described using a one-phase exponential decay function (Fig. 4B,F). Notably, the rate of extinction was comparable for both drugs. From the final 1.0 mg/kg/infusion self-administration session to the first extinction session, lever responding was increased (Fig. 4A,E) and lever accuracy was reduced (Fig. 4C,G left), suggesting the mice actively seek drug in this session. By the final extinction session, lever discrimination was lost in the cocaine group (Fig. 4C, middle) and was diminished in the remifentanil group (Fig. 4G, middle).
Extinction and cue-induced reinstatement of cocaine- and remifentanil-associated lever responding. In mice trained to self-administer cocaine or remifentanil at FR4, drug-associated lever responding was extinguished in consecutive sessions in which drugs and cues were withheld and subsequently reinstated by the reintroduction of the drug-paired cues. Data are represented as mean ± SEM. (A,E) Active and inactive lever responses during extinction. Active and inactive lever responding is presented by extinction session number for mice in the cocaine (A) and remifentanil (E) paradigms. Lever responding for 1 mg/kg/infusion of drug (i.e., self-administration, SA) is included for reference. (B,F) Total lever responses during extinction. Total lever responding is presented by extinction session number in gray bars for mice in the cocaine (B) and remifentanil (F) paradigms. Colored curves are one-phase exponential decay fits. (C,G) Lever accuracy at maintenance SA to first extinction session, at the first and final extinction sessions, and at the final extinction session and reinstatement. The percent of total lever responses occurring at the active lever is presented by session type for mice in the cocaine (C) and remifentanil (G) paradigms. SA indicates accuracy during the 1.0 mg/kg/infusion drug maintenance active self-administration session. (D,H) Active and inactive lever responses during SA, extinction, and reinstatement. Active and inactive lever responding is presented by session type for mice in the cocaine (D) and remifentanil (H) paradigms. SA indicates lever responding during the 1.0 mg/kg/infusion drug active self-administration session. Group means are represented by colored bars and individual values are shown as gray circles. For curve parameters and details on statistical comparisons, see Table S5.
Presenting drug-experienced animals or humans with paired contextual or environmental cues can reinstate drug seeking66, a phenomenon known clinically as relapse. In mice that completed extinction, re-introduction of drug-associated cues, including the cue light, syringe pump sounds, and lever movements, increased responding at the previously active lever and reinstated lever discrimination (Fig. 4C, right; G, right; D,H). These results suggest that mice trained to self-administer cocaine or remifentanil in once daily, 1-h sessions display characteristic operant behaviors observed in other paradigms and species. Hence, cocaine- and remifentanil-associated lever responding in mice with restricted daily access to drug can be extinguished by withholding the drug and reinstated by presentation of the drug-associated cues.
Dimension reduction of self-administration data identifies latent variables
Collecting multiple measures from a given subject and having groups of sufficient size permits the use of multivariate analysis. Our data provide an ideal large data set (i.e., n > 30 mice per condition) to assess the latent variables underlying drug taking and seeking in mice.
Self-administration behaviors both within and across sessions were related. These relationships were visualized in correlation matrix in heat maps and dendrograms for 11 key variables from each of the cocaine and remifentanil paradigms (Fig. 5, Tables S6, S7). These variables included measures of drug taking averaged across the dose–response curve (i.e., self-administered reinforcements, active and inactive lever responding, lever accuracy, and latency measures), as well as indices of drug seeking (i.e., lever responding during extinction and cue-induced reinstatement). The internal structures of the drug taking-associated behavioral measures were remarkably similar in the cocaine and remifentanil paradigms (Fig. 5, variables 1–8). Differences between drug classes were apparent, however, in the relationships between the drug seeking measures (Fig. 5, variables 9–11).
Relationships between drug taking- and drug seeking-associated self-administration variables. Drug taking- and drug seeking-associated variables are numbered according to the key on the far left. Numbers assigned to drug seeking-associated variables are enclosed in circles. (A) Heat maps of Pearson R correlation coefficients and corresponding p-values. The increasing saturation of orange and blue colors correspond to increasing absolute values of positive and negative Pearson correlation coefficients, respectively. The increasing saturation of green color corresponds to decreasing p-values. White indicates a correlation coefficient close to 0 and p-value close to 0.4. (B) Dendrograms. Results of hierarchical clustering. Correlation coefficient values and corresponding p-values are provided in Tables S6 and S7.
For interrelated datasets such as these, dimension reduction strategies are useful. Such strategies may identify a reduced number of latent, orthogonal constructs contributing to common variance. Indeed, this approach has previously been used to reduce the dimensionality of open field data in mice67 and of self-administration and locomotor activity data in rats68. Bartlett’s Test of Sphericity indicated that there was sufficient shared variance between the selected measures in Fig. 5 to warrant dimension reduction.
We conducted two within-drug exploratory factor analyses (EFAs) with 12 and 11 variables from the cocaine and remifentanil datasets, respectively (Fig. 6, Table S8). We extracted 2 factors explaining a cumulative 52% of the total variance from the model for cocaine and 55% of the total variance from the model for remifentanil (Fig. 6, Table S8). In loading plots (Fig. 6A,B), the more variance within a measure that is explained by a given factor, the higher the factor score and the further from the origin the variable is located. Variables that are correlated will load onto a common factor, with variables that are directly proportional loading onto the same side of the origin and variables that are inversely proportional loading onto opposite sides of the origin.
Feature discovery and predictive modeling of self-administration behaviors. (A,B) Variable factor loading. Factor loading plots for self-administration variables included in exploratory factor analyses for cocaine (A) and remifentanil (B). (C) Global factor scores. Factor scores for individual animals, obtained using regression, are represented as squares or circles. Ovals are drawn around animals in each drug/reinforcer paradigm. Each oval encompasses the genotype mean and contacts the closest 80% of individual values. (D) Comparison of factor scores by reinforcer. Factor scores for components 1 and 2 were averaged by drug paradigm and compared using unpaired, two-tailed student’s t-tests. Data are represented as mean ± SEM. For component 1: [t(70) = 9.4, p < 0.0001]. For component 1: [t(70) = 1.3, p = 0.2082]. (E,F) Heat maps of Pearson correlation coefficients for open field variables and factor scores. Correlation coefficients for cocaine (E) and remifentanil (F) are shown on top for heat maps in which the increasing saturation of orange and blue colors correspond to increasing absolute values for positive and negative Pearson correlation coefficients, respectively. Significant values are indicated by an asterisk. *p < 0.05. (G) Actual versus predicted plots for multiple linear regression models constructed for drug seeking during early extinction (top), late extinction (middle), and reinstatement (bottom) based on responses to novelty, drug-induced hyperlocomotion, and drug-taking behaviors. For factor loadings, see Table S8. For information on variables included in the regression models and details on model performance, see Tables S9, S10 and S11, and Figure S8.
The first factor extracted accounted for 31% (eigen value = 3.728) of the cocaine model and 31% (eigen value = 3.396) of the remifentanil model. Based on variable loading, we determined that this factor represented an incentive motivation construct. The same variables (i.e., active lever responses, earned reinforcements, latencies to first lever response, and first reinforcement during self-administration) loaded most heavily onto this factor in both models (Fig. 6A, Table S8). The positions of these loadings indicate that animals with higher active lever responses and earned reinforcements had lower latencies to their first lever response and reinforcement.
The second factor extracted accounted for 21% (eigen value = 2.562) of variance in the cocaine model and 24% (eigen value = 2.673) of variance in the remifentanil model. We determined that factor 2 represented a discriminative control dimension, as the variables that loaded most heavily on this factor in both models were lever accuracy and inactive lever responding during self-administration (Fig. 6A, Table S8). As expected, this finding indicates that animals with lower lever accuracy produced more responses at the inactive lever during self-administration.
We next examined how drug seeking during extinction and cue-induced reinstatement loaded onto these factors. For cocaine, while early extinction lever responding was well-explained by the incentive motivation factor 1, late extinction responding was not. Instead, late extinction responding loaded weakly on the discriminative control factor 2. For remifentanil, early and late drug seeking clustered together, but were only modestly well-explained by either factor. Interestingly, cue-induced reinstatement of cocaine seeking loaded exclusively on factor 1, while that of remifentanil seeking loaded exclusively on factor 2. Together, the correlation matrices and factor analysis suggest that while patterns for cocaine and remifentanil taking are similar, those for cocaine and remifentanil seeking are distinct. Drug seeking during early extinction, late extinction, and cue-induced reinstatement may be driven by distinct underlying biological and/or behavioral programs that differ by drug class.
Identification of drug class-specific self-administration phenotypes
To determine whether cocaine and remifentanil produced distinct self-administration phenotypes, a global exploratory factor analysis (EFA) was performed with mice from both drug paradigms (Fig. S7, Table S8). Individual mice were assigned regression factor scores (Fig. 6C) and factor scores were compared between reinforcers. Animals in the cocaine and remifentanil paradigms differed on incentive motivation factor 1, but not on factor 2 (Fig. 6D). This finding demonstrates that EFA can identify drug class-specific self-administration phenotypes.
Predicting self-administration performance from a priori open field assessments
Because self-administration is time- and resource-intensive, it would be desirable to identify predictors of self-administration behaviors in other, more facile behavioral assays, including novelty- and drug-induced motor activities in the open field. As such, we assessed whether there was a relationship between the a priori exploratory or drug-induced behaviors in the open field and self-administration performance, as determined by factor 1 and 2 scores (Fig. 6E,F). Drug-induced locomotion was positively and negatively associated with factor 1 for cocaine and remifentanil, respectively. Exploratory vertical episodes (i.e., rearing) correlated with factor 2 scores for remifentanil, but not for cocaine. These findings suggest that the relationship between open field motor activities and self-administration factor scores differ by drug class.
Predicting drug seeking from a priori open field assessments and drug taking behavior
Drug seeking during extinction and reinstatement are the most clinically-relevant paradigm stages, but they were not well described by the factors extracted in the EFA. To examine whether drug seeking during early extinction, late extinction, or reinstatement can be predicted by a mouse’s response to novelty, drug-induced hyperactivity, or self-administered drug intake, we applied machine learning algorithms. To remove multicollinearity, we performed principal component analyses on novelty-induced open field (Table S9) and drug-taking behaviors (Table S10). We then divided the data into training and test datasets using a random 80:20 split and built mulitple regression models based on the novelty-response component scores, drug-taking component scores, and drug-induced hyperlocomotion data of the training set. Predictive models were successfully generated for both drugs and all drug seeking stages, except for late extinction responding for cocaine (Fig. 6G, Table S11). Residuals for the training and test sets were comparable for the late extinction and reinstatement models and only modestly higher for the early extinction models (Fig. S8), suggesting these models are not overfit. Interestingly, the variables contributing predictive value to the models differed both by drug and drug-seeking stage. These data indicate that drug seeking can be predicted from a mouse’s response to novelty, sensitivity to drug-induced locomotion, and drug taking behavior, but that the relative importance of these measures differs by drug type and drug-seeking phase.
Discussion
Causal studies on the biological basis of drug addiction are not practical or ethically achievable in humans. Thus, understanding disease mechanisms requires animal models. The most versatile and practical models have been rats. Technical obstacles have largely precluded longitudinal, multi-stage self-administration studies in mice, the mammalian species for which the most genetic tools are available. Here, we have overcome historic obstacles and developed paradigms for cocaine and remifentanil in which mice with indwelling jugular catheters progress through the self-administration stages of acquisition, maintenance, extinction, and reinstatement in a systematic manner that permits comparison of behavioral parameters across drug class and the application of predictive modeling.
IVSA requires placement of catheters into the jugular vein. Historically, jugular catheter placement, maintenance of catheter patency, and prevention of infection secondary to their use have limited the extent and duration of self-administration studies in mice13. The jugular catheterization procedure in mice is not trivial, due in part to their small size13. We used a guide needle technique modified from69, and used polyurethane catheters that are less thrombogenic than those composed of polyethylene or polyvinyl chloride. The new-found longevity of surgically-implanted mice and catheters in the current paradigms has permitted longitudinal, multi-stage study designs.
In many self-administration paradigms, drug exposure is preceded by operant conditioning with food49,70. To reduce the total time required for these types of studies, to circumvent potential confounding effects of prior conditioning49, and to permit the future assessment of genotype differences in self-administration acquisition, we did not conduct prior operant training. In acquisition sessions, mice progressively learned active versus inactive lever discrimination and self-administered drugs paired with a cue light on FR1, FR2, and FR4 schedules. The extent of self-administration during this training period was consistent with reports of the dose, time-frame, and reinforcement requirements in mice for cocaine14,69 and remifentanil71. The observed increase in total response output with the increased response requirement is a hallmark of drug self-administration across species and drug class52, and it was not observed in mice given access to the vehicle rather than cocaine or remifentanil.
Notably, vehicle mice in this study did not display a preference for the active lever despite active lever pressing resulting in the same infusion-associated cues as the cocaine and remifentanil IVSA groups. C57BL/6J mice have been found to self-administer visual and auditory cues without prior training, a phenomenon known as operant sensation seeking (OSS)72,73. The extent of OSS is related to the variability74 and complexity75 of the cues, and varies by strain75. Relevant to the current study, OSS takes time to develop and varies with the frequency and predictability of visual cue presentation74. The lack of OSS in the vehicle group may be the result of several factors, including the limited number of sessions in which they were evaluated, the long duration (20 s per infusion), slow frequency (1 Hz) and predictable nature of the cue light presentation, and the 20-s delay between lever press and lever retraction.
The percent of mice achieving final cocaine self-administration acquisition criteria (~ 80%) was consistent with that previously reported for C57BL/6J mice, and it is significantly higher than that reported for other mouse strains14,15. In our experiments, mice achieved high levels of lever discrimination during training for both drugs, as evidenced by the percent of total responses occurring at the active lever.
Once cocaine or remifentanil self-administration was achieved, animals progressed through a maintenance dose–response phase in which the drug dose per infusion was systematically varied. For both cocaine and remifentanil, the number of reinforcements earned per hour and the extent of drug consumption across doses was consistent with published results14,69,71. Interestingly, except for latency to the first response, all behavioral indices showed dose-dependence for both drugs.
Following maintenance dose–response testing, animals moved into an extinction phase. Extinction is more protracted in mice than in rats and nonhuman primates49. The rate and extent of extinction in the present study was consistent with previous reports for C57BL/6 and A/J mice extinguished in the absence of cocaine-associated cues49. However, the extinction in the current study was more effective, in that mice achieved greater reductions in active lever responding than that reported in cocaine-conditioned C57BL/6J mice when extinction occurred in the presence of paired cues49. This indicates that the presence of paired cues may hinder behavioral extinction.
As documented in multiple species, including mice49,63, lever responding in our paradigm decreased with successive extinction sessions in a manner that was best fit by a two-phase model: rapidly at first (i.e., sessions 1–4) and then more slowly (i.e., sessions 5–20). At the time mice met final extinction criterion, active lever responding was not only reduced, but active versus inactive lever discrimination was lost for cocaine and significantly reduced for remifentanil. For cocaine, these results suggest an absence of goal-directed drug seeking and successful extinction of drug-associated lever responding.
Following extinction, mice completed a reinstatement session in which drug-associated cues were presented. This reintroduction of drug-paired cues reinstated both responding at the previously active lever and active compared to inactive lever discrimination. The finding that limited access to cocaine or remifentanil afforded by once daily, 1-h sessions sufficient to produce this behavior is consistent with emerging evidence from the rat literature suggesting that exposure to large quantities of cocaine is not necessary for escalation of intake, incentive-sensitization, or drug or cue-induced reinstatement of drug-seeking behavior76. Many reinstatement paradigms have been described, including those using various environmental cues, drug priming, and stress. Reinstatement of drug seeking induced by different stimuli (e.g., cue-induced, drug-induced, stress-induced) occurs through distinct mechanisms77. We elected to use drug-paired cues as the stimulus for reinstatement in our study because of clinical reports demonstrating a relationship between drug-associated cues and relapse78. In the future, this paradigm can be adapted for the study of reinstatement induced by other stimuli.
The longevity of the jugular catheters and mice in our experiments permitted a longitudinal study design. One advantage in using a longitudinal design with a larger sample size is the ability to examine the relationships between behaviors at different time-points79. This approach may be especially useful for self-administration studies, where later time-points, such as at the extinction and reinstatement stages, may be more clinically-relevant and more time-intensive, costly, and technically challenging to assess as compared to the earlier behavioral acquisition and maintenance phases. We performed a series of EFAs to examine how the variables examined in our studies are related. We extracted two components from this analysis and, based on variable loading that was consistent between the drug paradigms, termed the one with the highest explanative value “incentive motivation” and the one with the next highest explanative value “discriminative control.”
Several aspects of the resulting model are informative. The first is the high degree of similarity between the internal structure of the drug-taking measures for cocaine and remifentanil. The second is the contrast between the structures of cocaine- and remifentanil-seeking data and their differential relationships to active and inactive lever responding during self-administration.
In the cocaine EFA, variable loadings support the idea that there are two distinct phases of lever responding during the extinction phase of the longitudinal self-administration paradigm. While early extinction responding is highly correlated with the extent of self-administration and urgency to initiate drug taking, late extinction responding is more closely associated with variation in lever discrimination and inactive lever responding during self-administration. A two-phase extinction model is supported further by the shape of the total presses relative to the extinction-session number best-fit curve and by prior observations in support of the early- and late-stage extinction phases as biologically distinct. For example, loss of β-arrestin 2 in the infralimbic prefrontal cortex affects extinction responding only in its early stages (i.e., sessions 1–4), and not in subsequent sessions63. Another noteworthy observation is that the number of sessions required to reach final extinction criteria in the cocaine-administered mice was almost entirely unrelated to the extent of self-administration during the maintenance stage, suggesting that the rate of extinction learning is independent of the degree of cocaine consumption. Conversely, the rate of extinction was closely associated with the rate of self-administration acquisition, consistent with the notion that acquisition and extinction are both learning processes80.
Additionally, it is significant that late extinction responding in the cocaine cohort and both early and late extinction responding in the remifentanil cohort were related more closely to inactive lever responding than to active lever responding during the maintenance dose–response phase. At least two interpretations of these findings are possible. One is that lever responding in later extinction sessions does not reflect active drug seeking, but instead is a perseverative response manifested from poor action impulse control. An alternative interpretation is that responding at the inactive lever during the maintenance dose–response phase is not only a reflection of discriminative control, but it is also an indication of active drug seeking. Drug seeking during the cue-induced reinstatement session was explained moderately by Factor 1 (incentive motivation) in the cocaine paradigm and more strongly by Factor 2 (discriminative control) in the remifentanil paradigm, suggesting that patterns of drug-seeking behaviors may differ by drug class.
A correlation between novelty-induced locomotor activity and vulnerability to psychostimulant, but not opioid self-administration, has been reported in rats81,82. Here, we demonstrate a parallel phenomenon in mice. Exploratory (i.e., novelty-induced) locomotor activity was positively correlated with the extent of cocaine self-administration, captured by cocaine EFA Factor 1, but not with the extent of remifentanil self-administration, captured by remifentanil EFA Factor 1. Notably, drug-induced hyperlocomotion is a positive and negative predictor of Factor 1 scores for cocaine and remifentanil, respectively.
Identifying at-risk individuals prior to the development of a chemical addiction is a major clinical goal. Here, we present predictive models of drug seeking behaviors in mice. Our ability to build such models based on reactions to novelty, response to acute drug exposure, and patterns of drug intake suggest relationships among these behaviors can be leveraged to identify at-risk subjects. The observation that the best predictors differed by drug and by drug-seeking phase (e.g., early extinction vs. late extinction vs. cue-induced reinstatement) supports our results of the cluster analysis and EFA, and it indicates that cocaine and remifentanil seeking are driven by underlying biological and/or behavioral programs that are distinct and stage-specific.
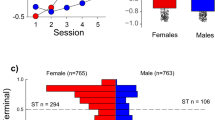
While sex effects on self-administration behaviors have been reported in rats, particularly in long-access paradigms (i.e., > 6 h)83, and in C57BL/6J mice on progressive ratio schedules84, no such effect on self-administration was identified in the current FR-based cocaine paradigm. This finding agrees with reports for cocaine84 and heroin23 self-administration, and others for alcohol85 and food reinforcement86, that sex differences are evident for C57BL/6 mice only when reinforcements are delivered on more behaviorally-demanding schedules. Interestingly, we identified a significant dose by sex interaction in the remifentanil paradigm. Female mice showed a marked reduction in IVSA at the lowest 0.01 mg/kg/infusion remifentanil dose, whereas males did not. This effect could be due to differences in the reinforcing efficacy of low doses of remifentanil between sexes, which could be manifested as a shift in the dose–response curve and altered break-point under a progressive ratio schedule of reinforcement. Future work will address this point. Sex as a biological variable in mouse IVSA studies continues to be understudied, with less than half of all studies from the last 5 years including both male and female animals (Supplemental Table S1).
While the present work represents a significant advance in the length and extent of IVSA studies in mice, attrition over the study duration and inter-animal variability have not been eliminated. Moreover, self-administration is not acquired by all mice. As such, for these types of longitudinal studies, one should expect to start with 40–45 mice for each longitudinal arm to finish with the 30 + cohort size required for sufficient statistical rigor. While studies of this magnitude are resource-intensive, they may help address the ongoing reproducibility crisis in animal research.
A limitation of the current study is the use of only a single mouse strain and, therefore, the inability to capture the effects of genetic variability in drug use. Genetic variation underlies the risk of substance use disorder87,88, that include for cocaine89 and opioid90 use disorders, and this variation drives the majority of the phenotypic variation in addiction-like behaviors in mice91,92. In a recent study, Bagley and colleagues92 assessed cocaine IVSA in 84 members of the hybrid mouse diversity panel and identified extreme phenotypic diversity between mouse strains. Cocaine IVSA was demonstrated to be heritable in mice, with between strain differences in cocaine intake dwarfing within-strain inter-animal differences. Notably, strain-dependent sex effects were identified. These reports with findings from the present study suggest that both a mouse’s environment and its genetics constitution can contribute the extent of drug intake. Studies using genetically-modified mice should be designed with these strain-specific IVSA phenotypes92 in mind and with an awareness that strain genetic background can modify the behavioral consequences of genetic perturbation93. Future studies are needed to expand these paradigms not only to other mouse strains, but also to other reinforcers. While remifentanil was studied here, it’s longer-lived analogue fentanyl is more commonly misused. We elected to study remifentanil over fentanyl in this paradigm because of its superior pharmacokinetics for sustaining operant responding. While both remifentanil and fentanyl are expected to be effective reinforcers, the relatively shorter half-life of remifentanil as compared to fentanyl was anticipated to result in mice exhibiting more lever responding behavior over the short, one-hour sessions.
Together, the present findings analyze cocaine- and remifentanil-reinforced behaviors in longitudinal self-administration paradigms in which cohorts of mice progressed through the stages of acquisition, maintenance, extinction, and reinstatement. The systematic progression of mice through these paradigms allows comparisons to be made across genetically-engineered mouse lines, reinforcing substances, and potential anti-addiction treatments. Incorporation of other drug access schedules (e.g., intermittent access) and reinforcement schedules (e.g., progressive or random ratio) will expand its utility. Critically, through the application of this multi-stage self-administration paradigm to the large and growing number of genetically-engineered mice, we can now conduct clinically-relevant, detailed studies on the contributions of genes to addiction. The knowledge gained from these studies may facilitate the identification of at-risk individuals and permit the development of mechanism-based therapeutics for a variety of substance use disorders.
Material and methods
Animals
All mouse studies were conducted in accordance with the National Institutes of Health Guidelines for Animal Care and Use and with approved animal protocols from the Duke University Institutional Animal Care and Use Committee. Methods are reported in accordance with ARRIVE guidelines. This study used adult male and female β-arrestin2flox/flox mice on a C57BL/6J background. These genetically-modified mice were selected for study because they could serve as controls for the conditional β-arrestin2 knock-out experiments, the results of which will be presented elsewhere. At the beginning of our experiments, animals were 12–24 weeks old and 19–30 g body weight. Prior to self-administration testing, mice were group-housed and maintained on a 12:12 h light:dark cycle. Following jugular catheter implantation, mice were singly housed and maintained on a 14:10 h light:dark cycle. Experiments were conducted during the light period, with the exception of extended access self-administration training, which took place predominantly during the dark period. To control for circadian effects94,95, the time of self-administration was held constant for individual animals throughout the study. Tap water and standard laboratory chow were supplied ad libitum, except during testing. Studies were conducted with 3 and 2 replicate cohorts of animals, for cocaine and remifentanil, respectively. Replicate cohorts were run sequentially.
Drug treatment
Cocaine hydrochloride (cocaine) was purchased from MilliporeSigma (St. Louis, MO) and remifentanil hydrochloride (remifentanil) was provided by the National Institute on Drug Abuse (NIDA), through the NIDA Drug Supply Program. Both drugs were dissolved in sterile physiological saline (Henry Schein, Raleigh, NC). Doses were calculated based on the weight of cocaine and remifentanil hydrochloride salts and were not adjusted for fractional base weight. For open field studies, cocaine (20 mg/kg, ip) or remifentanil (0.01 – 10 mg/kg, ip) was injected in a volume of 10 ml/kg body weight. The volume of injection was based on individual animal weight, and mice were weighed immediately prior to open field assessment. For iv self-administration studies, cocaine (0.1–3 mg/kg/infusion) or remifentanil (0.01–3 mg/kg/infusion) was administered intravenously in a volume of 18 µl over a 4 s period to animals with indwelling jugular catheters according to the test protocol and their lever responding. Separate drug solutions for infusion were made for male and female mice, with drug concentrations in the infusion solutions based on the mean male and mean female body weights. For example, for a group of male mice with a mean weight of 25 g requiring a 0.5 mg/kg/infusion cocaine dose, a 0.694 mg/ml cocaine infusion solution would be prepared: 0.5 mg/kg/0.018 ml X 0.025 kg. For a group of female mice with a mean weight of 20 g requiring a 0.5 mg/kg/infusion cocaine dose, a 0.556 mg/ml infusion solution would be prepared: 0.5 mg/kg/0.018 ml X 0.020 kg. Infusion solutions were prepared every 24–72 h based on body weight rounded to the nearest gram.
Jugular catheterization
Indwelling catheters (Instech Laboratories, Inc., Plymouth Meeting, PA) were implanted into the right jugular vein of adult male and female C57BL/6J mice, as described for CD-1 mice69, with several modifications. Specifically, changes were made in the type and route of anesthesia, the design/supplier of the catheters and vascular access buttons, the material of the vascular access buttons, and the size of the insertion needle. Briefly, mice were anaesthetized with a freshly prepared ketamine/xylazine (12/1 mg/ml, 10 ml/kg, i.p.) cocktail. Catheters were inserted into the right jugular vein and threaded under the skin of the shoulder to a mid-scapular vascular access button. Polyurethane catheters had a bead 1.2 cm from the catheter tip (Part #VAB62BS/25; Instech, Plymouth Meeting, PA). To prepare the catheters for insertion, the excess catheter tubing was cut 3.5 cm from the bead and affixed to a vascular access button (Part #KVAH62T; Instech). A modified 18-gauge, 1.5-inch guide needle (Part # BD-305196; Becton Dickinson, Franklin Lakes, NJ) was used for catheter insertion. This needle is larger than the more commonly used 20-gauge needle69, and the wider shaft provides a larger working area for catheter insertion. On post-surgery days 1–3, animals received amikacin at a dose of 10 mg/kg (s.c.) to prevent perioperative infection. To maintain patency and prevent infection for the duration of the study, catheters were flushed 1–2 × daily with 50 μl of a 30 U/ml heparin and 1 mg/ml gentamicin solution.
Jugular catheter patency testing
The patency and optimal placement of jugular catheters was determined by infusing (i.v) 20–30 μl of a 15 mg/ml ketamine solution in sterile saline69,96. In this test, the ketamine infusion resulted in rapid sedation (i.e., within 3 s), as evidenced by loss of muscle tone in animals with patent catheters. Catheter malfunctions were manifested as a delayed time for the onset of sedation (i.e., > 3 s)96,97 or an inability to infuse the ketamine; these mice were excluded from the study. Animals that showed evidence of sedation within 3 s of infusion were considered to have patent catheters. Patency testing was conducted with all mice on post-surgery day 7 to verify successful catheter placement later as according to behavioral performance criteria throughout the experiment, and at the completion of the study. Behavioral performance that qualified animals for catheter patency testing included a failure to progress toward training criteria after 4 consecutive sessions on the same protocol, loss of the active lever preference in trained animals, and/or inconsistencies in lever responding that were not concurrent with changes in the animal’s body weight, nesting activity, or motor activity level.
Locomotor activity
The VersaMax activity monitor (Omnitech Electronics, Inc., Columbus, OH) was used to assess locomotor activity, as previously described98. Male and female mice with indwelling jugular catheters were acclimated to the open field for 30 min prior to administration (ip) of cocaine (20 mg/kg, 10 ml/kg) or remifentanil (0.1, 1.0 and 10 mg/kg, 10 ml/kg, sequentially). After cocaine treatment, animals were immediately returned to the open field and locomotion was monitored over the next 90 min. After each successive remifentanil treatment, animals were immediately returned to the open field and activity was monitored over the next 20 min segments for each dose.
Self-administration
See Figs. S1 and S2 for protocol summaries.
Acquisition
Following a 7–14 day post-surgical recovery period, mice that completed a priori open field assessments and passed a ketamine test of catheter patency69 were trained to iv self-administer cocaine (0.5 mg/kg/infusion) or remifentanil (0.1 mg/kg/infusion) paired with a cue light through lever responding in operant chambers (Med Associates, Inc., Fairfax, VT). Active, reinforced lever(s) were denoted by a solid cue light. Reinforcements were delivered by a single- speed syringe pump (Med Associates) in a volume of 18 μl over a 4 s period. Each infusion was followed by a 40-s time-out period during which no additional reinforcements were provided. This time-out period was imposed to prevent adverse health consequences associated with excess cocaine or remifentanil administration99. For the first 20 s of this time-out period, the stimulus light blinked at a frequency of 1 Hz (i.e., 1 s on and 1 s off) and the levers remained available, but lever responses (i.e., time-out responses) had no programed consequences. For the following 20 s, the levers were retracted. Initial training began with an autoshaping session followed by a 12 h extended access session in which responding on either of the two levers resulted in reinforcement (i.e., ‘2 lever training’). Subsequently, in 1 h daily sessions, mice were trained to discriminate the active drug-delivering lever from the inactive non-drug-delivering lever (i.e., ‘1 lever training’) and the fixed lever response to reinforcement ratio (i.e., fixed ratio, FR) was increased from FR1 to FR2 to FR4. Training followed the contingent advancement protocols (see Figs. S1, S2). In all sessions, two levers were available in the chambers. During 2 lever training sessions, both levers were active and responding at either lever resulted in drug delivery. During 1 lever training sessions, responding at one lever (i.e., the active lever) resulted in a drug delivery while responding at the other lever (i.e., the inactive lever) did not. Responses at the inactive lever were recorded but had no programmed consequences. A small control group was given access to the vehicle (saline) rather than cocaine or remifentanil. This group completed a single autoshaping and a single extended-access session followed by 5 consecutive sessions of each training protocol. For the vehicle group, levers retracted and the cue light flashed as described for the mice lever pressing for cocaine and remifentanil.
Maintenance dose–response testing
After drug self-administration was acquired, stable lever responding at FR4 was assessed at 5 cocaine and 6 remifentanil doses. For cocaine, doses (mg/kg/infusion) were presented in order as: 0.5, 0.3. 0.1, 1.0, 3.0. The remifentanil, doses (mg/kg/infusion) were presented in the following order: 0.1, 0.03, 0.01, 0.3, 1.0, 3.0. Note, the 3.0 cocaine and remifentanil doses were added after the first replicate cohort had completed the study to better define the descending portion of the drug dose–effect curves. Stable responding was considered to have been achieved when self-administered reinforcements in two consecutive sessions varied by ≤ 20% with ≥ 50% of the total responses occurring at the active lever. Animals that failed to achieve or maintain stable responding and ≥ 50% active lever discrimination over 4 consecutive sessions were subjected to a patency test. Non-patent animals were removed from the study.
Extinction
After completing maintenance dose–response testing, mice were subjected to once daily, 1-h sessions in which the cue light was absent and lever responses were recorded but they had no programmed consequences. For the cocaine paradigm, daily extinction sessions continued until lever responses at the animal’s previously active lever were reduced to ≤ 20% of the animal’s previous responses for the 1 mg/kg/infusion cocaine dose over 2 consecutive sessions or until 40 total sessions were completed. Because preliminary cocaine data suggested the rate of extinction plateaued by session 20, in the remifentanil paradigm, all animals completed 20 daily extinction sessions regardless of performance.
Cue-induced reinstatement
After meeting extinction criteria, mice completed a single cue-induced reinstatement session. In this session, the drug-associated light was illuminated over the previously active lever and responses on this lever resulted in presentation of other drug-associated cues, including the cue light blinking, lever movements, and the sound of the syringe pump delivery, but in the absence of drug reinforcement.
Data capture and storage
Self-administration data were collected and managed in a customized REDCap (Research Electronic Data Capture) database created using REDCap electronic data capture tools hosted at Duke University100,101. Note, REDCap is a secure, clinically-utilized web-based software designed to support data capture for research studies.
Statistical analysis
All data are represented as mean ± SEMs, unless otherwise indicated. Primary behavioral data were analyzed and plotted using the software GraphPad Prism version 8.0. Information on curve fitting and statistical assessments are provided in the text, figure legends or supplemental tables. Statistical significance was assigned at p < 0.05.
Correlation matrices and hierarchical clustering
Pearson correlation coefficients for self-administration variables and novelty- and drug-induced activity open field variables were determined using the ‘cor’ function in R-Studio version 3. Agglomerative hierarchical clustering and dendrograms for self-administration variables were built using the R-Studio’s ‘hclust’ function with complete-linkage.
Exploratory factor analysis
To investigate the potential dimensional inter-relationships between self-administration behaviors at the stages of acquisition, maintenance, extinction, and cue-induced reinstatement, within-drug, correlation matrices and R-type exploratory factor analyses (EFAs) were performed using the IBM SPSS Statistics version 26 program (Chicago, IL). A total of 12 variables from the cocaine self-administration paradigm and 11 variables from the remifentanil self-administration paradigm were included in the models. Linear relationships were visually confirmed using bivariate scatterplots. All variables remained on their natural scales and the robustness of the models as to the assumption of multivariate normality was assessed. Missing values were replaced with the within-drug variable mean. Although 4 factors with eigen values > 1.0 were identified in the cocaine and remifentanil models, because the objective was dimension reduction, we used maximum likelihood estimation to extract only the two factors with the highest explanatory values. Unrotated loading plots and factor matrices were generated. Extracted factor 1 and extracted factor 2 scores were calculated for individual mice by regression. Only animals that met acquisition criteria and achieved stable responding at FR4 with 0.5 mg/kg/infusion of cocaine or 0.1 mg/kg/infusion of remifentanil were included in the models (n = 38 cocaine; n = 34 remifentanil), yielding a case to variable ratio of ~ 3:1.
To determine whether cocaine and remifentanil reinforcement produced distinct global self-administration phenotypes, a third factor analysis was performed that included data from 11 common variables with all mice irrespective of reinforcer (n = 72). Missing values were replaced with the global variable mean. The two factors with the highest eigen values were again extracted, by maximum likelihood estimation. Extracted factor 1 and extracted factor 2 scores from the unrotated solution were calculated for individual animals by regression. Factor 1 and 2 scores were plotted and compared by reinforcer.
To assess the relationship between behavior in the open field and self-administration parameters, Pearson’s R correlation coefficients were calculated between novelty- and drug-induced open field activity measures and within-drug factor scores (n = 28 cocaine; n = 34 remifentanil). Since open field data were not collected from an initial set of 10 mice in the cocaine self-administration paradigm, they were excluded from the analysis.
Predictive modeling of drug seeking
Machine learning was used to develop predictive models of early extinction, late extinction, and reinstatement lever responding based on novelty-induced responses in the open field, sensitivity to drug-induced hyperlocomotion, and drug-taking behavior. The data were split into a training and a test dataset using a random 80:20 split and models were built based on the training dataset. To remove multicollinearity in the novel open field and drug-taking data, principle component analysis (PCA) was performed in R-Studio version 3. Prior the PCA, data for 6 open field variables and 8 drug-taking-associated variables were first scaled using the scikit-learn package for Python. The number of components extracted was determined by the minimum required to cumulatively explain 97% of the variance. Extracted components and drug-induced locomotion data were treated as features to build multiple linear regressions using the ‘lm’ function in R-Studio. The ‘step’ function was used to select independent variables based on Akaike information criterion102,103. The trained models were applied to test datasets to make predictions. Mean absolute errors from the training and test datasets were calculated and compared to assess the model performance.
Figure illustration
The study overview figures presented in Fig. 1 were created using BioRender (Toronto, ON).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- IVSA:
-
Intravenous self-administration
- i.p.:
-
Intraperitoneal
- FR:
-
Fixed ratio
- EFA:
-
Exploratory factor analyses
References
Wilson, N., Kariisa, M., Seth, P., Smith, H. T. & Davis, N. L. Drug and opioid-involved overdose deaths—United States, 2017–2018. MMWR Morb. Mortal Wkly. Rep. 69, 290–297 (2020).
Florence, C. S., Zhou, C., Luo, F. & Xu, L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med. Care 54, 901–906 (2016).
Hedegaard, H., Bastian, B. A., Trinidad, J. P., Spencer, M. & Warner, M. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl. Vital Stat. Rep. 69, 1–13 (2018).
Volkow, N. D., Koob, G. F. & McLellan, A. T. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 374, 363–371 (2016).
Deneau, G., Yanagita, T. & Seevers, M. H. Self-administration of psychoactive substances by the monkey. Psychopharmacologia 16, 30–48 (1969).
Thompson, T. & Schuster, C. R. Morphine self-administration, food-reinforced, and avoidance behaviors in rhesus monkeys. Psychopharmacologia 5, 87–94 (1964).
Weeks, J. R. & Collins, R. J. Factors affecting voluntary morphine intake in self-maintained addicted rats. Psychopharmacologia 6, 267–279 (1964).
Weeks, J. R. Experimental morphine addiction: Method for automatic intravenous injections in unrestrained rats. Science 138, 143–144 (1962).
Woods, J. H. & Schuster, C. R. Reinforcement properties of morphine, cocaine, and spa as a function of unit dose. Int. J. Addict. 3, 231–237 (1968).
Panlilio, L. V. & Goldberg, S. R. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction 102, 1863–1870 (2007).
Lile, J. A. & Nader, M. A. The abuse liability and therapeutic potential of drugs evaluated for cocaine addiction as predicted by animal models. Curr. Neuropharmacol. 1, 21–46 (2003).
Balster, R. L. Drug abuse potential evaluation in animals. Br. J. Addict. 86, 1549–1558 (1991).
Chistyakov, V. S. & Tsibulsky, V. L. How to achieve chronic intravenous drug self-administration in mice. J. Pharmacol. Toxicol. Methods 53, 117–127 (2006).
Roberts, A. J., Casal, L., Huitron-Resendiz, S., Thompson, T. & Tarantino, L. M. Intravenous cocaine self-administration in a panel of inbred mouse strains differing in acute locomotor sensitivity to cocaine. Psychopharmacology 235, 1179–1189 (2018).
Georgiou, P. et al. Differential regulation of mGlu5 R and MuOPr by priming- and cue-induced reinstatement of cocaine-seeking behaviour in mice. Addict. Biol. 20, 902–912 (2015).
Grahame, N. J., Phillips, T. J., Burkhart-Kasch, S. & Cunningham, C. L. Intravenous cocaine self-administration in the C57BL/6J mouse. Pharmacol. Biochem. Behav. 51, 827–834 (1995).
Huyts, B., Brabant, C. & Tirelli, E. Pitolisant and intravenous cocaine self-administration in mice. Eur. J. Pharmacol. 851, 63–68 (2019).
DePoy, L. M. et al. Circadian-dependent and sex-dependent increases in intravenous cocaine self-administration in Npas2 mutant mice. J. Neurosci. 41, 1046–1058 (2021).
Han, X., DeBold, J. F. & Miczek, K. A. Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology 234, 2813–2821 (2017).
Windisch, K. A., Morochnik, M., Reed, B. & Kreek, M. J. Nalmefene, a mu opioid receptor antagonist/kappa opioid receptor partial agonist, potentiates cocaine motivation but not intake with extended access self-administration in adult male mice. Neuropharmacology 192, 108590 (2021).
Arena, D. T., Covington, H. E. 3rd., DeBold, J. F. & Miczek, K. A. Persistent increase of I.V. cocaine self-administration in a subgroup of C57BL/6J male mice after social defeat stress. Psychopharmacology (Berlin) 236, 2027–2037 (2019).
J. L. Huebschman, M. C. Davis, C. Tovar Pensa, Y. Guo, L. N. Smith, The fragile X mental retardation protein promotes adjustments in cocaine self-administration that preserve reinforcement level. Eur J Neurosci 54, 4920–4933 (2021).
Vollmer, K. M. et al. A novel assay allowing drug self-administration, extinction, and reinstatement testing in head-restrained mice. Front. Behav. Neurosci. 15, 744715 (2021).
Engeln, M. et al. Sex-specific role for Egr3 in nucleus accumbens D2-medium spiny neurons following long-term abstinence from cocaine self-administration. Biol. Psychiatry 87, 992–1000 (2020).
Penrod, R. D. et al. The activity-regulated cytoskeleton-associated protein, Arc/Arg3.1, influences mouse cocaine self-administration. Pharmacol. Biochem. Behav. 188, 172818 (2020).
Lopez, A. J. et al. An optimized procedure for robust volitional cocaine intake in mice. Exp. Clin. Psychopharmacol. 29, 319–333 (2021).
Martin, M. et al. Daidzein modulates cocaine-reinforcing effects and cue-induced cocaine reinstatement in CD-1 male mice. Psychopharmacology 238, 1923–1936 (2021).
Martini, M., Irvin, J. W., Lee, C. G., Lynch, W. J. & Rissman, E. F. Sex chromosome complement influences vulnerability to cocaine in mice. Horm. Behav. 125, 104821 (2020).
Campbell, R. R. et al. Cocaine induces paradigm-specific changes to the transcriptome within the ventral tegmental area. Neuropsychopharmacology 46, 1768–1779 (2021).
Thomsen, M., Crittenden, J. R., Lindsley, C. W. & Graybiel, A. M. Effects of acute and repeated administration of the selective M4 PAM VU0152099 on cocaine versus food choice in male rats. Addict. Biol. 27, e13145 (2022).
Nugent, A. L., Anderson, E. M., Larson, E. B. & Self, D. W. Incubation of cue-induced reinstatement of cocaine, but not sucrose, seeking in C57BL/6J mice. Pharmacol. Biochem. Behav. 159, 12–17 (2017).
Schoenrock, S. A. et al. Characterization of genetically complex collaborative cross mouse strains that model divergent locomotor activating and reinforcing properties of cocaine. Psychopharmacology 237, 979–996 (2020).
Delint-Ramirez, I., Segev, A., Pavuluri, A., Self, D. W. & Kourrich, S. Cocaine-induced synaptic redistribution of NMDARs in striatal neurons alters NMDAR-dependent signal transduction. Front. Neurosci. 14, 698 (2020).
Lujan, M. A., Cantacorps, L. & Valverde, O. The pharmacological reduction of hippocampal neurogenesis attenuates the protective effects of cannabidiol on cocaine voluntary intake. Addict. Biol. 25, e12778 (2020).
Dunn, A. et al. Modulation of cocaine-related behaviors by low doses of the potent KOR agonist nalfurafine in male C57BL6 mice. Psychopharmacology 237, 2405–2418 (2020).
Uhl, G. R. et al. Cocaine reward is reduced by decreased expression of receptor-type protein tyrosine phosphatase D (PTPRD) and by a novel PTPRD antagonist. Proc. Natl. Acad. Sci. U.S.A. 115, 11597–11602 (2018).
Heinsbroek, J. A. et al. Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Rep. 30, 2018–2027 (2020).
Stoll, K., Hart, R., Lindsley, C. W. & Thomsen, M. Effects of muscarinic M1 and M4 acetylcholine receptor stimulation on extinction and reinstatement of cocaine seeking in male mice, independent of extinction learning. Psychopharmacology 235, 815–827 (2018).
Jordan, C. J. et al. Xie2-64, a novel CB2 receptor inverse agonist, reduces cocaine abuse-related behaviors in rodents. Neuropharmacology 176, 108241 (2020).
Lujan, M. A., Castro-Zavala, A., Alegre-Zurano, L. & Valverde, O. Repeated Cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology 143, 163–175 (2018).
Galaj, E. et al. Dissecting the role of GABA Neurons in the VTA versus SNr in opioid reward. J. Neurosci. 40, 8853–8869 (2020).
Zhang, Y., Collins, D., Butelman, E. R., Blendy, J. A. & Kreek, M. J. Relapse-like behavior in a mouse model of the OPRM1 (mu-opioid receptor) A118G polymorphism: Examination with intravenous oxycodone self-administration. Neuropharmacology 181, 108351 (2020).
Towers, E. B., Tunstall, B. J., McCracken, M. L., Vendruscolo, L. F. & Koob, G. F. Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology 151, 189–194 (2019).
Severino, A. L. et al. μ-opioid receptors on distinct neuronal populations mediate different aspects of opioid reward-related behaviors. eNeuro https://doi.org/10.1523/ENEURO.0443-18.2019 (2020).
Bornebusch, A. B., Fink-Jensen, A., Wortwein, G., Seeley, R. J. & Thomsen, M. Glucagon-like peptide-1 receptor agonist treatment does not reduce abuse-related effects of opioid drugs. eNeuro https://doi.org/10.1523/ENEURO.0443-18.2019 (2019).
Zhang, Y. & Kreek, M. J. Nalfurafine modulates the reinforcing effects of oxycodone in male and female adolescent C57BL/6J mice. Neuropharmacology 176, 108244 (2020).
Hakimian, J. K. et al. Dietary supplementation with omega-3 polyunsaturated fatty acids reduces opioid-seeking behaviors and alters the gut microbiome. Nutrients 11, 447–455 (2019).
Koob, G. F. & Volkow, N. D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 3, 760–773 (2016).
Thomsen, M. & Caine, S. B. False positive in the intravenous drug self-administration test in C57BL/6J mice. Behav. Pharmacol. 22, 239–247 (2011).
Thomsen, M., Hall, F. S., Uhl, G. R. & Caine, S. B. Dramatically decreased cocaine self-administration in dopamine but not serotonin transporter knock-out mice. J. Neurosci. 29, 1087–1092 (2009).
Thomsen, M., Han, D. D., Gu, H. H. & Caine, S. B. Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J. Pharmacol. Exp. Ther. 331, 204–211 (2009).
Bigelow, G. E., Stitzer, M. L., Griffiths, R. R. & Liebson, I. A. Contingency management approaches to drug self-administration and drug abuse: Efficacy and limitations. Addict. Behav. 6, 241–252 (1981).
Mierzejewski, P., Koros, E., Goldberg, S. R., Kostowski, W. & Stefanski, R. Intravenous self-administration of morphine and cocaine: a comparative study. Pol. J. Pharmacol. 55, 713–726 (2003).
Howell, L. L., Wilcox, K. M., Lindsey, K. P. & Kimmel, H. L. Olanzapine-induced suppression of cocaine self-administration in rhesus monkeys. Neuropsychopharmacology 31, 585–593 (2006).
Haney, M. & Spealman, R. Controversies in translational research: drug self-administration. Psychopharmacology 199, 403–419 (2008).
Maccioni, P. et al. Elevated reinforcing and motivational properties of alcohol at the end of the nocturnal period in sP rats. Psychopharmacology 232, 3585–3595 (2015).
Green, J. L., Dykstra, L. A. & Carelli, R. M. Examination of cocaine dose in a preclinical model of natural reward devaluation by cocaine. Behav. Pharmacol. 26, 398–402 (2015).
King, C. P. et al. Premature responding is associated with approach to a food cue in male and female heterogeneous stock rats. Psychopharmacology 233, 2593–2605 (2016).
Markou, A. et al. Animal-models of drug craving. Psychopharmacology 112, 163–182 (1993).
Fuchs, R. A., Tran-Nguyen, L. T. L., Specio, S. E., Groff, R. S. & Neisewander, J. L. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology 135, 151–160 (1998).
Stewart, J. Conditioned and unconditioned drug effects in relapse to opiate and stimulant drug self-administration. Prog. Neuro-Psychopharmacol. 7, 591–597 (1983).
Ebner, S. R., Larson, E. B., Hearing, M. C., Ingebretson, A. E. & Thomas, M. J. Extinction and reinstatement of cocaine-seeking in self-administering mice is associated with bidirectional AMPAR-mediated plasticity in the nucleus accumbens shell. Neuroscience 384, 340–349 (2018).
Huang, B. et al. beta-Arrestin-biased beta-adrenergic signaling promotes extinction learning of cocaine reward memory. Sci. Signal. 11, eaam5402 (2018).
Karlsson, R. M., Kircher, D. M., Shaham, Y. & O’Donnell, P. Exaggerated cue-induced reinstatement of cocaine seeking but not incubation of cocaine craving in a developmental rat model of schizophrenia. Psychopharmacology 226, 45–51 (2013).
Millan, E. Z., Marchant, N. J. & McNally, G. P. Extinction of drug seeking. Behav. Brain Res. 217, 454–462 (2011).
Bossert, J. M., Marchant, N. J., Calu, D. J. & Shaham, Y. The reinstatement model of drug relapse: Recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology 229, 453–476 (2013).
Tanaka, S., Young, J. W., Halberstadt, A. L., Masten, V. L. & Geyer, M. A. Four factors underlying mouse behavior in an open field. Behav. Brain Res. 233, 55–61 (2012).
Belin, D., Berson, N., Balado, E., Piazza, P. V. & Deroche-Gamonet, V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36, 569–579 (2011).
Kmiotek, E. K., Baimel, C. & Gill, K. J. Methods for intravenous self administration in a mouse model. J. Vis. Exp. 70, e3739 (2012).
Caine, S. B., Negus, S. S. & Mello, N. K. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology 147, 22–24 (1999).
James, A. S. et al. Opioid self-administration results in cell-type specific adaptations of striatal medium spiny neurons. Behav. Brain Res. 256, 279–283 (2013).
Olsen, C. M. & Winder, D. G. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology 34, 1685–1694 (2009).
Olsen, C. M. & Winder, D. G. Operant sensation seeking in the mouse. J. Vis. Exp. 45, e2292 (2010).
Olsen, C. M. & Winder, D. G. Stimulus dynamics increase the self-administration of compound visual and auditory stimuli. Neurosci. Lett. 511, 8–11 (2012).
Dickson, P. E. & Mittleman, G. Stimulus complexity and mouse strain drive escalation of operant sensation seeking within and across sessions in C57BL/6J and DBA/2J mice. Front. Behav. Neurosci. 13, 286 (2019).
Kawa, A. B., Bentzley, B. S. & Robinson, T. E. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233, 3587–3602 (2016).
Farrell, M. R., Schoch, H. & Mahler, S. V. Modeling cocaine relapse in rodents: Behavioral considerations and circuit mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry 87, 33–47 (2018).
Childress, A. R. et al. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res. Monogr. 137, 73–95 (1993).
Short, R. & Horn, J. Some notes on factor-analysis of behavioral-data. Behaviour 90, 203–214 (1984).
Gass, J. T. & Chandler, L. J. The plasticity of extinction: Contribution of the prefrontal cortex in treating addiction through inhibitory learning. Front. Psychiatry 4, 46 (2013).
Mitchell, J. M., Cunningham, C. L. & Mark, G. P. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav. Neurosci. 119, 464–472 (2005).
Swain, Y., Muelken, P., LeSage, M. G., Gewirtz, J. C. & Harris, A. C. Locomotor activity does not predict individual differences in morphine self-administration in rats. Pharmacol. Biochem. Behav. 166, 48–56 (2018).
Algallal, H., Allain, F., Ndiaye, N. A. & Samaha, A. N. Sex differences in cocaine self-administration behaviour under long access versus intermittent access conditions. Addict. Biol. 25, e12809 (2019).
Griffin, W. C. 3rd., Randall, P. K. & Middaugh, L. D. Intravenous cocaine self-administration: individual differences in male and female C57BL/6J mice. Pharmacol. Biochem. Behav. 87, 267–279 (2007).
Middaugh, L. D. & Kelley, B. M. Operant ethanol reward in C57BL/6 mice: Influence of gender and procedural variables. Alcohol 17, 185–194 (1999).
Gentry, G. D. & Middaugh, L. D. Prenatal ethanol weakens the efficacy of reinforcers for adult mice. Teratology 37, 135–144 (1988).
Goldman, D., Oroszi, G. & Ducci, F. The genetics of addictions: Uncovering the genes. Nat. Rev. Genet. 6, 521–532 (2005).
Deak, J. D. & Johnson, E. C. Genetics of substance use disorders: A review. Psychol. Med. 51, 2189–2200 (2021).
Fernandez-Castillo, N., Cabana-Dominguez, J., Corominas, R. & Cormand, B. Molecular genetics of cocaine use disorders in humans. Mol. Psychiatry 27, 624–639 (2022).
Deak, J. D. et al. Genome-wide association study in individuals of European and African ancestry and multi-trait analysis of opioid use disorder identifies 19 independent genome-wide significant risk loci. Mol. Psychiatry https://doi.org/10.1038/s41380-022-01709-1 (2022).
Dickson, P. E. et al. Systems genetics of intravenous cocaine self-administration in the BXD recombinant inbred mouse panel. Psychopharmacology 233, 701–714 (2016).
Bagley, J. R., Khan, A. H., Smith, D. J. & Jentsch, J. D. Extreme phenotypic diversity in operant response to intravenous cocaine or saline infusion in the hybrid mouse diversity panel. Addict. Biol. 27, e13162 (2022).
Neuner, S. M., Heuer, S. E., Huentelman, M. J., O’Connell, K. M. S. & Kaczorowski, C. C. Harnessing genetic complexity to enhance translatability of Alzheimer’s disease mouse models: A path toward precision medicine. Neuron 101, 399–411 (2019).
Bass, C. E., Jansen, H. T. & Roberts, D. C. Free-running rhythms of cocaine self-administration in rats held under constant lighting conditions. Chronobiol. Int. 27, 535–548 (2010).
DePoy, L. M., McClung, C. A. & Logan, R. W. Neural mechanisms of circadian regulation of natural and drug reward. Neural Plast. 2017, 5720842 (2017).
Thomsen, M. & Caine, S. B. Chronic intravenous drug self-administration in rats and mice. Curr. Protoc. Neurosci. 32, 9.20.1-9.20.40 (2005).
Thomsen, M. & Caine, S. B. Intravenous drug self-administration in mice: practical considerations. Behav Genet 37, 101–118 (2007).
Slosky, L. M. et al. β-arrestin-biased allosteric modulator of NTSR1 selectively attenuates addictive behaviors. Cell 181, 1364–1379 (2020).
Bozarth, M. A. & Wise, R. A. Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA-J. Am. Med. Assoc. 254, 81–83 (1985).
Harris, P. A. et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009).
Harris, P. A. et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019).
Wang, T. et al. Social and anxiety-like behaviors contribute to nicotine self-administration in adolescent outbred rats. Sci. Rep. 8, 18069 (2018).
Yamashita, T., Yamashita, K. & Kamimura, R. A stepwise AIC method for variable selection in linear regression. Commun. Stat.-Theory Methods 36, 2395–2403 (2007).
Acknowledgements
We thank Xiuqin Zhang and Wendy L. Roberts for managing our mouse colony, and Dr. Ramona M. Rodriguiz for coordinating experimental schedules. This work was supported, in part, by NIH/NIDA grants P30DA029925, F32DA043931, UG3DA050316, and K99/R00DA048970.
Author information
Authors and Affiliations
Contributions
Study design: L.M.S., A.P., Y.B., N.B.C., V.M.P., K.T., W.C.W., L.S.B. and M.G.C. Obtained funding: L.M.S., L.S.B., M.G.C. Data collection: L.M.S., A.P., Y.B., N.B.C., K.T. and F.P. Data analysis: L.M.S., A.P., N.B.C., F.P., E.H., Y.Z. and X.C. Data interpretation: L.M.S., E.R.H., V.M.P., K.T., J.D.G., W.C.W., L.S.B. and M.G.C. Drafted paper: L.M.S. Revised paper critically for intellectual content: L.M.S., E.R.H., J.D.G., V.M.P., W.C.W., L.S.B. and M.G.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Slosky, L.M., Pires, A., Bai, Y. et al. Establishment of multi-stage intravenous self-administration paradigms in mice. Sci Rep 12, 21422 (2022). https://doi.org/10.1038/s41598-022-24740-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24740-2
This article is cited by
-
Head-mounted central venous access during optical recordings and manipulations of neural activity in mice
Nature Protocols (2024)
-
Intravenous fentanyl self-administration in male and female C57BL/6J and DBA/2J mice
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.