Abstract
Here we present a comprehensive mass cytometry analysis of peripheral innate lymphoid cell (ILC) subsets in relapsing/remitting MS (RRMS) patients prior to and after onset of cladribine tablets (CladT). ILC analysis was conducted on CyTOF data from peripheral blood mononuclear cells (PBMC) of MS patients before, 2 and 6 months after onset of CladT, and non-MS controls. Dimensionality reduction was used for immunophenotyping ILC subsets. CladT reduced all ILC subsets, except for CD56bright NK cells and ILC2. Furthermore, CD38+ NK cell and CCR6+ ILC3 were excluded from CladT-induced immune cell reductions. Post-CladT replenishment by immature ILC was noted by increased CD5+ ILC1 proportions at 2 months, and boosted CD38−CD56bright NK cell numbers at 6 months. CladT induce immune cell depletion among ILC but exclude CD56bright NK cells and ILC2 subsets, as well as CD38+ NK cell and CCR6+ ILC3 immunophenotypes. Post-CladT ILC expansions indicate ILC reconstitution towards a more tolerant immune system phenotype.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is an autoimmune disease causing inflammatory demyelination in the central nervous system (CNS)1. Disease-modifying therapy (DMT) aims to curb MS disease activity by modulating the immune system2.
DMT with cladribine tablets (CladT) causes immune reconstitution by short-term, semi-selective immune cell depletion, resulting in the emergence of a new, expectedly more tolerant, immune system2. Once CladT are absorbed and intracellularly phosphorylated to cladribine triphosphate, cladribine triphosphate acts as a nucleoside analogue of deoxyadenosine, and thereby inhibits DNA synthesis and repair, resulting in consecutive cell apoptosis3.
Among innate lymphoid cells (ILC), the effects of CladT have thus far only been investigated on NK cells, which rapidly declined from baseline and demonstrated a nadir at week 54. However, the impacts of CladT on other ILC subsets have not been studied.
ILC encompass five subtypes: NK cells, lymphoid tissue inducer cells, and three helper ILC subsets, namely ILC1, ILC2 and ILC35. Together, ILC are characterized by their lymphoid morphology, but concomitant absence of adaptive antigen-specific receptors and myeloid/dendritic cell markers6. ILC constitute rapid innate immune reactions to viruses, tumors, extracellular microbes, and allergens5,6. Moreover, they are involved in tissue repair, tissue homeostasis and epithelial integrity6,7. Notwithstanding, imbalanced ILC enhance chronic inflammatory diseases7. ILC are therefore implicated in autoimmune diseases, such as type 1 diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, psoriasis, and spondylarthritis8,9.
To date, the role of ILC in MS is yet to be clarified in detail9. Increased pro-inflammatory ILC1 and/or ILC3 have been reported in peripheral blood of untreated MS patients10,11 as well as in CSF during early stages of the disease12. On the contrary, CD56bright NK cells have been proposed as immunoregulatory, and increase after various DMTs13.
Here we interrogate the impacts of CladT on NK cell and helper ILC subsets in MS patients by mass cytometry. CladT provoked marked reductions in the majority of circulating ILC subsets. However, certain ILC immunophenotypes were unaffected or even increased following CladT treatment, implying ILC-specific resets of the immune system towards more tolerogenicity, and thereby offering potential future therapeutic targets.
Patients and methods
Study participants
In this study relapsing–remitting MS (RRMS) patients were recruited from Neurology clinics at Central West Neurology and Neurosurgery, Orange, NSW and from the University of Sydney’s MS Clinic at the Brain and Mind Centre (BMC). MS was defined by McDonald 2017 criteria14. Age- and sex-matched blood donors without MS or other autoimmune disease (non-MS controls) were recruited from friends and relatives of MS patients, or other healthy volunteers. Patient data are shown in Supplementary Tables S1 and S2.
ILC derived from peripheral blood mononuclear cells (PBMC) were analyzed prior to and during the 6 months after the first dose of oral cladribine tablets (CladT), which comprised 5 consecutive days of CladT, followed by another consecutive 5 days 1 month later; a cumulative dose of 1.75 mg/kg was given. PBMC for mass cytometry analysis in this study were taken at baseline (prior to first dose of CladT), and at 2 (2M) and 6 months (6M) after their first intake of CladT (Supplementary Tables S2, S3). Untreated non-MS control subjects had blood taken at the same intervals as CladT-treated MS patients.
Furthermore, demographics (age, gender, date of first diagnosis), EDSS and MRI reviews were collected at various timepoints (Supplementary Table S3).
Standard protocol approvals, registrations and patient consent
This study was performed according to the Declaration of Helsinki. Ethical consent for the study was obtained from the Research Integrity and Ethics Administration of the University of Sydney (2018/377). Written informed consent was obtained from all participants.
Sample collection and preparation
Up to 50 mL of venous blood were collected in EDTA vacuette tubes (Greiner Bio-One International, Kremsmünster, Austria) from patients and controls. Blood was taken from MS patients prior to and 2, and 6 months after their first dose of CladT. Concurrently, the same numbers of blood tubes were also taken from age- and sex-matched non-MS control subjects at baseline, and then 2, and 6 months later. PBMC were then isolated from the blood using a Ficoll-Paque Plus (GE Healthcare, Chicago, Illinois, US) density separation gradient. Samples were cryopreserved in 5% DMSO/FBS for storage in liquid nitrogen prior to mass cytometry staining. Thawed cryopreserved Ficoll-isolated PBMCs were labelled with isotope-conjugated antibodies and examined by mass cytometry using the CyTOF2 system, Helios.
Cell staining and analysis by mass cytometry
2.5 × 106 cells were thawed at 37 °C and washed in RPMI medium15. Individual patient/timepoint samples were first barcoded with anti-human CD45 (on 4 different metal isotopes) and blocked with purified human FcR binding inhibitor (eBioscience Inc., San Diego, USA) for 30 min, such that four independent samples could be combined for further staining. To control for batch variability, PBMC taken from a single non-MS control (taken from a single timepoint) were included within each batch as an internal control, used across 22 batches. Samples were combined (7.5–10 × 106 cells) and stained with cisplatin (Fluidigm, South San Francisco, CA, USA) for 5 min as a live/dead marker. Cells were stained with antibodies specific for the markers in Supplementary Table S4 for 30 min. These antibodies were purchased unlabeled in a carrier-protein-free and conjugated with the indicated metal isotope using the × 8 MaxPAR conjugation kit (Fluidigm) according to the manufacturer’s protocol. Conjugations done by the Ramaciotti Facility for Human Systems Biology are indicated in Supplementary Table S4. Cells were fixed in 4% paraformaldehyde (PFA) for 20 min prior to being incubated in Foxp3 permeabilisation buffer (eBioscience Inc.) for 15 min. Cells were then stained with anti-RORgt conjugated to AF647 for 45 min at room temperature (20–24 °C). After washing, cells were then stained with an intracellular antibody cocktail (indicated in Supplementary Table S4) for 45 min at room temperature. Cells were finally resuspended in DNA intercalator mix (500 nM iridium intercalator (Fluidigm) in 4% PFA) and left in the fridge until running on a CyTOF 2 Helios mass cytometer (Fluidigm) within 7 days. Cells were washed in Maxpar® Cell Acquisition Solution (CAS) (Fluidigm) before being resuspended in 10% EQ four element beads (Fluidigm) in CAS at a concentration of 0.8 × 106 cells mL–1.
Analysis of CyTOF data
Data files (.fcs files), which had been acquired on the CyTOF 2 Helios mass cytometer (Fluidigm), were imported into FlowJo software (version v10.8.0, Becton Dickinson, Ashland, OR, USA). Samples were initially gated as shown in Supplementary Fig. S1 using FlowJo to identify CD45+ live single cells15. Of all acquired events, beads were first excluded with the use of 140Ce isotope. Cell aggregates were then removed by gating 191Ir DNA signal (from staining with an Ir-loaded DNA intercalator) versus event length. Live cells were identified based on cisplatin. CD45+ live PBMC were subsequently identified by barcoding with 4 different isotopes (104Pd, 106Pd, 108Pd, 110Pd).
ILC were then defined as live CD45+ PBMC, negative for lineage markers (Lin−) CD3, CD19, CD14, CD11c, CD123, CD34, FcεRIa, and TCRαβ. Lin− cells were then sorted into Lin−CD56+CD94+ NK cells (and further subdivided into CD56bright and CD56dim NK cells), and Lin−CD56−CD94−CD127+ helper ILC (which were then subdivided into CD294-CD117− ILC1, CD294+CD117+/− ILC2 and CD294−CD117+ ILC3) (Fig. 1). Dimensionality reduction was done on all Lin−CD56−CD94−CD127+ helper ILC and Lin−CD56+CD94+ NK cells using ‘FIt-SNE’16, as part of the ‘Spectre’ package in R15,17. A subsample of up to 70,000 cells from all study participants (non-MS controls, MS patients from all timepoints and batch controls) were included for the generation of FIt-SNE plots. Dimensionality reduction plots were calculated using the following markers: CD1a, CD1c, CD5, CD21, CD23, CD38, CD56, CD80, CD86, CD94, CD103, CD117 (c-kit), CD120a, CD120b, CD161, CD184 (CXCR4), CD196 (CCR6), CD213α1, CD213α2, CD274 (PD-L1), CD294 (CRTH2), CD335 (NKp46), CD336 (NKp44), HLA-DR, GATA3, PAF-R (platelet-activating factor receptor), RORγt, T-bet (Supplementary Table S4). ILC immunophenotypes, which were identified with the help of FIt-SNE plots, were further analyzed in FlowJo software. To distinguish between positive versus negative surface marker expression of FIt-SNE-depicted ILC immunophenotypes, live CD45+ PBMC samples demonstrating low basal marker expression were considered as a negative control (Supplementary Fig. S2).
Gating strategy on CD45+ live PBMC to identify ILC subtypes. (a) Biaxial gating for Lin−(CD3−CD19−CD14−CD11c−CD123−CD34−FcεRIa−TCRab−) live CD45+ PBMC (peripheral blood mononuclear cells). (b) Natural killer (NK) cells were defined as Lin-CD56+CD94+ and subdivided into CD56dim and CD56bright NK cells. (c) Helper ILC were defined as Lin−CD56−CD94−CD127+ and subdivided into innate lymphoid cell (ILC) type 1 (CD294−CD117−), ILC2 (CD294+CD117+/−) and ILC3 (CD294−CD117+).
To calculate cell numbers, the percentage of the ILC as proportions of total PBMC were multiplied by PBMC counts of each study participant.
The median signal intensity of CD38 among total CD56+CD94+ and CD127 among Lin-CD56−CD94−CD127+ helper ILC was calculated and compared between groups.
Statistical analysis
All statistics were calculated using packages available within R18, using Type III Sum of Squares for permuational multivariate analysis of variance (PERMANOVA)15.
To calculate differences between timepoints and disease status, a linear mixed-effects model was applied using the ‘lme4’ package19, in which the timepoints (prior, 2M, 6M) and disease status (MS or non-MS) were considered fixed effects, and patients were random effects. 4999 permutations were then run using the ‘permanova.lmer’ function as part of the ‘predictmeans’ R package to calculate p-values20. Permutational tests are powerful non-parametric tests, as they do not assume normal distribution or homogeneity of variance, and only assume data are exchangeable15,21. 4999 permutations were done to generate p-values. Holm’s corrections were used for multiple comparisons which were performed to compare various study timepoints (prior vs. 2M, 2M vs. 6M, prior vs. 6M, non-MS prior vs. non-MS 2M, non-MS 2M vs. non-MS 6M, non-MS prior vs. non-MS 6M, prior vs. non-MS prior, 2M vs. non-MS 2M, 6M vs. non-MS 6M).
A p-value < 0.05 was considered statistically significant. All plots were generated using GraphPad Prism software (GraphPad Software, San Diego, CA, USA) version 9.3.1 and 9.4.1.
Results
To investigate the impacts of CladT on peripheral ILC in MS patients (Supplementary Tables S1, S2), we established a 43-parameter CyTOF panel (Supplementary Table S4), which allowed us to differentiate ILC subtypes in PBMC from MS patients at baseline (prior), as well as 2 (2M) and 6 months (6M) following the onset of CladT treatment (Supplementary Tables S2, S3). Furthermore, PBMC for ILC analysis were derived from non-MS controls (Supplementary Table S2) at baseline, as well as 2 and 6 months thereafter.
In total, 12 MS patients and 10 non-MS patients were analyzed in this study. No MS patients were on DMT at the time of blood sampling at study entry. MS patients prior to CladT treatment (prior, n = 12) were treatment-naïve (n = 4) or free from treatment for a minimum of 2 months prior to CladT (n = 8). All MS patients had low disability (EDSS range 0–2). MS activity was defined as new T2 and/or T1 Gadolinium-enhancing lesions in the 6 months before the onset of CladT (active MS: n = 9, inactive MS: n = 3).
ILC were defined as live CD45+ PBMC (Supplementary Fig. S1), negative for lineage markers (Lin−) CD3, CD19, CD14, CD11c, CD123, CD34, FcεRIa and TCRαβ (Fig. 1a). Lin− cells were gated into Lin−CD56+CD94+ NK cells (CD56bright and CD56dim NK cells) (Fig. 1b), and Lin−CD56−CD94−CD127+ helper ILC (CD294−CD117− ILC1, CD294+CD117+/− ILC2 and CD294−CD117+ ILC3) (Fig. 1c).
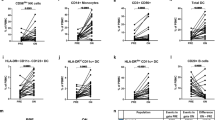
Firstly, CladT treatment induced significant reductions in PBMC numbers at 2 (Fig. 2a) and 6 months post-administration (Fig. 2a). The decrease in PBMC was resembled by total and CD56dim NK cells at the 2M (Fig. 2b) but not 6M timepoint. At the same time, no marked alterations in CD56bright NK cells were apparent (Fig. 2b). Helper ILC and ILC1 reflected the significant decrease in PBMC at 6M (Fig. 2c) but not 2M. In contrast, ILC3 were diminished at the 2M (Fig. 2c) but not 6M timepoint, while ILC2 remained unaffected (Fig. 2c). Non-MS controls did not demonstrate any changes in PBMC or ILC throughout the study (Supplementary Fig. S3) and did not significantly differ from MS patients at any timepoint (Supplementary Fig. S4). Furthermore, the observed trends in helper ILC subset levels were not reflected by expression levels of CD127 on these cell types (measured as median signal intensity) (Supplementary Fig. S5).
CladT reduce ILC but exempt CD56bright NK and ILC2 subsets. (a) Peripheral blood mononuclear cell (PBMC) count (106/mL) across patients at baseline (prior, n = 12), and 2 (2M, n = 10) and 6 months (6M, n = 12) following the first administration of CladT. (b) Cell counts (cells/mL) of CD56+CD94+, CD56dim and CD56bright NK cells at prior, 2M and 6M. NK cells were defined as live CD45+Lin−(CD3−CD19−CD14−CD11c−CD123−CD34−FcεRIa−TCRab−) CD56+CD94+, and further subdivided into CD56dim and CD56bright subpopulations. (c) Cell counts (cells/mL) of helper ILC (defined as live CD45+Lin−(CD3−CD19−CD14−CD11c−CD123−CD34−FcεRIa−TCRab−) CD56−CD94−CD127+helper ILC), and ILC1 (CD294−CD117−), ILC2 (CD294+CD117+/−) and ILC3 (CD294−CD117+) subsets at prior (n = 12), 2M (n = 10) and 6M (n = 12). A linear mixed-effects model was calculated to compare between MS patients before and after treatment. 4999 permutations were then run to calculate p-values. Holm’s corrections were used for multiple comparisons which were performed to compare the different timepoints (prior, 2M & 6M). p-values of p < 0.1 are indicated on the graphs.
To further elucidate the observed shifts in ILC upon CladT, ILC immunophenotypes were classified by FIt-SNE dimensionality reduction and assessed at all study timepoints.
FIt-SNE of total NK cells revealed CD38+/− NK cell subpopulations (Fig. 3a). CD38 expression levels were not related to high or low expression of NK-cell defining markers CD56 or CD94 among total CD56+CD94+ NK cells (Supplementary Fig. S6), which was further reflected by similarly distributed CD38+ populations among NK cell subsets (34.90% of CD56+CD94+, 33.31% of CD56bright and 34.86% of CD56dim NK cells were CD38+ on average). Strikingly, CD38+NK cells were not affected by CladT (Fig. 3b). In contrast, CD38− total and CD38−CD56dim NK cells were significantly diminished at 2M (Fig. 3c), reflecting the above-mentioned abatements in total and CD56dim NK cells upon CladT (Fig. 2b). Like CD56bright NK cells, CD38−CD56bright NK cells did not undergo any significant changes from prior to 2M (Fig. 3c). However, they displayed an increment from 2M to 6M (Fig. 3c), which was not observed in total CD56bright NK cells (Fig. 2b). Furthermore, clear enhancements in CD38 median signal intensity among total CD56+CD94+, CD56bright and CD56dim NK cells were observed from prior to 2M and prior to 6M, with a slight decline from 2M to 6M (Fig. 3d), which were not apparent among non-MS participants (Supplementary Fig. S7).
CD38+ but not CD38− NK cells remain unaffected by CladT. (a) Expression levels of CD38 among CD56+CD94+ NK cells on Fast Interpolation-based t-stochastic neighbor embedding (FIt-SNE) plots. A subsample of up to 70,000 cells from all study participants (non-MS controls, MS patients from all timepoints and batch controls) were included for the generation of FIt-SNE plots. (b) CD38+CD56+CD94+, CD38+CD56dim, CD38+CD56bright NK cells as cells/mL in MS patients at baseline (prior, n = 12), and 2 (2M, n = 10), and 6 months (6M, n = 12) following administration of CladT. (c) CD38-CD56+CD94+, CD38−CD56dim and CD38−CD56bright NK cells as cells/mL in MS patients prior (n = 12), 2M (n = 10), and 6M (n = 12) following administration of CladT. (D) CD38 median signal intensity of MS patients at prior (n = 12), 2M (n = 10), and 6M (n = 12) following administration of CladT. A linear mixed-effects model was calculated to compare between MS patients before and after treatment. 4999 permutations were then run to calculate p-values. Holm’s corrections were used for multiple comparisons which were performed to compare the different timepoints (prior, 2M & 6M). p-values of p < 0.1 are indicated on the graphs.
Next, FIt-SNE was performed on helper ILC to elucidate ILC immunophenotypes that are potentially involved in the observed ILC1 and ILC3 shifts upon CladT treatment. FIt-SNE differentiated helper ILC subsets ILC2 and ILC3 by their expression of CD294+ (ILC2) and CD117+ (ILC3), while ILC1 did not express these markers (Supplementary Fig. S8). FIt-SNE plots also identified small helper ILC populations of high HLA-DR, CD103, and CD335 expression, but did not demonstrate positive expression of co-stimulatory signals CD80 and CD86, or any expression of natural cytotoxicity receptor CD336 (Supplementary Fig. S9). However, FIt-SNE plots depicted clear bimodal CCR6 and CD5 expression by helper ILC immunophenotypes (Fig. 4a,b), which were further investigated.
FIt-SNE depicts CCR6 (CD196) and CD5 expression on helper ILC. Expression levels of (a) CCR6 (CD196) and (b) CD5 among Lin−CD56−CD94−CD127+ helper ILC on Fast interpolation-based t-stochastic neighbor embedding (Fit-SNE) plots. A subsample of up to 70,000 cells from all study participants (non-MS controls, MS patients from all timepoints and batch controls) were included for the generation of FIt-SNE plots.
16.60% of total helper ILC were identified as CCR6+, of which 14.03% were ILC1, and 27.25% ILC3. Reductions in helper ILC1 and ILC3 upon CladT (Fig. 2c) were reflected by CCR6+ and CCR6- ILC1 (Fig. 5a), as well as CCR6- ILC3 (Fig. 5b). Strikingly, CCR6+ ILC3 were excluded from CladT-caused decreases in helper ILC (Fig. 5b). No significant shifts were observed among CCR6+/− helper ILC1 and ILC3 when analyzed as a proportion of total helper ILC (Supplementary Fig. S10).
CCR6+ ILC3 are exempted from CladT-induced reductions in helper ILC. (a) CCR6+/− ILC1, and (b) CCR6+/− ILC3 as cells/mL in MS patients at baseline (prior, n = 11), and 2 (2M, n = 10), and 6 months (6M, n = 11) following administration of CladT. A linear mixed-effects model was calculated to compare between MS patients before and after treatment. 4999 permutations were then run to calculate p-values. Holm’s corrections were used for multiple comparisons which were performed to compare the different timepoints (prior, 2M & 6M). p-values of p < 0.1 are indicated on the graphs.
Furthermore, 43.09% of helper ILC were identified as CD5+. Most CD5+ ILC were ILC1 (54.19% CD5+ ILC1), whereas merely 2.93% of ILC3 were CD5+. Similar to total ILC1 counts (Fig. 2c), CD5+ and CD5– ILC1 numbers exhibited marked declines at 6M (Fig. 6a). Interestingly, when CD5+ ILC were analyzed as a percentage of helper ILC, they demonstrated a significant increase from baseline to 2M (Fig. 6b), and a subsequent decline from 2M to 6M (Fig. 6b). In contrast, CD5− ILC1 significantly decreased from prior to 2M (Fig. 6b), but recovered from 2M to 6M (Fig. 6b).
CD5+ ILC1 demonstrate short-term increments upon CladT within the helper ILC compartment. (a) CD5+ ILC1 and CD5− ILC1 as cells/mL in MS patients at baseline (prior, n = 12), and 2 (2M, n = 10), and 6 months (6M, n = 12) following administration of CladT. (b) CD5+ ILC1 and CD5−ILC1 as %Lin−CD56−CD94−CD127+ helper ILC (%Helper ILC) in MS patients at prior (n = 12), 2M (n = 10), and 6M (n = 12) following administration of CladT. A linear mixed-effects model was calculated to compare between MS patients before and after treatment. 4999 permutations were then run to calculate p-values. Holm’s corrections were used for multiple comparisons which were performed to compare the different timepoints (prior, 2M & 6M). p-values of p < 0.1 are indicated on the graphs.
Discussion
We present here a comprehensive mass cytometry analysis of circulating ILC subsets (CD56bright & CD56dim NK cells, ILC1, ILC2 & ILC3) in CladT-treated RRMS patients.
By semi-selective immune cell depletion, CladT is thought to induce the reconstitution of an immune system more tolerant of the CNS2. This likely occurs through the expansion of immune cells that remain despite immune reconstitution therapeutics, such as CladT2.
Among NK cells, CladT spared CD56bright NK cells, which are known for autologous CD4+ T cell suppression22. This function is impaired in MS patients22 but is reversed by several DMTs through numeral, proportional and/or functional enhancements in CD56bright NK cells13,22. Similarly, Moser et al.23 reported CD56bright regulatory NK cells with non-significant numeral expansions upon CladT. In contrast, enhanced circulating CD56dim NK cell numbers24 and proportions24,25 are associated with MS disease activity24,25, while therapy-naïve MS patients with stable disease course demonstrate reduced CD56dim NK cells compared to healthy subjects26. Therefore, CladT-induced reductions of CD56dim NK cells but unaltered CD56bright NK cell levels imply an MS-beneficial immune reconstitution within the NK cell compartment.
Furthermore, we found that CD38+ NK cells were resistant to CladT-induced immune cell depletion and demonstrated enhanced CD38 expression upon CladT. CD38+ NK cells may impose regulatory functions in MS, as CD38+CD56bright NK cells are implicated in adenosine production, which consequently inhibits autologous CD4+ T cell proliferation22. Interestingly, the anti-CD25 monoclonal antibody daclizumab increases CD38 expression by CD56bright NK cells27. Moreover, CD38 characterizes mature NK cells, which demonstrate efficacious cytokine production and cytolytic functions28. We also noted expansions of CD38−CD56bright from 2M to 6M. Thus, immune cell depletion by CladT may foster subsequent expansion of immature CD38−CD56bright NK cells, which, once matured to CD38+ NK cells, have MS-beneficial effects.
Similarly, CladT caused selective depletion of helper ILC subsets. Only ILC1 and ILC3, but not ILC2, were significantly reduced upon CladT. Coherently, observations in MS patients and/or experimental autoimmune encephalomyelitis (EAE) models have pointed towards pathogenic influences of ILC1 and ILC310,11,12,29, but beneficial impacts of ILC229.
In addition, we delineated CCR6 and CD5+ helper ILC immunophenotypes by FIt-SNE, which are of particular interest in CladT-treated MS patients.
Firstly, CCR6+ ILC3 infiltrate the CNS, where they stimulate myelin-specific T cells in EAE30. Concomitantly, cladribine impedes lymphocyte transition across the blood brain barrier31,32. Hence, our observations in which CCR6+ ILC3 were excluded from CladT-induced peripheral immune cell reduction suggest reduced migration of CCR6+ ILC3 to the CNS following treatment with CladT.
Secondly, our reported increases in CD5+ ILC1 proportions among helper ILC imply short-term replenishment of helper ILC upon CladT-induced immune cell depletion, as CD5+ ILC encompass immature ILC which however downregulate expression of CD5 once they acquire effector functions33. Hence, proportional expansions in CD5+ ILC1 suggest that CladT reduces mature ILC while exempting their immature precursors.
Limitations to our study include a relatively low n-number of study participants, which did not enable separate analysis of active versus non-active MS subjects. Furthermore, the low occurrence of ILC in circulating blood was another challenge in our analyses. No MS patients relapsed during the observational period. Hence, the effect on the ILC compartment during MS relapse needs to be addressed in future studies.
Moreover, ratios of deoxycytidine kinase (DCK) and 5′-nucleotidase (5′-NT), which activate and inactivate CladT metabolites respectively, are presumed as the determinant of its cell type-specificity3,4. To our knowledge, DCK:5′-NT ratios have so far only been measured among NK cells4,34 but not helper ILC. Strikingly, CD56bright and CD56dim NK cells show comparable DCK:5′-NT ratios4,34, but were affected differently by CladT in our study. Possibly, CladT-induced effects on other immune cells influence ILC subtypes indirectly, especially as ILC display essential crosstalk to other immune cells5. Lastly, future studies should evaluate DCK enzyme activity34, and ADA enzyme levels among ILC35, which may also influence vulnerability to CladT34,35.
In summary, CladT caused dramatic reductions in the majority of ILC, but spared potentially MS-inhibitory CD56bright NK cells, ILC2, and CD38+ NK cells. Consistent peripheral levels of CCR6+ ILC3 may further depict CladT-altered migration of pro-inflammatory immune cells to the CNS. Replenishment of the ILC compartment upon CladT is expected, as enhancements in CD38−CD56bright NK cells and CD5+ ILC1 are likely to comprise of immature ILC. Hence, we suggest ILC-specific resets of the immune system towards more tolerogenicity, with influential implications for the development of future therapeutic targets.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2M :
-
2 months after cladribine tablets
- 5′-NT:
-
5′-Nucleotidase
- 6M :
-
6 months after cladribine tablets
- BMC:
-
Brain and Mind Centre
- CAS:
-
Cell Acquisition Solution
- CNS:
-
Central nervous system
- CladT:
-
Cladribine tablets
- DCK:
-
Deoxycytidine kinase
- DMT:
-
Disease-modifying therapy
- EAE:
-
Experimental autoimmune encephalomyelitis
- ILC:
-
Innate lymphoid cell
- MS:
-
Multiple sclerosis
- PFA:
-
Paraformaldehyde
- PBMC:
-
Peripheral blood mononuclear cells
- PERMANOVA:
-
Permuational multivariate analysis of variance
- RRMS:
-
Relapsing/remitting MS
References
Baecher-Allan, C., Kaskow, B. J. & Weiner, H. L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 97, 742–768. https://doi.org/10.1016/j.neuron.2018.01.021 (2018).
Lunemann, J. D., Ruck, T., Muraro, P. A., Bar-Or, A. & Wiendl, H. Immune reconstitution therapies: Concepts for durable remission in multiple sclerosis. Nat. Rev. Neurol. 16, 56–62. https://doi.org/10.1038/s41582-019-0268-z (2020).
Meuth, S. G., Ruck, T., Aktas, O. & Hartung, H. P. Cladribine tablets: Oral immunotherapy of relapsing-remitting multiple sclerosis with short yearly treatment periods. Nervenarzt 89, 895–907. https://doi.org/10.1007/s00115-018-0498-0 (2018).
Voo, V. T. F., Butzkueven, H., Stankovich, J., O’Brien, T. & Monif, M. The development and impact of cladribine on lymphoid and myeloid cells in multiple sclerosis. Mult. Scler. Relat. Disord. 52, 102962. https://doi.org/10.1016/j.msard.2021.102962 (2021).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066. https://doi.org/10.1016/j.cell.2018.07.017 (2018).
Spits, H. et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149. https://doi.org/10.1038/nri3365 (2013).
Sonnenberg, G. F. & Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 21, 698–708. https://doi.org/10.1038/nm.3892 (2015).
Yang, Y., Day, J., Souza-Fonseca Guimaraes, F., Wicks, I. P. & Louis, C. Natural killer cells in inflammatory autoimmune diseases. Clin. Transl. Immunol. 10, e1250. https://doi.org/10.1002/cti2.1250 (2021).
Xiong, T. & Turner, J. E. Innate lymphoid cells in autoimmunity and chronic inflammatory diseases. Semin. Immunopathol. 40, 393–406. https://doi.org/10.1007/s00281-018-0670-4 (2018).
Perry, J. S. et al. Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci. Transl. Med. 4, 145. https://doi.org/10.1126/scitranslmed.3004140 (2012).
Gross, C. C. et al. Distinct pattern of lesion distribution in multiple sclerosis is associated with different circulating T-helper and helper-like innate lymphoid cell subsets. Mult. Scler. 23, 1025–1030. https://doi.org/10.1177/1352458516662726 (2017).
Degn, M. et al. Increased prevalence of lymphoid tissue inducer cells in the cerebrospinal fluid of patients with early multiple sclerosis. Mult. Scler. 22, 1013–1020. https://doi.org/10.1177/1352458515609795 (2016).
Laroni, A. & Uccelli, A. CD56bright natural killer cells: A possible biomarker of different treatments in multiple sclerosis. J. Clin. Med. 9, 1450. https://doi.org/10.3390/jcm9051450 (2020).
Thompson, A. J. et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17, 162–173. https://doi.org/10.1016/s1474-4422(17)30470-2 (2018).
Marsh-Wakefield, F. et al. Peripheral B cell dysregulation is associated with relapse after long-term quiescence in multiple sclerosis patients. Immunol. Cell Biol. 100, 453–467. https://doi.org/10.1111/imcb.12552 (2022).
Linderman, G. C., Rachh, M., Hoskins, J. G., Steinerberger, S. & Kluger, Y. Fast interpolation-based t-SNE for improved visualization of single-cell RNA-seq data. Nat. Methods 16, 243–245. https://doi.org/10.1038/s41592-018-0308-4 (2019).
Ashhurst, T. M. et al. Integration, exploration, and analysis of high-dimensional single-cell cytometry data using Spectre. Cytometry A 101, 237–253. https://doi.org/10.1002/cyto.a.24350 (2022).
R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
Luo, D., Ganesh, S. & Koolaard, J. predictmeans: Calculate predicted means for linear models. R Package Version 1.0.6 (2021).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x (2001).
Gross, C. C. et al. Regulatory functions of natural killer cells in multiple sclerosis. Front. Immunol. 7, 606. https://doi.org/10.3389/fimmu.2016.00606 (2016).
Moser, T. et al. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann. Clin. Transl. Neurol. 7, 2199–2212. https://doi.org/10.1002/acn3.51206 (2020).
Caruana, P., Lemmert, K., Ribbons, K., Lea, R. & Lechner-Scott, J. Natural killer cell subpopulations are associated with MRI activity in a relapsing-remitting multiple sclerosis patient cohort from Australia. Mult. Scler. 23, 1479–1487. https://doi.org/10.1177/1352458516679267 (2017).
Hawke, S. et al. Selective modulation of trans-endothelial migration of lymphocyte subsets in multiple sclerosis patients under fingolimod treatment. J. Neuroimmunol. 349, 577392. https://doi.org/10.1016/j.jneuroim.2020.577392 (2020).
Gross, C. C. et al. Impaired NK-mediated regulation of T-cell activity in multiple sclerosis is reconstituted by IL-2 receptor modulation. Proc. Natl. Acad. Sci. U.S.A. 113, E2973–E2982. https://doi.org/10.1073/pnas.1524924113 (2016).
Ranganath, T. et al. Characterization of the impact of daclizumab beta on circulating natural killer cells by mass cytometry. Front. Immunol. 11, 714 (2020).
Le Gars, M. et al. CD38 contributes to human natural killer cell responses through a role in immune synapse formation. BioRxiv 53, 3343. https://doi.org/10.1101/349084v2 (2019).
De Pasquale, C., Campana, S., Bonaccorsi, I., Carrega, P. & Ferlazzo, G. ILC in chronic inflammation, cancer and targeting with biologicals. Mol. Aspects Med. 80, 100963. https://doi.org/10.1016/j.mam.2021.100963 (2021).
Grigg, J. B. et al. Antigen-presenting innate lymphoid cells orchestrate neuroinflammation. Nature 600, 707–712. https://doi.org/10.1038/s41586-021-04136-4 (2021).
Balasa, R., Barcutean, L., Mosora, O. & Manu, D. Reviewing the significance of blood-brain barrier disruption in multiple sclerosis pathology and treatment. Int. J. Mol. Sci. 22, 8370. https://doi.org/10.3390/ijms22168370 (2021).
Kopadze, T., Dobert, M., Leussink, V. I., Dehmel, T. & Kieseier, B. C. Cladribine impedes in vitro migration of mononuclear cells: A possible implication for treating multiple sclerosis. Eur. J. Neurol. 16, 409–412. https://doi.org/10.1111/j.1468-1331.2008.02433.x (2009).
Alisjahbana, A. et al. CD5 surface expression marks intravascular human innate lymphoid cells that have a distinct ontogeny and migrate to the lung. Front. Immunol. 12, 752104. https://doi.org/10.3389/fimmu.2021.752104 (2021).
Carlini, F. et al. Different susceptibility of T and B cells to cladribine depends on their levels of deoxycytidine kinase activity linked to activation status. J. Neuroimmune Pharmacol. https://doi.org/10.1007/s11481-021-09994-3 (2021).
Ceronie, B. et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J. Neurol. 265, 1199–1209. https://doi.org/10.1007/s00415-018-8830-y (2018).
Acknowledgements
FM-W is supported by the International Society for the Advancement of Cytometry (ISAC) Marylou Ingram Scholars program. This work contributed to an MD thesis by F.A-L (Leitner F. Innate Lymphoid Cells in Multiple Sclerosis. Medical University of Vienna; 2022). We gratefully acknowledge subsidised access to the Ramaciotti Facility for Human Systems Biology and Sydney Cytometry and thank the support staff in this core facility for their assistance.
Funding
This work was supported by a Research Grant from Multiple Sclerosis Research Australia (17-0140) and from Merck Healthcare Pty. Ltd. (MS700568_0030), Macquarie Park, Australia, an affiliate of Merck KGaA (CrossRef Funder ID: https://doi.org/10.13039/100009945).
Author information
Authors and Affiliations
Contributions
F.A.-L. wrote the main manuscript, analyzed data, prepared figures. P.J., A.J., F.M.-W. generated data. S.B., S.H., G.G., F.M.-W. supervised project, intellectual input. S.H. recruited patients. F.A.-L., P.J., A.J., S.H., G.G., F.M.-W. reviewed manuscript. G.G. and F.M.-W. contributed equally as senior authors. Written informed consent was obtained from all participants.
Corresponding authors
Ethics declarations
Competing interests
Dr Hawke has received research funding, travel, and educational grants from Merck Healthcare Pty. Ltd., Macquarie Park, Australia, an affiliate of Merck KGaA. Other authors have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aglas-Leitner, F.T., Juillard, P., Juillard, A. et al. Mass cytometry reveals cladribine-induced resets among innate lymphoid cells in multiple sclerosis. Sci Rep 12, 20411 (2022). https://doi.org/10.1038/s41598-022-24617-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24617-4
This article is cited by
-
The role of CD56bright NK cells in neurodegenerative disorders
Journal of Neuroinflammation (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.