Abstract
Tracking and differentiating small insects at the individual levels requires appropriate marking materials because of their small size. This study proposes and investigates the use of fluorescent silica nanoparticles (FSNPs) as an internal marker owing to their good optical properties and biocompatibility. FSNPs were prepared using the water-in-oil reverse microemulsion technique with Rubpy dye as a fluorophore. The obtained particles were spherical, monodispersed in nanosize and exhibited bright orange luminescence under ultraviolet (UV) light. Internal marking was accomplished in fruit flies (Drosophila melanogaster) through feeding. The result shows that the fruit flies exhibit bright luminescence in their abdomen when exposed to UV light. The marking persistence duration of FSNPs in the fruit fly bodies is longer than those of other fluorescent dyes. Fruit flies fed with FSNPs have a longer lifespan than those fed with Rubpy dye. There was no difference in fertility and negative geotaxis response among the treatment and control groups. These findings demonstrate that FSNPs can be used as an internal marker in fruit flies, and are possibly applied with other small insects with a translucent abdomen.
Similar content being viewed by others
Introduction
Marking is a crucial step in understanding the behavior, activities, and dispersal of actively mobile animals since it allows researchers to distinguish and identify them at the individual level1. Various techniques have been used for marking, depending on budget and target organisms2. Painting, tagging with bands or collars, mutilation, and tattooing are inexpensive and easy to implement3,4,5,6,7,8. Using more advanced technology, such as radio and satellite telemetry, offers more accurate data on animal movement and habitat use9,10. Some of these techniques have been applied to insects, but only to large species such as beetles, butterflies, bees, and dragonflies11,12,13,14,15,16. However, marking small insects is considerably more challenging owing to their size9.
Fluorescent marking is a technique used for small insects, where materials with luminescent properties are employed to mark outside or inside the targets. Individuals marked can be identified visually by their luminosity against a dark background when exposed to ultraviolet (UV) or short-wavelength light. Fluorescent dust is a conventional technique for marking small insects using particles that can glow under UV light. The procedure is straightforward: place the insects and dust in a container and shake until the dust sticks to the insect surface17,18. However, long-term studies might not be suitable because of the limited persistence lifetime, and too much dust may obstruct spiracles, affecting insect survival and behavior1.
Internal marking using fluorescent dye is another technique to overcome the drawbacks of fluorescent dust, where insects easily obtain the dye through feeding. This technique suits insects whose integument is thin and translucent enough to allow excitation and emission light to pass through19,20. Since dye molecules directly contact animal tissues and are possibly absorbed through the digestive tract, impacts on physiology are hardly inevitable and should be concerned21. Fluorescence quenching is another issue in which the fluorescence emission gradually fades out during the excitation period, mainly because of the reaction to oxygen in the surrounding environment22,23. This phenomenon limits the efficiency of fluorescent dyes when long-term observation is required.
Nanomaterials are currently gaining attention in biological science owing to their small size and unique properties. Quantum dots (QDs) and fluorescent silica nanoparticles (FSNPs) are two prominent types of fluorescent nanoparticles (NPs) that are useful for marking and labeling. QDs are semiconductor nanocrystals that emit bright luminescence under UV light24. Many colors are available depending on their elemental composition and size. External marking using QDs has been successfully used in Trilobium castaneum25. Additionally, using indirect marking, bee pollination has been tracked by labeling pollen with QDs26,27. However, most QDs are composed of heavy metals, especially cadmium, which is not environmentally friendly, and their toxicity to biological systems is still debated24,28.
FSNPs are another type of NPs in which numerous fluorophores are incorporated inside an amorphous matrix of silica particles, resulting in a significantly magnified optical signal compared to a single dye molecule29. Moreover, because the dye is contained within the silica matrix, which acts as an efficient barrier between the dye and the surrounding environment, the dye has great photostability22,23. Because of their great photostability, these nanoparticles are well suited for applications requiring high intensity or sustained excitations, such as detecting bacteria, cancer cells, and biomolecules30,31,32,33,34. However, there have been no reports of FSNPs being used as insect markers.
It is possible that using FSNPs as a fluorescent marking material in insects might be an effective approach. Therefore, this study evaluates the possibility of using FSNPs as a marker in small insects. The FSNPs are prepared, and their physical properties are characterized. The efficacy of marking and its influence on biological aspects are investigated using fruit flies, Drosophila melanogaster, as a model organism.
Results
Characteristics of FSNPs
Approximately 70 mg of dried FSNPs were obtained from each time of synthesis. Amount of fluorescent dye found in the supernatant after sysnthesis was very low (< 1% of total dye in the reaction system) which indicated that almost all of the dye molecules were trapped inside the NPs.
FSNPs were highly spherical and monodispersed (Fig. 1a), and NPs were uniform in size, with an average diameter of 65.78 ± 4.38 nm (Fig. 1b). True density of FSNPs was 1.763 g/cm3 and the concentration was 3.804 × 1015 particles/g of dried NPs. Based on calculation, the number of dye molecules trapped in a single NP was 43,407 molecules. The average zeta-potential of dispersed 1 mg/mL FSNPs in deionized water (pH = 5.8) was − 31.5 ± 0.76 mV (n = 6), indicating moderate stability. However, when FSNPs were dispersed in the liquid food for rearing fruit flies (pH = 5.8), zeta-potential increased to − 10.85 ± 0.40 mV (n = 6), indicating incipient instability.
The FSNP suspensions glowed bright orange luminescence under UV light (Fig. 2a). Absorption spectra of FSNPs and the Rubpy dye solution was similar with maximal absorption wavelengths at 454 and 452 nm, respectively (Fig. 2b). Maximal emission wavelength of FSNPs was 618 nm which was slightly blue-shifted relative to that of the Rubpy solution (624 nm) under a 460 nm excitation band (Fig. 2c). However, the fluorescence intensity of FSNPs was higher at equal dye molar concentrations. FSNPs showed greater photostability than that of the Rubpy dye solution, with their fluorescence brightness changing very little over the course of 2 h of excitation under a 150 W mercury vapour lamp (Fig. 2d).
(a) Orange luminescence of FSNP suspension and Rubpy dye solution under UV light, (b) absorption spectra of 0.1 mg/mL FSNPs and 0.027 mM Rubpy dye solution, (c) emission spectra of 1 mg/mL FSNPs and 0.27 mM Rubpy dye solution when excited with 460 nm excitation band, and (d) fluorescence stability of 1 mg/mL FSNPs suspension compared to 0.27 mM Rubpy dye solution after being exposed to a 150 W mercury vapor lamp for 2 h. Arrows indicate maximal absorption or maximal emission wavelengths.
Appropriate quantity of FSNPs for marking
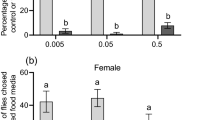
The marking efficiency varied significantly across NPs concentrations, increasing efficiency with increasing FSNP concentrations until 1.00 mg/mL (Fig. 3). The efficiency was not different between sex, and interactions between concentration and sex were not detected statistically (Table 1).
Marking the efficiency of FSNPs at different concentrations in Drosophila melanogaster (n of each treatment = 10, males and females combined). The horizontal lines within each box indicate the median, the box edges indicate the 75th and 25th percentiles, the whiskers show the maximum and minimum, and the circles represent outliers. Treatments with a common letter are not significantly different from each other as revealed by the aligned rank test at the 5% level of significance.
Feeding acceptance
Feeding acceptance measured from the amount of food consumed indicated a significant difference between male and female flies, but not among marker types (Table 1). Average consumption of all tested foods ranged between 0.89 and 0.91 µL/individuals/day for males and between 0.94 and 1.03 µL/individuals/day for females.
Marking persistence
Fluorescein- and FSNP-fed flies showed distinctively brighter luminescence in the abdomen than Rubpy-fed flies under UV light (Fig. 4). However, Rubpy-fed flies showed pale luminescence, which was difficult to distinguish from autofluorescence in non-marked flies. The duration of detectable luminescence from flies varied among marker types and sexes (Table 1). Luminescence from FSNP-fed flies could be detected longer than those with fed fluorescein and Rubpy dye (Fig. 5). However, luminescence in most flies did not persist longer than 10 h after feeding, except for some FSNP-fed females. During the experiment, bright fecal spots were noticed on the inner wall of vials from the first hour of feeding.
Drosophila melanogaster under a 40 W UV light bulb (365–400 nm wavelength) at a distance of 30 cm away after feeding liquid food containing different fluorescent markers for 1 h, (a) liquid food without a marker, (b) liquid food containing 0.27 mM Rubpy dye, (c) liquid food containing 0.27 mM fluorescein, and (d) liquid food containing 1 mg/mL FSNPs.
Detectable luminescence duration of Drosophila melanogaster after feeding liquid food containing different markers. The horizontal lines within each box indicate the median, the box edges indicate the 75th and 25th percentiles, the whiskers show the maximum and minimum, and the circles represent outliers. Treatments with a common letter are not significantly different from each other as revealed by the aligned rank test at the 5% level of significance. The numbers above the boxes are the sample size of each treatment.
Longevity
The effects of FSNPs on the longevity of adult flies were tested and compared with those of other dyes. Survivorship of fruit flies was significantly different among treatment groups (χ2 = 362, df = 3, P < 0.001). The survival probability of Rubpy-fed flies was lower than that of other groups of the same age (Fig. 6a). Overall, females had a slightly longer lifespan than males in all treatments. Median survival times of control, fluorescein-, Rubpy- and FSNP-fed flies were 41, 32.5, 16, and 34 days for males and 41, 40, 17, and 39.5 days for females, respectively (Fig. 6b).
(a) Survival curves of Drosophila melanogaster fed with liquid food containing different markers and (b) lifespans of the fruit fly in each treatment. The horizontal lines within each box indicate the median, the box edges indicate the 75th and 25th percentiles, the whiskers show the maximum and minimum, and the circles represent outliers. Treatments with a common letter are not significantly different from each other as revealed by the aligned rank test at the 5% level of significance.
Fertility
The number of offspring developed into adults was highly varied but not significantly different among treated males, treated females, and untreated fly pairs (χ2 = 11.85, df = 6, P = 0.07) (Table 2). The offspring sex ratio of these tested pairs was slightly female-biased, and it was not significantly different among treatment groups (χ2 = 2.19, df = 6, P = 0.90) (Table 2).
Negative geotaxis
The percentages of flies with negative geotaxis and the ability to traverse a 7 cm line in the control, fluorescein, Rubpy, and FSNP treatments were all 30% for males and 40%, 30%, 40%, and 50% for females, respectively. However, there was a significant difference between sex, but not among treatments (Table 1).
Discussion
Bright luminescence, high photostability, and low toxicity are important features of fluorescent markers when applied to living organisms or biological systems1,29. In this study, the observed optical properties of FSNPs and free dye were slightly different. The shifts of absorption and emission peaks of FSNPs relative to those of free dye might be due to polarity of the medium surrounding dye molecules which is different between inner and outer sides of NPs35. The fluorescence signal from FSNPs was brighter than that of the Rubpy dye solution at the equivalent-molar concentration (Figs. 2c and 4). Excellent brightness of FSNPs has been reported previously23,30,31. Numerous fluorophores are incorporated inside an amorphous matrix of silica particles, resulting in a significantly magnified optical signal compared to a single dye molecule29. Silica nanostructures serve as a barrier protecting dye molecules from interacting with fluorescence quenchers (e.g. oxygen) in the surrounding environment, and thus maintaining the high fluorescence quantum yields22,23,36.
FSNPs did not appear to accumulate in the gut passage of fruit flies, and they were gradually excreted in the first hour after being consumed. The experiment in previous studies using FD&C Blue No. 1 mixed in the food indicated that fruit flies could excrete the food and dye consumed within 30 min37,38. This indicated that food retention time in the guts of fruit flies was very fast. However, females had longer marking persistence time than males (Table 1 and Fig. 5). This could be explained by the variation of gut sizes between sex in which females typically have larger guts than males, resulting in longer food and dyes (or NPs) retention time in the guts39. Nevertheless, the absorption of NPs can occur because of electrostatic interaction between the surface charge of NPs and the membrane in the guts. For example, under acidic conditions in the middle midgut of fruit flies, positively charged NPs can pass quickly while absorbed and stay longer in the alkaline posterior segment of the midgut40,41. The observation of these interactions has been achieved using fluorescent polymeric NPs41. According to zetapotential analysis in this study, surface of FSNPs was negatively charged which might have electrostatic between the NPs and the negatively charged cell membrane in the guts, and might also be influenced by van der Waals forces and hydrogen bonds42.
Not only are dye molecules protected from the surrounding environment, but silica nanostructure also prevents the direct contact between the dye and living cells, which could negatively affect the target organisms29. This could explain the rapid decline of fly survival in the Rubpy-fed group, whereas the flies treated with FSNPs lived longer and had a lifespan comparable to that of the control group (Fig. 6a). Although no studies on the toxicity of Rubpy dye in living organisms have been conducted, it is hypothesized that this fluorescent dye has a negative effect on fly survival and that silica nanostructure could prevent this effect.
There are still discrepancies regarding the toxicity of silica NPs. For example, amorphous silica NPs are currently being developed as food additives and drug-delivery agents owing to their safety for human health, low toxicity, and compatibility with biological systems23,43,44,45,46. However, it has been reported that FSNPs increased apoptosis of human lung epithelial cells and showed cytotoxicity in MRC-5 cells47,48. Even when only FSNPs were prepared using the same process and fluorescent dye (Rubpy dye) as in this study, reports of toxicity varied among tested organisms. For example, they had no negative effect on zebrafish embryos but promoted apoptosis in human lung epithelial cells45,47. Silica NPs seem to have distinctively entomocidal effects and are developed to be pesticides. Furthermore, when used as dust, they absorb water from insect cuticles and cause abrasion on the wax layer, eventually leading to death from desiccation48,49,50. D. melanogaster larvae exposed to silica NPs undergo adverse effects from gut membrane destabilization, mouthparts deformation, and oxidative stress51,52. However, note that the toxicity of silica NPs depends on many factors, including the preparation technique, functional groups on the particle surface, particle size, dosage, and target cells or organisms53,54.
The FSNPs in this study aggregate faster in the liquid food than in deionized water. This phenomenon was confirmed by zeta-potential, which showed that when the particles were dispersed in liquid food containing sugar and peptone, the value was close to zero. Aggregation is a concerning issue when NPs are applied in the aqueous phase. This problem can be prevented by modifying functional groups on particle surfaces, making them suitable for applications in biological systems55. Therefore, investigating the effects of surface modification in vivo is recommended for future studies.
Conclusion
This study shows the achievement of using FSNPs synthesized using the water-in-oil reverse microemulsion (WORM) technique as an internal fluorescent marker in fruit flies. FSNPs were highly spherical and monodispersed, with an average diameter of 65.78 ± 4.38 nm. Fruit flies can easily intake the NPs through liquid food, yielding bright orange luminescence in the abdomen under UV light. Additionally, no adverse effects on survival and fertility were detected compared to the control group. The particle surface modification can be used to increase the persistence of NPs in the insect body or to design NPs with specific binding to target tissues/organs. More studies on the interactions between NPs and living organisms are required for future research.
Methods
Synthesis of FSNPs
FSNPs were prepared using the WORM technique with slight modifications from the previous studies by using Rubpy dye (tris (2,2′-bipyridyl)ruthenium(II) chloride) as a fluorophore due to well-embedded in the silica network, and there is no need of pre-modification of the dye molecules before NP synthesis31,32,34,56. Next, 15 mL cyclohexane, 3.6 mL 1-hexanol, 3.54 mL Triton™ X-100, and 0.96 mL of 20 mM Rubpy dye solution were mixed and stirred for 20 min. Then, 0.2 mL tetraethyl orthosilicate (TEOS) was added and stirred for 10 min. After the formation of WORM in the system, 0.2 mL of 28–30% ammonium solution was added to catalyze the reaction and stirred further for 24 h at room temperature under dark conditions.
To reduce dye leakage from NPs, the product was subsequently coated with a silica shell by repeating the reaction for one more cycle57. Then, 0.96 mL deionized water, 0.2 mL TEOS, and 0.2 mL of 28–30% ammonium solution were added to the slurry and stirred for 24 h. After the reaction was completed, FSNPs were separated from the solution with acetone precipitation and then washed with 95% ethanol and distilled water to remove the solvent and surfactant. Dye leakage was measured from the absorbance of the supernatant after precipitating the NP product at 460 nm using a spectrophotometer (Dlab, SP-V1000), and compared with the standard curve of Rubpy dye solution. The washed FSNPs were stored in a suspension in the dark at 4 °C.
Characterization of FSNPs
FSNPs were examined under a transmission electron microscope (TEM; JEOL JEM-1400). The diameter was measured from TEM images (three independent samples, 90 particles per sample) using ImageJ software (version 1.53o, NIH, USA; https://imagej.nih.gov/ij). True density of NPs was measured by helium pycnometry using a Ultrapycnomter 1000 (Quantachrome Instruments). The aggregation of NPs in an aqueous solution was measured in terms of zeta-potential using a zeta-sizer (Malvern Panalytical, Nano ZSP). The higher the value (positive or negative), the more stable the particle dispersion, with 0 to ± 10 mV indicating that the NPs are unstable and tend to aggregate, ± 10 to ± 30 mV incipiently instable, ± 30 to ± 40 mV moderately stable, ± 40 to ± 60 mV well stable, and values beyond ± 60 mV excellently stable58. Absorption spectra were analyzed using a spectrophotometer (Dlab, SP-V1000), and emission spectra were analyzed using a spectrofluorometer (Shimadzu, RF-6000) at a maximal excitation wavelength of 460 nm32. To test fluorescence stability, 1 mg/mL of FSNP suspension was exposed to a 150 W mercury vapour lamp, and its fluorescence intensity was measured every 10 min for 2 h.
FSNP concentration and the number of dye molecules embedded in a particle
Concentration of FSNPs was expressed as the number of NPs in 1 g of dried NPs, which was calculated from the formula for the volume of a sphere, as shown in Eq. (1):
where VNP is the volume of a NP and r is the average radius of FSNPs in the unit of cm.
The number of FSNPs (NNP) in 1 g was calculated according to Eq. (2):
where D is the true density of FSNPs in the unit of g/cm3.
To estimate the number of dye molecules trapped in a single NP, calculation was performed based on the assumption that all dye molecules were trapped inside FSNPs and each synthesis yielded 70 mg of dried NPs.
Fly culture and husbandry
Wild-type fruit flies, Drosophila melanogaster, collected from natural populations in Bangkok, Thailand, were bred and maintained on a standard cornmeal-sucrose-yeast agar medium (100 g/L cornmeal, 50 g/L sucrose, 50 g/L yeast powder, 20 g/L agar, 0.65 g/L sodium propionate, and 0.1 g/L methylparaben) at 25 °C ± 1 °C, 60% ± 10% relative humidity under a 12/12 h light/dark cycle. This condition was used throughout the study.
Appropriate FSNP concentration for marking
An appropriate concentration of FSNPs for marking fruit flies was measured in terms of marking efficiency. FSNPs were mixed in liquid food (100 g/L sucrose and 10 g/L yeast extract) and adjusted to five concentrations: 0.25, 0.5, 1.0, 1.5, and 2.0 mg/mL. Groups of 20–40 flies with 3 to 5 days of adult age were starved for 18–20 h in 240 mL glass bottles containing 1.5% water agar before the experiment. The experiment was conducted in 240 mL glass bottles with eight feeders made of 200 µL pipette tips hanging from the bottle lid (8 tips per bottle, loading 20 µL liquid food per tip). The flies were allowed to feed on the food for 2 h before being freeze-killed. The luminescence of flies due to FSNPs intake, which was visible through the cuticle of the abdomen, was visually inspected at a distance of 30 cm away from the flies under a 40 W UV light bulb (365–400 nm wavelength). Fluorescent brightness was scored ranging from 0 to 2: 0 = no fluorescence, 1 = slight fluorescence, and 2 = bright fluoscence. Marking efficiency was calculated following the formula described in a previous study17, as shown in Eq. (3):
where nF is the number of flies with each score value, b is the brightness score, B is the highest possible score (here: 2), and NF is the total number of scored flies. The experiment was conducted with five replications.
Feeding acceptance
Feeding acceptance was measured as the quantity of food intake through capillary feeders during a 3 day period59. Groups of five flies with 3–5 days of adult age were kept in 45 mL glass vials containing 1.5% water agar. Liquid food containing 1 mg/mL FSNPs was provided to flies through two 5 µL capillary feeders hanging from the cotton plug. After 24 h, the quantity of food intake was measured, and the old capillaries were replaced by new ones. The reduction of liquid food from evaporation was determined from feeders in the vials without flies. Feeding acceptance of FSNPs was compared with the feeding of other markers, i.e., liquid food containing 0.27 mM fluorescein, liquid food containing 0.27 mM Rubpy dye, and liquid food without marker (control). The concentration of dye solution at 0.27 mM was selected because this concentration was equivalent to the amount of dye presented in 1 mg/mL FSNPs based on calculation.
Marking persistence
Fruit flies with 3–5 days of adult age were starved for 18–20 h in 45 mL glass vials containing 1.5% water agar. The test began at 08:00 h by allowing flies to feed on liquid food containing different markers as described earlier through feeders for 1 h. Then, the markers were replaced by normal liquid food. Luminescence from the flies was observed under UV light every hour until the luminescence was not detected. Flies without luminescence at the beginning (indicating no feeding) were excluded from the analysis.
Longevity experiment
Groups of 10 flies consisting of five males and females with an adult age less than 12 h were kept in 45 mL glass vials containing 1.5% water agar. Liquid food containing different markers described earlier was provided to the flies ad libitum through 200 µL pipette tips and renewed every other day. The numbers of dead and alive flies were checked daily, and the live flies were transferred to new vials every week to prevent fungal infection until all individuals died.
Effects on fertility
Newly emerged virgin flies (adult age less than 6 h) of the same sex were treated by rearing with tested food containing different markers in the same way as the longevity experiment for 7 days. To identify the effects of dyes or FSNPs on reproduction in relation to the sex, treated (fed with food containing the marker) and untreated (fed with normal food) flies were paired, i.e., treated male × untreated female and untreated male × treated female. The flies were reared with CYS agar one pair per vial and transferred to fresh vials every five days for 10 days. The vials were kept under standard rearing conditions until all offspring developed into adults. The number of offspring was counted, and the sex was identified.
Negative geotaxis
The effects of markers on locomotion and behavioral response were investigated from negative geotaxis using a climbing assay. The rearing, food, and feeding apparatus were conducted similarly to the longevity experiment. After 2 weeks of the feeding tested foods, groups of 10 flies of the same sex were transferred to new glass vials, and the vials were marked at 7 cm above the bottom. Flies were left without disturbance for 1 h before conducting the behavioral test. Then, the flies were tapped to the bottom three times and given 10 s to climb upward to the top. The numbers of flies above and below the 7 cm mark were recorded. The experiment was repeated for three trials with 1 min intervals. Ten replications were conducted per treatment.
Statistical analyses
The effects of markers, sex, and their interaction on marking parameters and characteristics of fruit flies were analyzed using an aligned rank test60. The effects of markers on longevity were visualized by plotting survival curves using Kaplan–Meier analysis, and differences in survival rates were tested using the log-rank test. The number of offspring and offspring sex ratio were compared among treatments using Kruskal–Wallis test. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using the base, ARTool, survival, and survminer packages in R, version 4.1.361.
Data availability
All data generated and analyzed within this study are available from the corresponding author upon reasonable request.
References
Hagler, J. R. & Jackson, C. G. Methods for marking insects: current techniques and future prospects. Annu. Rev. Entomol. 46, 511–543. https://doi.org/10.1146/annurev.ento.46.1.511 (2001).
Thomas, B., Holland, J. D. & Minot, E. O. Wildlife tracking technology options and cost considerations. Wildl. Res. 38, 653–663. https://doi.org/10.1071/WR10211 (2011).
De Souza, A. R., Ribeiro, B., José, N. & Prezoto, F. Paint marking social wasps: An evaluation of behavioral effects and toxicity. Entomol. Exp. Appl. 144, 244–247 (2012).
Fisher, P. Review of using Rhodamine B as a marker for wildlife studies. Wildl. Soc. Bull. 27, 318–329 (1999).
Schmera, D., Szentesi, Á. & Jermy, T. Within field movement of overwintered Colorado potato beetle: A patch-based approach. J. Appl. Entomol. 131, 34–39 (2007).
Unruh, T. & Chauvin, R. Elytral punctures: A rapid, reliable method for marking Colorado potato beetle. Can. Entomol. 125, 55–63 (1993).
Paterson, W. et al. Assessment of flipper tag site healing in gray seal pups using thermography. Mar. Mamm. Sci. 27, 295–305. https://doi.org/10.1111/j.1748-7692.2010.00400.x (2011).
McGregor, M. & Jones, D. Tattoo pens as a low-cost approach to in-field permanent identification of medium-sized mammals. Wildl. Soc. Bull. 40, 169–173. https://doi.org/10.1002/wsb.631 (2016).
Daniel Kissling, W., Pattemore, D. E. & Hagen, M. Challenges and prospects in the telemetry of insects. Biol. Rev. 89, 511–530. https://doi.org/10.1111/brv.12065 (2014).
Whitford, M. & Klimley, A. P. An overview of behavioral, physiological, and environmental sensors used in animal biotelemetry and biologging studies. Anim. Biotelem. 7, 26. https://doi.org/10.1186/s40317-019-0189-z (2019).
Fisher, K. E., Adelman, J. S. & Bradbury, S. P. Employing very high frequency (VHF) radio telemetry to recreate monarch butterfly flight paths. Environ. Entomol. 49, 312–323. https://doi.org/10.1093/ee/nvaa019 (2020).
Moskowitz, D. & May, M. Adult tiger spiketail (Cordulegaster erronea Hagen) habitat use and home range observed via radio-telemetry with conservation recommendations. J. Insect Conserv. 21, 885–895. https://doi.org/10.1007/s10841-017-0027-7 (2017).
Rink, M. & Sinsch, U. Radio-telemetric monitoring of dispersing stag beetles: Implications for conservation. J. Zool. 272, 235–243. https://doi.org/10.1111/j.1469-7998.2006.00282.x (2007).
Tini, M. et al. Detection of stag beetle oviposition sites by combining telemetry and emergence traps. Nat. Conserv. 19, 81 (2017).
Tini, M. et al. Use of space and dispersal ability of a flagship saproxylic insect: A telemetric study of the stag beetle (Lucanus cervus) in a relict lowland forest. Insect Conserv. Divers. 11, 116–129 (2018).
Wikelski, M. et al. Large-range movements of neotropical orchid bees observed via radio telemetry. PLoS ONE 5, e10738. https://doi.org/10.1371/journal.pone.0010738 (2010).
Clymans, R., Van Kerckvoorde, V., Beliën, T., Bylemans, D. & De Clercq, P. Marking Drosophila suzukii (Diptera: Drosophilidae) with fluorescent dusts. Insects 11, 152. https://doi.org/10.3390/insects11030152 (2020).
Russell, T. L. et al. Anopheles farauti is a homogeneous population that blood feeds early and outdoors in the Solomon Islands. Malar. J. 15, 1–7. https://doi.org/10.1186/s12936-016-1194-9 (2016).
Sarkar, D., Muthukrishnan, S. & Sarkar, M. Fluorescent marked mosquito offer a method for tracking and study mosquito behaviour. Int. J. Mosq. Res. 4, 5–9 (2017).
Gayahan, G. G. & Tschinkel, W. R. Fire ants, Solenopsis invicta, dry and store insect pieces for later use. J. Insect Sci. 8, 1. https://doi.org/10.1673/031.008.3901 (2008).
Alford, R. et al. Toxicity of organic fluorophores used in molecular imaging: Literature review. Mol. Imag. 8, 341–354 (2009).
Yang, H.-H. et al. Nanometer fluorescent hybrid silica particle as ultrasensitive and photostable biological labels. Analyst 128, 462–466. https://doi.org/10.1039/B210192K (2003).
Cho, E. B., Volkov, D. O. & Sokolov, I. Ultrabright fluorescent mesoporous silica nanoparticles. Small 6, 2314–2319. https://doi.org/10.1002/smll.201001337 (2010).
Gidwani, B. et al. Quantum dots: Prospectives, toxicity, advances and applications. J. Drug Deliv. Sci. Technol. 61, 102308. https://doi.org/10.1016/j.jddst.2020.102308 (2021).
Gurdasani, K., Li, L., Rafter, M. A., Daglish, G. J. & Walter, G. H. Nanoparticles as potential external markers for mark–release–recapture studies on Tribolium castaneum. Entomol. Exp. Appl. 169, 575–581. https://doi.org/10.1111/eea.13040 (2021).
Konzmann, S., Kluth, M., Karadana, D. & Lunau, K. Pollinator effectiveness of a specialist bee exploiting a generalist plant—tracking pollen transfer by Heriades truncorum with quantum dots. Apidologie 51, 201–211. https://doi.org/10.1007/s13592-019-00700-0 (2020).
Minnaar, C. & Anderson, B. Using quantum dots as pollen labels to track the fates of individual pollen grains. Methods Ecol. Evol. 10, 604–614. https://doi.org/10.1111/2041-210X.13155 (2019).
Wang, Z. & Tang, M. The cytotoxicity of core-shell or non-shell structure quantum dots and reflection on environmental friendly: A review. Environ. Res. 194, 110593. https://doi.org/10.1016/j.envres.2020.110593 (2021).
Wei, W., Wei, M. & Liu, S. Silica nanoparticles as a carrier for signal amplification. Rev. Anal. Chem 31, 163–176. https://doi.org/10.1515/revac-2012-0021 (2012).
Palantavida, S., Guz, N. V., Woodworth, C. D. & Sokolov, I. Ultrabright fluorescent mesoporous silica nanoparticles for prescreening of cervical cancer. Nanomed. Nanotechnol. Biol. Med. 9, 1255–1262. https://doi.org/10.1016/j.nano.2013.04.011 (2013).
Lian, W. et al. Ultrasensitive detection of biomolecules with fluorescent dye-doped nanoparticles. Anal. Biochem. 334, 135–144. https://doi.org/10.1016/j.ab.2004.08.005 (2004).
Santra, S., Zhang, P., Wang, K., Tapec, R. & Tan, W. Conjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkers. Anal. Chem. 73, 4988–4993. https://doi.org/10.1021/ac010406+ (2001).
Chen, Z.-Z. et al. Indirect immunofluorescence detection of E. coli O157: H7 with fluorescent silica nanoparticles. Biosens. Bioelectron. 66, 95–102. https://doi.org/10.1016/j.bios.2014.11.007 (2015).
Songvorawit, N., Tuitemwong, P., Tuchinda, K. & Tuitemwong, K. Fluorescent dye-doped silica nanoparticles with polyclonal antibodies for the rapid detection of Salmonella spp. Chiang Mai Univ. J. Nat. Sci. 12, 25–33 (2013).
Ma, D., Kell, A. J., Tan, S., Jakubek, Z. J. & Simard, B. Photophysical properties of dye-doped silica nanoparticles bearing different types of dye–silica interactions. J. Phys. Chem. C 113, 15974–15981 (2009).
Kalaparthi, V., Palantavida, S. & Sokolov, I. The nature of ultrabrightness of nanoporous fluorescent particles with physically encapsulated fluorescent dyes. J. Mater. Chem. C 4, 2197–2210 (2016).
Deshpande, S. A. et al. Quantifying Drosophila food intake: comparative analysis of current methodology. Nat. Methods 11, 535–540. https://doi.org/10.1038/nmeth.2899 (2014).
Shell, B. C. et al. Measurement of solid food intake in Drosophila via consumption-excretion of a dye tracer. Sci. Rep. 8, 11536. https://doi.org/10.1038/s41598-018-29813-9 (2018).
Millington, J. W. & Rideout, E. J. Sexual dimorphism: Ecdysone modulates sex differences in the gut. Curr. Biol. 30, R1327–R1330. https://doi.org/10.1016/j.cub.2020.08.088 (2020).
Pappus, S. A. & Mishra, M. in Cellular and molecular toxicology of nanoparticles Vol. 1048 (eds Q Saquib, M Faisal, A Al-Khedhairy, & A Alatar) 311–322 (Springer, Cham, 2018).
Jiang, S. et al. Oral administration and selective uptake of polymeric nanoparticles in Drosophila larvae as an in vivo model. ACS Biomater. Sci. Eng. 1, 1077–1084. https://doi.org/10.1021/acsbiomaterials.5b00163 (2015).
Kettiger, H., Québatte, G., Perrone, B. & Huwyler, J. Interactions between silica nanoparticles and phospholipid membranes. Biochim. Biophys. Acta Biomembr. 1858, 2163–2170. https://doi.org/10.1016/j.bbamem.2016.06.023 (2016).
Go, M.-R., Bae, S.-H., Kim, H.-J., Yu, J. & Choi, S.-J. Interactions between food additive silica nanoparticles and food matrices. Front. Microbiol. 8, 1013. https://doi.org/10.3389/fmicb.2017.01013 (2017).
Wang, Y. et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 11, 313–327. https://doi.org/10.1016/j.nano.2014.09.014 (2015).
Fent, K., Weisbrod, C. J., Wirth-Heller, A. & Pieles, U. Assessment of uptake and toxicity of fluorescent silica nanoparticles in zebrafish (Danio rerio) early life stages. Aquat. Toxicol. 100, 218–228. https://doi.org/10.1016/j.aquatox.2010.02.019 (2010).
Chandra, S., Beaune, G., Shirahata, N. & Winnik, F. M. A one-pot synthesis of water soluble highly fluorescent silica nanoparticles. J. Mater. Chem. B 5, 1363–1370. https://doi.org/10.1039/C6TB02813F (2017).
Jin, Y., Kannan, S., Wu, M. & Zhao, J. X. Toxicity of luminescent silica nanoparticles to living cells. Chem. Res. Toxicol. 20, 1126–1133. https://doi.org/10.1021/tx7001959 (2007).
Debnath, N., Das, S., Patra, P., Mitra, S. & Goswami, A. Toxicological evaluation of entomotoxic silica nanoparticle. Toxicol. Environ. Chem. 94, 944–951. https://doi.org/10.1080/02772248.2012.682462 (2012).
Shoaib, A. et al. Entomotoxic effect of silicon dioxide nanoparticles on Plutella xylostella (L.)(Lepidoptera: Plutellidae) under laboratory conditions. Toxicol. Environ. Chem. 100, 80–91. https://doi.org/10.1080/02772248.2017.1387786 (2018).
Debnath, N. et al. Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.). J. Pest Sci. 84, 99–105. https://doi.org/10.1007/s10340-010-0332-3 (2011).
Pandey, A., Chandra, S., Chauhan, L. K. S., Narayan, G. & Chowdhuri, D. K. Cellular internalization and stress response of ingested amorphous silica nanoparticles in the midgut of Drosophila melanogaster. Biochim. Biophys. Acta - Gen. Subj. 1830, 2256–2266. https://doi.org/10.1016/j.bbagen.2012.10.001 (2013).
Galal, O. & El-Samahy, M. Genetical effects of using silica nanoparticles as biopesticide on Drosophila melanogaster. Egypt J. Genet. Cytol. 41, 87–106. https://doi.org/10.21608/ejgc.2012.10561 (2012).
Croissant, J. G., Butler, K. S., Zink, J. I. & Brinker, C. J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 5, 886–909. https://doi.org/10.1038/s41578-020-0230-0 (2020).
Kim, I.-Y., Joachim, E., Choi, H. & Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 11, 1407–1416. https://doi.org/10.1016/j.nano.2015.03.004 (2015).
Bagwe, R. P., Hilliard, L. R. & Tan, W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 22, 4357–4362. https://doi.org/10.1021/la052797j (2006).
Gubala, V., Giovannini, G., Kunc, F., Monopoli, M. P. & Moore, C. J. Dye-doped silica nanoparticles: Synthesis, surface chemistry and bioapplications. Cancer Nanotechnol. 11, 1–43. https://doi.org/10.1186/s12645-019-0056-x (2020).
Yoo, H. & Pak, J. Synthesis of highly fluorescent silica nanoparticles in a reverse microemulsion through double-layered doping of organic fluorophores. J. Nanopart. Res. 15. 1609. https://doi.org/10.1007/s11051-013-1609-2 (2013).
Kumar, A. & Dixit, C. K. in Advances in Nanomedicine for the Delivery of Therapeutic Mucleic Acids (eds Surendra Nimesh, Ramesh Chandra, & Nidhi Gupta) 43–58 (Woodhead Publishing, 2017).
Diegelmann, S. et al. The CApillary FEeder assay measures food intake in Drosophila melanogaster. J. Vis. Exp. 121, 55024. https://doi.org/10.3791/55024 (2017).
Seaman, J. W. Jr., Walls, S. C., Wise, S. E. & Jaeger, R. G. Caveat emptor: Rank transform methods and interaction. Trends Ecol. Evol. 9, 261–263. https://doi.org/10.1016/0169-5347(94)90292-5 (1994).
R Core Team. (R Foundation for Statistical Computing, Vienna, Austria, 2022).
Acknowledgements
We thank the Petroleum and Petrochemical College, Chulalogkorn University, and Oral Biology Research Center, Chulalogkorn University for providing equipment for analyses. This research was funded by Ratchadapiseksompotch Fund Chulalongkorn University (CU_GR_62_61_23_24).
Author information
Authors and Affiliations
Contributions
N.S. initiated the research idea, acquired the funding, designed and performed most of the experiments, analyzed the data, and wrote the manuscript. P.P. and T.K. reared fruit flies and collected the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Songvorawit, N., Phengphuang, P. & Khongkhieo, T. Fluorescent silica nanoparticles as an internal marker in fruit flies and their effects on survivorship and fertility. Sci Rep 12, 19745 (2022). https://doi.org/10.1038/s41598-022-24301-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24301-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.