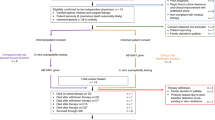
Abstract
The neutropenic thigh infection model is one of the standard models in pharmacokinetic/ pharmacodynamic (PK/PD) characterization of novel antibacterials which are urgently needed due to the rise of antimicrobial resistance. The model enables to investigate PK/PD parameters crucial for translation of animal results towards humans. However, the neutropenic thigh infection model can result in moderate to severe discomfort of the animals, especially when high inocula are used. Tramadol has been proven to reduce pain effectively. This study investigates if tramadol influences the bacterial burden in the primary organ, the thighs, and organs affected by secondary seeding. Therefore, several strains of the ESKAPE pathogens, namely S. aureus, P. aeruginosa, K. pneumoniae, E. coli, A. baumannii and E. faecalis were examined. It was shown that tramadol did not influence the bacterial burden neither in thighs nor in organs affected by secondary seeding for the strains of E. faecalis, S. aureus, P. aeruginosa, K. pneumoniae and E.coli tested here, whereas secondary seeding seemed to be affected by tramadol for the tested strain of A. baumannii. Consequently, it was demonstrated that tramadol is an option to reduce discomfort in the untreated group for the strains of five out of the six tested ESKAPE pathogens and, thereby, contributes to the refinement of one of the standard PK/PD models.
Similar content being viewed by others
Introduction
Antimicrobial resistance is a silent and deadly pandemic with several million individuals affected every day around the world1,2. Therefore, antimicrobial resistance in the so-called ESKAPE pathogens, namely Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa as well as Enterobacter species, is of high concern. As a result, those pathogens have been listed as priority 1 (critical priority) or 2 (high priority) pathogens by the WHO3,4. New treatment options are urgently needed to enable to keep—or at least try to keep—pace with the pathogens developing new strategies to overcome the mechanisms of action of current antibiotics in the market5. During preclinical development, the evaluation of the performance of a novel antibiotic or even a completely new treatment option6, such as siderophores (e.g. cefiderocol7) or antimicrobial conjugates8, is key. For that purpose, standard pharmacodynamic models are used, such as the neutropenic lung and the neutropenic thigh infection model. Both of them are deployed to determine efficacy of novel treatment strategies in vivo and, thus, constitute an important milestone during early preclinical development9. Neutropenic mice are used as neutropenia hampers the immune response and prevents rapid clearance of the infection by the immune system10. Consequently, this allows to study and determine the PK/PD (pharmacokinetic/pharmacodynamics) index which helps to elucidate which pharmacokinetic parameter drives the effect of a novel drug, i.e. Cmax/MIC, time over MIC or AUC/MIC. The determination of the PK/PD index is crucial for effective translation into humans11,12.
The neutropenic thigh infection model, first described in the early 1950s13, results in moderate discomfort, especially for those animals just receiving vehicle or no treatment, due to inflammation and swelling of thighs in the course of the infection. This can lead to earlier sacrifice of animals when the humane endpoint is reached before the actual endpoint if very high inocula are used14,15. Of note, only one study deploying the neutropenic thigh infection model was published, in which a drug for pain relief, namely buprenorphine, was used16. However, no pain relieving drug has been investigated systematically in the neutropenic thigh infection model for its capacity to reduce pain as well as discomfort in the vehicle or untreated group without affecting bacterial burden in the primary organ as well as other organs possibly affected by secondary seeding, such as kidneys and lungs.
In this study, tramadol was chosen as a pain reducing agent. Tramadol belongs to the group of µ-receptor agonists, but has a different side effect profile than classical µ-receptor-agonists, such as morphine or fentanyl: it is a weak µ-opioid receptor agonist with non-opioid-related effects, e.g. on serotonin and noradrenaline transporters17,18. Moreover, it has been frequently used for animal experimentation for the treatment of pain in several indications19. Here, it is investigated if tramadol has an impact on bacterial burden in the primary organ, the thighs, but also if it has an impact on the bacterial distribution towards different organs compared to a group not receiving pain reducing treatment in the neutropenic thigh infection model. This assessment is important as it would enable to use tramadol in the vehicle or untreated group only, supposing that groups treated with antibiotic will not experience discomfort due to the active substance reducing bacterial burden. There are three reports about a potential antibacterial activity of tramadol against different ESKAPE pathogens without elucidating what the mechanism of action is20,21,22. Consequently, it is necessary to assess if tramadol exerts an effect at the dose and under the specific infection conditions in this study.
Therefore, several ESKAPE strains including Gram-positive as well as Gram-negative bacteria are tested, namely S. aureus (two different strains), P. aeruginosa, K. pneumoniae, A. baumannii, E.coli and E. faecalis. Moreover, it is assessed, specifically for P. aeruginosa, if there is a difference for the same strain when the model is extended up to 48 h. To the best of one’s knowledge, this is the first study investigating the use of a pain reducing agent only in the untreated control groups of the neutropenic thigh infection model with the aim to determine if the readout of the model was affected. Finally, it was shown that tramadol did not influence bacterial burden or secondary seeding to other organs for the strains of E. faecalis, S. aureus, E. coli, P. aeruginosa and K. pneumoniae used in this study. For the strain of A. baumannii used in this study no significant influence on bacterial burden in thigh was detected, but secondary seeding to other organs seemed to occur more frequently in the tramadol-treated group.
Results
Independent of the inoculum: tramadol treatment does neither affect bacterial burden in thighs nor organ distribution in the neutropenic thigh infection model with E. faecalis ATCC 29212
First, animals were infected after receiving cyclophosphamide at days -4 and -1 to render them neutropenic, with two different inocula of E. faecalis strain ATCC 29212, 1 × 107 cfu/ml and 1 × 108 cfu/ml. One group received tramadol at 20 mg/kg subcutaneously whereas the other group did not receive a pain reducing agent. Bacterial burden was assessed in thigh as the primary organ, but also determined in kidneys and lungs to evaluate if secondary seeding occurred. An inoculum of 1 × 107 cfu/ml resulted in a median 6.0 log10 cfu/g tissue for the untreated and 6.3 log10 cfu/g tissue for the tramadol-treated group, whereas an inoculum of 1 × 108 cfu/ml resulted in a median burden of 7.2 vs. 7.3 log10 cfu/g tissue for the untreated and the tramadol-treated group, respectively (Fig. 1a,d; Table 1, p = 0.7301 (low inoculum) and p = 0.9895 (high inoculum)). Consequently, tramadol treatment did not influence bacterial burden in the primary organ, the thighs. Moreover, it was determined if bacteria seed to secondary tissues in the course of the infection. Therefore, burden in lungs as well as in kidneys was assessed as well. For both inocula deployed in this study, only individual animals showed secondary seeding into the kidneys. No significant differences were detected between untreated and tramadol groups in kidneys (Fig. 1c,f). Additionally, bacterial loads in lung were determined. For the low inoculum of 1 × 107 cfu/ml a median burden of 2.9 log10 cfu/g tissue was observed in the untreated group versus 3.7 log10 cfu/g tissue in the tramadol-treated group (Fig. 1b,e; Table 2). For the high inoculum of 1 × 108 cfu/ml, only a median difference of about 0.4 log10 cfu/g tissue was shown in lung for the two groups. Neither for the low (p = 0.3517) nor for the high inoculum (p = 0.9737) this difference was significant. Moreover, bacterial loads in lung tissue were quite low (between 2 to 3 log10 cfu/g tissue). Finally, tramadol treatment did not affect the primary readout of the model, bacterial burden in the thighs, with the E. faecalis strain tested at different inocula sizes. No significant difference was detected between the untreated and the tramadol-treated group for organs affected by secondary seeding, either.
Bacterial burden in untreated and tramadol-treated groups in the neutropenic thigh infection model with two different inocula of E. faecalis. Bacterial burden expressed as cfu/g tissue was assessed for groups that were treated with either 20 mg/kg tramadol subcutaneously or left untreated (n = 9 per group). All groups were infected with E. faecalis at a low inoculum (1 × 107 cfu/ml, a-c) or at a high inoculum (1 × 108 cfu/ml, d-f). Bacterial burden was assessed in thigh (a,d), lung (b,e) and kidney (c,f). Statistical testing was performed using a Kolmogorov–Smirnov test. Ns not significant.
Independent of the specific strain? Tramadol does not affect the readout for the neutropenic thigh infection model with S. aureus strains ATCC 33591 and ATCC 29213
Next, the effect of tramadol in the neutropenic thigh infection model with S. aureus was assessed. Two different strains, a methicillin-sensitive S. aureus (MSSA, ATCC 29213) and a methicillin-resistant S. aureus (MRSA, ATCC 33591) strain, were used for this model, but with the same inoculum size. For the MSSA-strain a median burden of around 8.3 vs. 8.7 log10 cfu/g tissue was observed in thigh for the untreated and the tramadol-treated group, respectively. For the MRSA-strain a median burden of 7.2 vs. 7.8 log10 cfu/g tissue was detected for the untreated and the tramadol-group, respectively (Fig. 2a,c; Table 1). No significant difference in the bacterial burden in thighs for both strains was seen (p = 0.1429 (MRSA) and p = 0.1429 (MRSA)). With respect to secondary seeding, no bacteria were found in kidneys, whereas lung was affected in both strains with a burden of around 5.3 vs. 5.2 log10 cfu/g tissue for MRSA and around 5.8 vs. 5.9 log10 cfu/g tissue for MSSA for the untreated vs. the tramadol-treated group, respectively. Again, no difference was detected between tramadol- and untreated group for both strains (Fig. 2b,d; Table 2, p = 0.9307 (MSSA) and p = 0.4740 (MRSA))). Finally, for the two different S. aureus strains tested here, no impact of tramadol on bacterial burden in thighs or on distribution was observed.
Bacterial burden in untreated and tramadol-treated groups in the neutropenic thigh infection model with two different strains of S. aureus. Bacterial burden expressed as cfu/g tissue was assessed for groups that were treated with either 20 mg/kg tramadol subcutaneously or left untreated (n = 6 per group). All groups were infected with MSSA (a,b) or MRSA (c,d). Bacterial burden was assessed in thigh (a,c) and lung (b,d). Statistical testing was performed using a Kolmogorov–Smirnov test. Ns not significant.
Independent of timing? Impact of tramadol in the neutropenic thigh infection model with P. aeruginosa at different readout time points
Next, the impact of tramadol on the readout of the neutropenic thigh infection model was investigated when the same strain was used, but the animals were infected for either 24 h or 48 h. After 24 h a slightly higher burden of around 5.0 log10 cfu/g tissue in thigh tissue was detected in the untreated group compared to the tramadol-treated group (3.2 log10 cfu/g tissue) (Fig. 3a; Table 1). However, this was not significant upon statistical testing using a Kolmogorov–Smirnov-test which also takes into account the distribution in the individual groups (p = 0.1429). After 24 h a similar bacterial burden was found in lung tissue with of median 4.6 log10 cfu/g tissue (untreated) versus 4.9 log10 cfu/g tissue (tramadol) (Fig. 3b; Table 2, p = 0.4740). Next, the focus was on the possible impact of tramadol on the readout after 48 h of infection with the same strain. In thigh, a median burden of 3.4 log10 cfu/g tissue was seen in the untreated group compared to 3.5 log10 cfu/g tissue in the tramadol-treated group (Fig. 3c; Table 1, p = 0.9119). Moreover, in lung tissue a median burden of 4.7 log10 cfu/g tissue was found in the untreated treated group compared to 4.8 log10 cfu/g tissue in the tramadol-treated group (Fig. 3d; Table 2, p = 0.9048). Thus, no significant difference was detected in both tissues for both groups. In summary, tramadol treatment was similar to the untreated control groups when tested at different readout time points.
Bacterial burden in untreated and tramadol-treated groups in the neutropenic thigh infection model with two different readout time points of the same strain of P. aeruginosa. Bacterial burden expressed as cfu/g tissue was assessed for groups that were treated with either 20 mg/kg tramadol subcutaneously or left untreated. All groups were infected with P. aeruginosa either for 24 h ((a,b); n = 6 per group) or for 48 h ((c,d); n = 18 per group). Bacterial burden was assessed in thigh (a,c) and lung (b,d). Statistical testing was performed using a Kolmogorov–Smirnov test. Ns not significant.
Neutropenic thigh infection model with Gram-negative ESKAPE bacteria—impact of tramadol
In a next step, the influence of tramadol in the neutropenic thigh infection model with E. coli, A. baumannii and K. pneumoniae was determined. In the neutropenic thigh infection model with E. coli a median burden of around 9.3 log10 cfu/g tissue was observed in thigh for both groups (Fig. 4a; Table 1). Secondary seeding towards lung and kidneys occurred as well. In lung, a median burden of 5.9 log10 cfu/g tissue was found for both groups (Fig. 4b; Table 2), whereas in kidneys, a median burden of around 5.0 log10 cfu/g tissue was determined for both groups (Fig. 4c; Table 3). No significant differences were detected between both groups in the neutropenic thigh infection model with E. coli, neither in the primary readout organ, thigh (p = 0.9372), nor in the organs affected by secondary seeding (lung (p = 0.9372) and kidneys (p > 0.9999)).
Bacterial burden in untreated and tramadol-treated groups in the neutropenic thigh infection model with three different species of the ESKAPE pathogens. Bacterial burden expressed as cfu/g tissue was assessed for groups that were treated with either 20 mg/kg tramadol subcutaneously or left untreated (n = 6 per group). All groups were either infected with E. coli (a-c) or with A. baumannii (d-f) or K. pneumoniae (g-i). Bacterial burden was assessed in thigh (a,d,g), lung (b,e,h) and kidney (c,f,i). Statistical testing was performed using a Kolmogorov–Smirnov test. Ns not significant.
Then, the effect of tramadol in the neutropenic thigh infection model with A. baumannii was investigated. Similarly to the E. coli model, no significant difference between the untreated and the tramadol group was observed with a median burden of around 7.6 log10 cfu/g tissue in thigh for both tested groups (Fig. 4d; Table 1, p = 0.4740). Secondary seeding to kidneys and lung tissue also took place to a lower extent. In lung, a median burden of 3.6 log10 cfu/g tissue was found in the tramadol group (Fig. 4e; Table 2), whereas in kidneys, a median burden of 1.2 log10 cfu/g tissue was detected in the tramadol group. However, not all animals were affected by secondary seeding into the kidneys or in the lungs. In the untreated group no secondary seeding into the kidneys was detected at all (Fig. 4f). Moreover, only two animals in the untreated group showed secondary seeding into the lung (Fig. 4e, p = 0.4156). No significant difference was detected in the different groups using the Kolmogorov–Smirnov-test.
Finally, the effect of tramadol in the neutropenic thigh infection model with K. pneumoniae was assessed. In thigh, a median burden of 5.3 log10 cfu/g tissue was determined in the untreated group versus 7.1 log10 cfu/g tissue in the tramadol group (Fig. 4g; Table 1, p = 0.4740). However, both groups exhibited a similar standard deviation. In lung, a median burden of 4.6 log10 cfu/g tissue was detected in the untreated group versus 4.3 log10 cfu/g tissue in the tramadol group (Fig. 4h; Table 2, p = 0.9307). In kidneys, the median burden for the untreated group was 3.2 log10 cfu/g tissue whereas the tramadol-treated group showed a median burden of 3.3 log10 cfu/g tissue (Fig. 4i; Table 3, p = 0.9307). The differences between the two tested groups were not significant in all tested organs. In conclusion, tramadol was similar to the untreated control group for the strains of the three bacterial species tested here with respect to bacterial burden in the primary, but also in organs affected by secondary seeding.
Discussion
The neutropenic thigh infection model is a standard model for pharmacodynamic characterization of novel compounds and determination of the PK/PD driver via dose fractionation studies9,11. However, this model can cause moderate and, possibly at high inocula sizes, also severe discomfort for animals. This is indicated by studies showing that the vehicle-treated control group was euthanized before the actual planned endpoint due to animals of this group reaching the humane endpoint14,15. Therefore, in line with the 3R principle, established by Russell and Burch in the late 1950s, this study is the first one, to the best of one’s knowledge, that evaluated if a pain reducing agent can be used without influencing the bacterial burden in the primary readout organ, the thighs. One study was found that used buprenorphine three times a day for all groups in a neutropenic thigh infection model16. However, this study did not inform about the influence on bacterial burden in general. As all groups, also those investigated for their antibacterial potency, underwent buprenorphine, potential treatment effects on bacterial burden of buprenorphine per se might be negligible. Nevertheless, use of buprenorphine for all groups, including the treated ones, bears a high drug-drug-interaction potential, especially concerning drugs mainly metabolized via the CYP enzyme 3A4 as buprenorphine is mainly metabolized via this enzyme23. In this study, influence of tramadol on bacterial burden was investigated with the aim to enable treatment of only the untreated control group with tramadol. As a result, treatment groups do not need to receive tramadol-treatment for reduction of discomfort if substances show effect. This avoids drug-drug-interactions in study groups as a result of deployment of the pain reducing agent.
Dissemination in the neutropenic thigh infection model towards distant vital organs has been observed by others before10,24. The mechanism of this dissemination has not been determined. The thigh is a well perfused tissue so that it is conceivable that bacteria are capable to transition from the thigh to the blood stream and are then transported to other organs. As tramadol might influence the process of inflammation in the thigh, the perfusion of the non-inflamed thigh tissue could be different to the one in the untreated control group. One could speculate that this could result in a different degree of dissemination. Thus, it is crucial that tramadol does not influence the distribution of bacterial burden in the organs, potentially affected by secondary seeding, either.
In general, two classes of pain reducing agents appear to be suitable: cyclooxygenase (COX)-inhibitors and opioids. In the neutropenic thigh infection model described in this study, the kidneys were frequently affected by secondary seeding in the course of the infection. It is known that cyclooxygenase-inhibitors directly influence inflammation via the cyclooxygenase and, moreover, impact glomerular filtration via the cyclooxygenase-225,26,27. It was shown in this study that secondary seeding from the primary infection site occurred for all species investigated here. For E. faecalis and A. baumannii, bacterial burden was detected in the kidneys of some individuals, whereas for E. coli and K. pneumoniae, a considerable burden was determined. Wang and colleagues showed that upon administration of a COX-inhibitor, endotoxins reduced the glomerular filtration rate, whereas this was not the case when endotoxins were administered without a COX-inhibitor28. This suggests that COX-inhibitors might contribute to increased severity and higher bacterial burden in the kidneys during the neutropenic thigh infection model. As a pain reducing agent is needed that does not influence the course of the infection, COX-inhibitors were excluded in this study right from the beginning. Therefore, tramadol was chosen as pain reducing agent: Due to different receptor affinities, tramadol does not bear the same potential to interfere with inflammation and pain compared to the full (morphine) or partial µ-opioid (buprenorphine) receptor agonists which also contributes to less side effects for tramadol17,29,30. Finally, tramadol has also been used extensively in animal models for pain so that doses administered via the subcutaneous route assure rapid analgesia lasting for several hours31,32 which was important for the neutropenic thigh infection model studied here.
In this study, only a small number of strains from different species was used to assess the potential effects of tramadol on bacterial burden. It was shown for several of the ESKAPE pathogens, Gram-positive as well as Gram-negative species, that tramadol did not influence the bacterial burden neither in thigh nor in organs affected by secondary seeding, such as lung and kidney. In general, all strains tested in this study caused secondary seeding at least into lung tissue. Only A. baumannii as well as both inocula of E. faecalis showed low inoculation of lung tissue with a bacterial burden lower than 4 log10 cfu/g tissue. With respect to kidney tissue, only infection with K. pneumoniae and E. coli resulted in a considerable burden.
By using different inocula sizes of the same strain of E. faecalis, it was demonstrated that the outcome of the infection upon treatment with tramadol was inocula-independent in both organs, thigh and lung. In this study, only two different inocula sizes were tested for E. faecalis which could be considered as a limitation. However, these inocula sizes are commonly used for neutropenic thigh infection models with E. faecalis33. Next, it was investigated if using two different strains of the same species, namely S. aureus, resulted in distinct outcomes. Again, no difference was observed for the primary organ thigh as well as for lung tissue affected by secondary seeding. This suggested that tramadol usage did not influence bacterial burden when different strains of the same species were used. It is evident that this has to be investigated further using several strains of the same species. Nevertheless, this first investigation is promising towards tramadol deployment for the untreated or vehicle group in the neutropenic thigh infection model.
When the thigh infection model has a prolonged endpoint at 48 h, as shown with P. aeruginosa, tramadol treatment does also not influence the bacterial burden. Nonetheless, tramadol-treatment resulted in a lower median burden in thigh tissue upon infection with P. aeruginosa for 24 h. This finding was not significant. Moreover, the untreated group showed a much higher standard deviation compared to the tramadol-treated group. Furthermore, no difference was detected between both groups in lungs after 24 h. There are two reports about the anti-pseudomonal and anti-staphylococcal activity of tramadol in vitro20 and in vivo21. In the in vivo study, tramadol was injected at high concentrations of about 62.5 mg/kg (based on an estimation of 20 g for the weight of Balb/c mice which were used in that study) directly into the site of infection21. Similarly, the in vitro study used concentrations of 12.5 mg/ml of tramadol to detect bacterial killing over 24 h20. By contrast, in the neutropenic thigh infection model, tramadol is injected at a different site and not injected together with the bacteria intramuscularly. A recent in vitro study also investigated the antibacterial properties of tramadol against the same strains of E. coli, S. aureus and P. aeruginosa used in this study and against a K. pneumoniae strain22. Only at concentrations of 500 µg/ml 100% killing of K. pneumoniae was achieved, whereas that concentration did not result in complete killing of E. coli, S. aureus, and P. aeruginosa22 suggesting that concentrations in the range of more than 500 µg/ml might be necessary to see complete eradication. Based on pharmacokinetic information about tramadol after subcutaneous administration31, these concentrations were not reached in this study. Therefore, a direct effect of tramadol on the bacterial infection in the neutropenic thigh infection model was unlikely.
In addition to the Gram-negative bacterium P. aeruginosa, also E. coli, A. baumannii and K. pneumoniae were tested. Whereas burden in all three organs (thigh, lung and kidney) was nearly identical for E. coli upon treatment with tramadol in comparison to untreated animals, A. baumannii showed the same burden only in thigh. For A. baumannii some individual animals were affected by secondary seeding. In lung tissue, it appeared that tramadol-treated animals seemed to bear a higher bacterial load compared to untreated animals. This was also reflected in kidney. It should be investigated further if tramadol treatment facilitates dissemination from the thigh tissue into other distant organs. Although the mechanism of secondary seeding is not elucidated, one could speculate that the reduced inflammation in thigh tissue as a result of effective tramadol treatment leads to a higher degree of secondary seeding. Although the differences in kidney and lung tissue were not statistically significant, one has to bear in mind that the analysis for detection of differences in bacterial load was primarily powered for thigh. Thus, it is possible that a potential effect of tramadol on the seeding in A. baumannii might not be detected as the secondary seeding to other organs goes in hand with a higher standard deviation. This would require a higher number of animals to retrieve statistically significant differences as well and could be subject to future studies. In the neutropenic thigh infection model with K. pneumoniae a higher bacterial load upon tramadol treatment was found in thighs which was not statistically significant due to the similar standard deviation in both groups. When looking into lung and kidney tissue, no differences were seen for both groups.
In conclusion, this study showed for a first small subset of different species and conditions, like different inocula sizes, different strains, different endpoints, that tramadol is a viable option to enhance animal welfare during the model and, thus, contributes to the refinement of one of the standard models in the field of PK/PD. Therefore, this study emboldens to use tramadol for those groups not receiving treatment to reduce discomfort as the bacterial burden is not affected. This study does not claim that tramadol does not exert an effect on bacterial burden for every specific bacterial strain and species used in neutropenic thigh infection models around the globe. However, it does encourage everyone using this model for determination of PK/PD effects of novel antibacterials to assess if tramadol is an option to reduce discomfort and to enhance the welfare of animals. Finally, this study fortifies that the usage of a pain reduction agent does not per se influence the outcome in this established in vivo model. It is rather necessary to investigate potential effects. This should encourage everyone using in vivo models resulting in pain, distress or discomfort for the animals to explore if a pain reduction agent is beneficial and able to reduce discomfort without influencing crucial parameters important for readout.
Methods
Strains
The following strains were used for in vivo studies: S. aureus ATCC 29213 (MSSA), S. aureus ATCC 33591 (MRSA), K. pneumoniae ATCC 43816, P. aeruginosa ATCC 27853, E. coli ATCC 25922, A. baumannii ATCC 19606, E. faecalis ATCC 29212.
Preparation of the inoculum for infection with S. aureus strains ATCC 29213 (MSSA) and ATCC 33591 (MRSA)
The respective strain was streaked out from a glycerol culture onto a blood agar plate and incubated at 37 °C for 8 h. One colony was inoculated in 20 ml BHI medium (BD) and incubated for 15–16 h at 120 rpm and 37 °C. Then, the bacterial culture was diluted in fresh BHI medium to yield an OD600 of < 0.1 and incubated at 120 rpm at 37 °C until it reached an OD600 between 0.45 and 0.55. Next, the culture was centrifuged for 15 min at 4000 rpm and 4 °C. The pellet was washed twice using PBS and centrifuged for 15 min at 4000 rpm and 4 °C. Finally, the pellet was resuspended in PBS and the OD600 was adjusted to 1. Aliquots were prepared and frozen at − 80 °C. One day before infection one aliquot was plated onto BHI agar and incubated overnight at 37 °C. The following day, the colonies were counted to adjust the infection inoculum accordingly by dilution with PBS. For infection with S. aureus ATCC 29213 an inoculum of 1 × 106 cfu/ml was used. For infection with S. aureus ATCC 33591 an inoculum of 1 × 106 cfu/ml was used.
Preparation of the inoculum for infection with K. pneumoniae ATCC 43816
The strain was streaked out from a glycerol culture onto a blood agar plate and incubated at 37 °C overnight. A few colonies were inoculated in a mixture of MHB diluted in water (one part MHB and nine parts water) and incubated for 14–15 h at 120 rpm and 37 °C. The following day, the culture was centrifuged at 4000 rpm and 4 °C for 15 min when it reached an OD600 between 0.5 and 0.7. Then it was washed twice with PBS and centrifuged for 15 min at 4000 rpm and 4 °C. Finally, the pellet was resuspended in PBS and the OD600 was adjusted to 1. Then, the inoculum was diluted 1:50,000 in PBS. For infection with K. pneumoniae an inoculum of 1 × 104 cfu/ml was used.
Preparation of the inoculum for infection with E. coli ATCC 25922
The strain was streaked out from a glycerol culture onto a blood agar plate and incubated at 37 °C overnight. A few colonies were inoculated in a mixture of TSB diluted in water (one part TSB and fourteen parts water) and incubated for 13–15 h at 130 rpm and 37 °C. The following day, the culture was centrifuged at 4000 rpm and 4 °C for 15 min when it reached an OD600 between 0.7 and 0.8. Then it was washed twice with PBS and centrifuged for 15 min at 4000 rpm and 4 °C. Finally, the pellet was resuspended in PBS and the OD600 was adjusted to 1. Then, the inoculum was diluted 1:100 in PBS. For infection with E. coli an inoculum of 3 × 106 cfu/ml was used.
Preparation of the inoculum for infection with P. aeruginosa ATCC 27853
The inoculum was prepared as follows: on day-1 the P. aeruginosa strain was streaked out onto a blood agar plate and incubated at 37 °C. Then one single colony was inoculated into MHB medium (diluted 1:2 in water) and incubated at 120 rpm and 37 °C. On day 0 bacteria were centrifuged for 15 min at 4000 rpm and washed twice in PBS. Then they were adjusted to an OD of 1 and diluted 1:5000 in PBS for the infection until 48 h and 1:1000 n PBS for the infection until 24 h. For infection with P. aeruginosa until 24 h an inoculum of 2 × 105 cfu/ml was used whereas an inoculum of 3 × 104 cfu/ml was used for an infection until 48 h.
Preparation of the inoculum for infection with A. baumannii ATCC 19606
The strain was streaked out from a glycerol culture onto a blood agar plate and incubated at 37 °C overnight. A few colonies were inoculated in a mixture of MHB diluted in water (one part MHB and four parts water) and incubated for 14–15 h at 120 rpm and 37 °C. The following day, the culture was centrifuged at 4000 rpm and 4 °C for 15 min when it reached an OD600 between 0.7 and 0.8. Then it was washed twice with PBS and centrifuged for 10 min at 4000 rpm and 4 °C. Finally, the pellet was resuspended in PBS and the OD600 was adjusted to 1. Then, the inoculum was diluted 1:10 in PBS. For infection with A. baumannii an inoculum of 3 × 107 cfu/ml was used.
Preparation of the inoculum for infection with E. faecalis ATCC 29212
The strain was streaked out from a glycerol culture onto a blood agar plate and incubated at 37 °C for 8 h. One colony was inoculated in 20 ml BHI medium (BD) and incubated for 15–16 h at 120 rpm and 37 °C. Then, the bacterial culture was diluted in fresh BHI medium to yield an OD600 of < 0.1 and incubated at 120 rpm at 37 °C until it reached an OD600 between 0.3 and 0.6. Next, the culture was centrifuged for 15 min at 4000 rpm and 4 °C. The pellet was washed twice using PBS and centrifuged for 15 min at 4000 rpm and 4 °C. Finally, the pellet was resuspended in PBS and the OD600 was adjusted to 1. Aliquots were prepared and frozen at − 80 °C. One day before infection one aliquot was plated onto BHI agar and incubated overnight at 37 °C. The following day, the colonies were counted to adjust the infection inoculum accordingly by dilution with PBS. For infection with E. faecalis an inoculum of 1 × 108 cfu/ml was used.
Animals
The animal studies were conducted in accordance with the recommendations of the European Community (Directive 86/609/EEC, 24 November 1986). All animal procedures were performed in strict accordance with the German regulations of the Society for Laboratory Animal Science (GV- SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). Animals were excluded from further analysis if sacrifice was necessary according to the humane endpoints established by the ethical board. All experiments were approved by the ethical board of the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Oldenburg, Germany. Animals were kept in individually ventilated cages with a 10 h/14 h dark/light cycle and had access to food and water ad libitum. The study is reported in accordance with the ARRIVE guidelines.
Neutropenic thigh infection with E. faecalis, S. aureus, P. aeruginosa, E. coli, K. pneumonia or A. baumannii for 24 h
Male, 6 weeks-old, CD-1 mice (Charles River, Germany) were rendered neutropenic by administration of 150 mg/kg and 100 mg/kg cyclophosphamide intraperitoneally on day -4 and -1, respectively. Mice were grouped as follows per strain (n = 6 for S. aureus, P. aeruginosa, E. coli, K. pneumonia or A. baumannii and n = 9 for E. faecalis): (1) untreated group, (2) tramadol group (receiving 20 mg/kg subcutaneously t = 0 h post infection). On the day of infection (day 0), mice received 30 µl of the respective strain into each lateral thigh under isoflurane anesthesia. 24 h after infection, mice were euthanized, blood was removed from the heart, lung and thighs were aseptically removed. 24 h after infection the clinical score of every individual animal was assessed. Clinical scoring comprised assessment of different parameters such as spontaneous behavior, posture, appearance, provoked behavior and body weight. Each parameter was assessed with a score of 0 to 3 (ascending severity from 0 to 3). The humane endpoint was reached when the clinical score of one parameter was 3 or if the clinical score was higher than 8. Whole blood was collected into Eppendorf tubes coated with 0.5 M EDTA and immediately spun down at 13.000 rpm for 10 min at 4 °C. The plasma was transferred into a new Eppendorf tube and then stored at − 80 °C until analysis. Organs were homogenized in 0.9% NaCl-solution and plated onto agar plates in duplicates in serial dilutions and incubated at 37 °C for 24 h.
Neutropenic thigh infection model with P. aeruginosa for 48 h
Male, 6 weeks-old, CD-1 mice (Charles River, Germany) were rendered neutropenic by administration of 150 mg/kg and 100 mg/kg cyclophosphamide intraperitoneally on day -4, -1 and + 1, respectively. The P. aeruginosa strain ATCC 27853 was used. The inoculum was plated onto MHB agar plates in serial dilutions and incubated at 37 °C. Mice were grouped into the following (n = 18 for each group): (1) untreated group, (2) tramadol group (receiving 20 mg/kg tramadol subcutaneously t = 0 and 24 h post infection). On the day of infection (day 0), mice received 30 µl of the P. aeruginosa strain ATCC 27853 into each lateral thigh (inoculum: 5 × 104 cfu/ml) under isoflurane anesthesia. 48 h after infection, mice were euthanized, blood was removed from the heart, lung and thighs were aseptically removed. 24 and 48 h after infection the clinical score of every individual animal was assessed. Clinical scoring comprised assessment of different parameters such as spontaneous behavior, posture, appearance, provoked behavior and body weight. Each parameter was assessed with a score of 0 to 3 (ascending severity from 0 to 3). The humane endpoint was reached when the clinical score of one parameter was 3 or if the clinical score was higher than 8. Whole blood was collected into Eppendorf tubes coated with 0.5 M EDTA and immediately spun down at 13.000 rpm for 10 min at 4 °C. The plasma was transferred into a new Eppendorf tube and then stored at − 80 °C until analysis. Organs were homogenized in 0.9% NaCl-solution and plated onto MHB agar plates in duplicates in serial dilutions and incubated at 37 °C for 24 h.
Statistical testing
Testing for statistical significance was performed using a Kolmogorov–Smirnov-test (unpaired, two-tailed) using GraphPad Prism 9.3.1 software. Results were considered statistically significant when p-values were below 0.05 (95% confidence interval).
Data availability
The author confirms that the data supporting the findings of this study are available within the article.
References
Oldenkamp, R., Schultsz, C., Mancini, E. & Cappuccio, A. Filling the gaps in the global prevalence map of clinical antimicrobial resistance. Proc. Natl. Acad. Sci. 118, e2013515118 (2021).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet https://doi.org/10.1016/S0140-6736(21)02724-0 (2022).
Antimicrobial resistance: global report on surveillance. (World Health Organization, 2014).
Beyer, P. & Paulin, S. The antibacterial research and development pipeline needs urgent solutions. ACS Infect. Dis. 6, 1289–1291 (2020).
Miethke, M. et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. https://doi.org/10.1038/s41570-021-00313-1 (2021).
Fleitas Martínez, O., Cardoso, M. H., Ribeiro, S. M. & Franco, O. L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell Infect. Microbiol. 9, 74 (2019).
Lee, Y. R. & Yeo, S. Cefiderocol, a new siderophore cephalosporin for the treatment of complicated urinary tract infections caused by multidrug-resistant pathogens: Preclinical and clinical pharmacokinetics, pharmacodynamics efficacy and safety. Clin. Drug Investig. 40, 901–913 (2020).
Klahn, P. & Brönstrup, M. Bifunctional antimicrobial conjugates and hybrid antimicrobials. Nat. Prod. Rep. 34, 832–885 (2017).
Zhao, M., Lepak, A. J. & Andes, D. R. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg. Med. Chem. 24, 6390–6400 (2016).
Rodriguez, C. A., Agudelo, M., Gonzalez, J. M., Vesga, O. & Zuluaga, A. F. An optimized mouse thigh infection model for enterococci and its impact on antimicrobial pharmacodynamics. Antimicrob. Agents Chemother. 59, 233–238 (2014).
Ambrose, P. G. et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 44, 79–86 (2007).
Andes, D. R. & Lepak, A. J. In vivo infection models in the pre-clinical pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Curr. Opin. Pharmacol. 36, 94–99 (2017).
Selbie, F. R. & Simon, R. D. Virulence to mice of Staphylococcus pyogenes: Its measurement and its relation to certain in vitro properties. Br. J. Exp. Pathol. 33, 315–326 (1952).
Lepak, A. J., Marchillo, K., Craig, W. A. & Andes, D. R. In vivo pharmacokinetics and pharmacodynamics of the lantibiotic NAI-107 in a neutropenic murine thigh infection model. Antimicrob. Agents Chemother. 59, 1258–1264 (2015).
Sabet, M., Tarazi, Z. & Griffith, D. C. Pharmacodynamics of meropenem against acinetobacter baumannii in a neutropenic mouse thigh infection model. Antimicrob. Agents Chemother. 64, e02388-e2419 (2020).
Bernhard, F. et al. Pharmacokinetics-pharmacodynamics of enmetazobactam combined with cefepime in a neutropenic murine thigh infection model. Antimicrob. Agents Chemother. 64, e00078-e120 (2020).
Raffa, R. B. et al. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J. Pharmacol. Exp. Ther. 260, 275–285 (1992).
Desmeules, J. A., Piguet, V., Collart, L. & Dayer, P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br. J. Clin. Pharmacol. 41, 7–12 (1996).
Vazzana, M. et al. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 70, 234–238 (2015).
Tamanai-Shacoori, Z. et al. The antibacterial activity of tramadol against bacteria associated with infectious complications after local or regional anesthesia. Anesth. Analg. 105, 524–527 (2007).
Farzam, H. et al. Antibacterial effect of tramadol against Staphylococcus aureus and Pseudomonas aeruginosa: An in vivo study. New Microbes New Infect. 24, 42–46 (2018).
Unlu, O., Bingul, E., Kesi̇ci̇, S. & Demirci, M. Investigating antimicrobial features and drug interactions of sedoanalgesics in intensive care unit: An experimental study. ADMET DMPK 9, 219–226 (2021).
Picard, N., Cresteil, T., Djebli, N. & Marquet, P. In vitro metabolism study of buprenorphine: Evidence for new metabolic pathways. Drug Metab. Dispos. 33, 689–695 (2005).
Zuluaga, A. F., Agudelo, M., Cardeño, J. J., Rodriguez, C. A. & Vesga, O. Determination of therapeutic equivalence of generic products of gentamicin in the neutropenic mouse thigh infection model. PLoS ONE 5, e10744 (2010).
Dinchuk, J. E. et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378, 406–409 (1995).
Tegeder, I., Pfeilschifter, J. & Geisslinger, G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 15, 2057–2072 (2001).
Nørregaard, R., Kwon, T.-H. & Frøkiær, J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res. Clin. Pract. 34, 194–200 (2015).
Wang, W. et al. Prostacyclin in endotoxemia-induced acute kidney injury: Cyclooxygenase inhibition and renal prostacyclin synthase transgenic mice. Am. J. Physiol.-Renal Physiol. 293, F1131–F1136 (2007).
Andrade, P. et al. Role of TNF-alpha during central sensitization in preclinical studies. Neurol. Sci. 32, 757 (2011).
Bravo, L., Mico, J. A. & Berrocoso, E. Discovery and development of tramadol for the treatment of pain. Expert Opin. Drug Discov. 12, 1281–1291 (2017).
Evangelista Vaz, R. et al. Preliminary pharmacokinetics of tramadol hydrochloride after administration via different routes in male and female B6 mice. Vet. Anaesth. Analg. 45, 111–122 (2018).
Evangelista-Vaz, R., Bergadano, A., Arras, M. & Jirkof, P. D. Analgesic efficacy of subcutaneous-oral dosage of tramadol after surgery in C57BL/6J mice. J. Am. Assoc. Lab. Anim. Sci. 57, 368–375 (2018).
Kidd, J. M., Abdelraouf, K., Asempa, T. E., Humphries, R. M. & Nicolau, D. P. Pharmacodynamics of daptomycin against enterococcus faecium and enterococcus faecalis in the murine thigh infection model. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.00506-18 (2018).
Acknowledgements
KR thanks Dr. Pia Empting for advice in planning of animal models with respect to animal welfare. KR thanks Andrea Ahlers, Janine Schreiber and Jennifer Wolf for excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. KR receives support from the German Centre for Infection Research (DZIF, TTU 09.719, No. 8004709719) and from the German Federal Ministry of Education and Research (BMBF, SIAM-APH, No. 16GW0235, grant number: 01KI2126B).
Author information
Authors and Affiliations
Contributions
K.R. conceived the study, conducted the experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rox, K. Influence of tramadol on bacterial burden in the standard neutropenic thigh infection model. Sci Rep 12, 19606 (2022). https://doi.org/10.1038/s41598-022-24111-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24111-x
This article is cited by
-
Aerosolized delivery of ESKAPE pathogens for murine pneumonia models
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.