Abstract
Anopheles darlingi is the main malarial vector in the Brazilian Amazon region. An. nuneztovari s.l., An. triannulatus s.l., An. evansae, and An. benarrochi s.l. do not have a defined role as malarial vectors, although they have been found to be naturally infected with Plasmodium vivax, and some develop oocysts. In this study, we evaluated the importance of low numbers of oocysts in sporozoite salivary gland invasion and transmission. Field-collected mosquitoes were experimentally infected with P. vivax. The infection rates and oocyst and sporozoite infection intensities were evaluated and compared with those of An. aquasalis. We found the highest number of oocysts in An. darlingi (mean = 39.47) and the lowest in An. nuneztovari s.l. (mean = 2). The highest number of sporozoites was observed in An. darlingi (mean = 610) and lowest in An. benarrochi s.l. (mean = 30). Plasmodium vivax DNA was detected in the saliva of all mosquito species after a blood meal. Regardless of the number of oocysts, all species transmitted sporozoites during blood meals. Considering the abundance of these mosquitoes and transmission of sporozoites, it is logical to assume that An. nuneztovari s.l. and An. triannulatus s.l. are involved in the transmission of P. vivax.
Similar content being viewed by others
Introduction
Plasmodium vivax is the second most common cause of malaria in the world1. It is transmitted to humans through the bite of anopheline mosquitoes. However, not all Anopheles species can transmit the parasite, as transmission depends on a combination of factors, such as vectorial competence and capacity2,3. To be considered a competent malarial vector, the mosquito needs to allow the parasite to complete its life cycle, beginning with the ingestion of gametocytes during a blood meal in an infected host, passing through the zygote and ookinete phases in the lumen of the midgut and oocysts in the intestinal epithelium, to the sporozoites, which, after being released into the hemocoel, migrate to the salivary gland and invade it, and finally, the inoculation of infective sporozoites during the blood meal in the vertebrate host2,4.
During this journey, which takes an average of 14 days depending on the Anopheles and Plasmodium species, the parasite finds the physical and immunological barriers that it must go through to survive. The first barrier that the parasite must cross is the lumen of the midgut, where hundreds of gametocytes arrive after an infected blood meal, but less than 5% develop into oocysts5. The second major barrier to Plasmodium development is the hemocoel cavity, where sporozoites are released after oocyst rupture, but only approximately 25% of them migrate to and invade the mosquito salivary glands5. Therefore, this complex and highly toxic journey leads to the death of some parasites and survival of others. In other words, the conditions are neither completely toxic nor completely favorable. Although it is known that this miscellany of conditions can cause a mechanism known as refractoriness, the mechanism by which some mosquitoes are able to resist infection is still unknown6.
The Brazilian Amazon region is home to a high diversity of mosquitoes with different epidemiological importance in malaria transmission. Although 49 species of Anopheles have been described in the Brazilian Amazon region, only 19 were found to be naturally infected with Plasmodium spp.7,8. Anopheles darlingi is an important malaria vector in the Amazon region; however, the role of other species as malaria vectors, such as An. nuneztovari s.l., An. triannulatus s.l., and An. benarrochi s.l., which have been found to be naturally infected with P. vivax, has not yet been demonstrated9,10,11,12,13. Ríos-Velásquez et al.14 proposed that in the Brazilian Amazon region, An. nuneztovari s.l. is involved in the transmission of P. vivax owing to the abundance of this species in the areas where An. darlingi is also abundant, in addition to the high intensity of infection observed in the laboratory. However, owing to the low number of oocysts, the authors reinforced the idea that An. triannulatus is not involved in the transmission of P. vivax, although this species is more abundant than An. nuneztovari s.l.
Therefore, it is of great interest to understand the significance of the number of oocysts in the mosquito midgut for sporozoite salivary gland invasion and P. vivax transmission to vertebrate hosts. In this study, five field-collected Anopheles species frequently recorded in peri-urban areas of the city of Manaus were used to evaluate the importance of low or high number of oocysts in sporozoite salivary gland invasion and transmission. Anopheles aquasalis, a vector in the coastal regions of Brazil that colonizes under laboratory conditions, has been used as a reference species.
Results
Field collections
At all 2.224 mosquitoes were collected and reared in laboratory to obtain F1 females. Only five species were collected during the fieldwork at the studied localities. The most abundant species were An. triannulatus s.l. with 996 individuals (44.78%), followed by An. darlingi with 467 (20.99%), An. nuneztovari s.l. with 366 (16.45%), An. evansae with 298 (13.39%), and An. benarrochi s.l. with 97 (4.36%).
Mosquitoes
A total of 913 adult female mosquitoes corresponding to six F1 Anopheles species (An. triannulatus s.l., An. nuneztovari s.l., An. evansae, An. benarrochi s.l. and An. darlingi), and An. aquasalis were used to evaluate the susceptibility and transmission of P. vivax (Table 1, Fig. 1). In addition, six malaria-infected patients were included in the experimental infections using the membrane feeding assay.
Infection rate
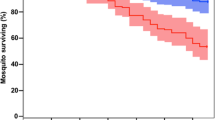
All Anopheles species were susceptible to P. vivax infection; however, the infection rates differed among them (Table 1, Fig. 2). Anopheles aquasalis had the highest infection rate (mean IR = 76.42), followed by An. darlingi (mean IR = 76), An. nuneztovari s.l. (mean IR = 20), An. triannulatus s.l. (mean IR = 15.51), An. evansae (mean IR = 7.81), and An. benarrochi s.l. (mean IR = 5). Compared with the reference species, An. aquasalis, there were no statistically significant differences in the infection rate of An. darlingi (p = 0.9477), but there was a significant difference in An. nuneztovari s.l. (p = 0.0003), An. triannulatus s.l. (p < 0.0001), and An. evansae (p < 0.0001) (Table 1, Fig. 2). Infection rates were not significantly different among An. nuneztovari s.l., An. triannulatus s.l., and An. evansae (p = 0.1450); however, the infection rate of An. nuneztovari s.l. was higher.
Infection rates and number of P. vivax oocysts in the midgut of six Anopheles species. ***p < 0.0001: Statistically significant; ns: statistically non-significant difference; dpi: days post-infection. Plotted with GraphPad Prism v.6.00 software (San Diego, CA, USA, https://www.graphpad.com/).
Intensity of oocyst infection
The infection intensity, measured as the number of oocysts per infected mosquito, varied significantly among the species studied (Table 1, Fig. 1). The highest oocyst infection intensity was observed in An. darlingi (mean = 39.47, median = 20.5, oocyst range = 0–218), followed by An. aquasalis (mean = 39.18, median = 19.5, oocyst range = 0–238), An. evansae (mean = 14, median = 7, oocyst range = 0–45), An. triannulatus s.l. (mean = 5.16, median = 2.5, oocyst range 0–36), An. benarrochi s.l. (mean = 3, median = 3, oocyst range = 0–3), and An. nuneztovari s.l. (mean = 2, median = 2, oocyst range = 0–3). When comparing the oocyst infection intensity of the studied species with that of the reference species, there was no statistically significant difference between An. darlingi (p = 0.8273) and An. evansae (p = 0.2158); however, there was a significant difference for An. triannulatus s.l. (p < 0.0001) and An. nuneztovari s.l. (p < 0.0001).
The infection intensity varied between species and was lower than that of An. aquasalis. Anopheles nuneztovari s.l. had 19.59-fold fewer oocysts than An. aquasalis, followed by An. benarrochi s.l. with 13.06-fold fewer oocysts, An. triannulatus s.l. with 7.59-fold fewer oocysts, and An. evansae with 2.79-fold fewer oocysts; however, An. darlingi presented 0.98-fold more oocysts than did An. aquasalis.
Sporozoite infection
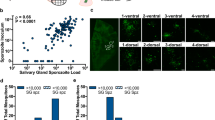
The salivary glands of all studied species were infected by P. vivax sporozoites; however, the number of sporozoites varied among them (Table 1, Fig. 3). A higher intensity of sporozoites per mosquito was observed in An. darlingi (mean = 610, median = 700, range = 375–800), followed by An. aquasalis (mean = 390, median = 200, range = 25–1.000), An. nuneztovari s.l. (mean = 204, median = 254, range = 150–357), An. triannulatus s.l. (mean = 141, median = 150, range = 25–294), An. evansae (mean = 88, median = 88, range = 25–150), and An. benarrochi s.l. (mean = 30, median = − 30, range = 30–30) (Table 1). Comparing the sporozoite infection intensity of the evaluated species with that of the reference species, no statistically significant difference was observed in An. darlingi (p = 0.3051) and An. nuneztovari s.l. (p = 0.4928); however, it was significantly different for An. triannulatus s.l. (p = 0.0089) and An. evansae (p = 0.0067).
Heat map showing the relative amounts of P. vivax sporozoites in the salivary glands of Anopheles species. Mean number of sporozoites per mosquito. Each row shows the sporozoite quantification per replicate and each column shows the sporozoite number arranged from top to bottom according to species. Plotted with GraphPad Prism v.6.00 software (San Diego, CA, USA, https://www.graphpad.com/).
Although An. nuneztovari s.l., An. triannulatus s.l., An. evansae, and An. benarrochi s.l. had few oocysts, they all had salivary glands invaded by P. vivax sporozoites at 14 dpi (Fig. 4). Compared with the reference species An. benarrochi s.l. had 12.97-fold fewer sporozoites, followed by An. evansae with 4.44-fold fewer sporozoites, An. triannulatus s.l. with 2.76-fold fewer sporozoites, and An. nuneztovari s.l. with 1.91-fold fewer sporozoites. Anopheles darlingi had a 1.56-fold higher number of sporozoites than did An. aquasalis.
Comparison between the number of sporozoites and oocysts in six species of Anopheles infected with P. vivax. Plotted with GraphPad Prism v.6.00 software (San Diego, CA, USA, https://www.graphpad.com/).
Correlation analyses between oocysts and sporozoites revealed a moderate to strong correlation among An. aquasalis (r = 0.536, p = 0.215), An. darlingi (r = 0.968, p = 0.159), An. triannulatus s.l. (r = 0.231, p = 0.659), An. nuneztovari s.l. (r = 1, p < 0.001), and An. evansae (r = 1, p < 0.001). Because of the low number of individuals available for experimentation, analyzing the correlations for An. benarrochi s.l. was not possible.
Detection of P. vivax
P. vivax DNA was detected in the saliva of all the Anopheles species studied after the salivation test. A DNA fragment of 100 bp was detected, corresponding to the Pv18S gene pattern (Fig. 5). Original gels are presented in Supplementary Fig. 1.
Polymerase chain reaction for detection of P. vivax in the saliva of six species of Anopheles. (L) DNA Ladder; (1) An. triannulatus; (2) An. nuneztovari; (3) An. benarrochi; (4) An. evansae; (5) An. darlingi; (6) An. aquasalis; (C + G) Positive control (salivary gland + P. vivax); (C + M) Positive control (midgut + P. vivax); (C-) Negative control. Original gels are presented in Supplementary Fig. 1.
Discussion
The diversity of Anopheles species is high in the Brazilian Amazon region, with approximately 49 species recorded to date, 19 of which have been found infected with human Plasmodium species7,8,15. Anopheles darlingi is an important malaria vector in the Amazon region16. Other species including An. albitarsis s.l., An. deaneorum, An. oswaldoi, An. mediopunctatus s.l., An. triannulatus s.l., An. nuneztovari s.l., and An. marajoara are potentially regional vectors because they are commonly collected at high densities in the Amazon region, show anthropophilic behavior, and have been found naturally infected with human Plasmodium species17. However, the vector status of these species is yet to be elucidated.
In this study, we selected six Anopheles species to evaluate the significance of having either few or many oocysts from the viewpoint of sporozoite salivary gland infection and transmission. The species selected were An. aquasalis, which was used as a reference species because it is colonized under laboratory conditions, and the other five species (An. darlingi, An. nuneztovari s.l., An. triannulatus s.l., An. benarrochi s.l., and An. evansae) were most frequently collected in peri-urban areas of the city of Manaus. Mosquitoes were fed on blood samples obtained from malaria-infected patients using a membrane feeding assay, which although an experimental method, has been shown to be suitable for mosquito susceptibility studies14,18,19,20.
An. darlingi showed high infection rates and oocyst intensities similar to those of An. aquasalis, but higher than those reported by Rios-Velásquez et al.14. Similar oocyst and sporozoite production rates were observed in field populations of An. darlingi from the Peruvian Amazon, with an oocyst infection intensity of 13 (range of 1–93) and a sporozoite yield per mosquito of 1080 (range, 170–7380)21.
Anopheles aquasalis and An. darlingi are considered important malaria vectors in different regions of the Americas, including the Amazon Region3,7; therefore, they are expected to be highly susceptible to human Plasmodium species such as P. vivax. Variations in infection rates and intensities are related to human hosts, parasites, and mosquito vector factors, such as genetics, immunity, biology, and ecology, which can vary among individuals and populations2,22,23.
Anopheles nuneztovari s.l. and An. triannulatus s.l. showed substantially lower infection rates and oocyst and sporozoite intensities than did An. aquasalis or An. darlingi but similar to those observed by Rios-Velasquez et al.14. These two species are considered important malaria vectors in some places in Latin America16; however, their vectorial competence in the Amazon region has not been elucidated and they are considered as occasional vector in some localities. Anopheles triannulatus s.l. had the fourth highest infection rate (15.51%) and oocyst intensity (5.16). Furthermore, an average of 141 sporozoites were counted per salivary gland pair. In Brazil, An. triannulatus s.l. has been found to be naturally infected by P. vivax and P. falciparum, with infection rates between 0.23 and 0.56%15,24. Under experimental conditions, the infection rate was 8.8%14. Although in Brazil An. triannulatus showed low susceptibility to P. vivax infection14,25, it is a vector in Colombia, Peru, and Venezuela26,27,28. Anopheles triannulatus s.l. has preferential zoophilic habits, and this behavior is probably the limiting factor for the transmission of human malaria. However, at high densities, it can behave as an opportunistic species, and depending on the availability of vertebrate hosts, it can play a role in malaria transmission. In the present study, these species showed attractiveness to humans, as observed in other studies29,30. The evidence that those two species can bite humans in addition to the susceptibility to P. vivax and the capacity to develop sporozoites indicates that they must be important for maintenance of malaria transmission cycles.
Anopheles nuneztovari s.l. showed infection rates and intensities of 20% and 2, respectively. Furthermore, an average of 204 sporozoites were counted per salivary gland pair. Although statistically different from An. aquasalis and An. darlingi, An. nuneztovari s.l., it was not statistically different from An. triannulatus s.l., An. evansae, or An. benarrochi s.l. (Table 1). The importance of vectors that are considered secondary or occasional has increased markedly over the years. This species is considered epidemiologically unimportant; however, outside of the Brazilian Amazon, An. nuneztovari s.l. is associated with malaria transmission in Venezuela, Colombia, and Peru26,31,32.
Our results showed that An. nuneztovari s.l and An. triannulatus s.l were permissive for P. vivax development in the midgut and salivary glands, and for the transmission of sporozoites during blood meals. Despite the lower rates of oocysts in An. nuneztovari s.l and An. triannulatus s.l., sporozoite production was higher than that observed in An. evansae, which had a higher mean oocyst count and a lower quantity of sporozoites.
Anopheles evansae and An. benarrochi s.l. that showed the lowest infection rates among the studied species, have not been considered potential vectors, and are found in low abundance in the field. Anopheles benarrochi s.l. was collected for the first time infected naturally by P. vivax in the Brazilian Amazon region13. An. evansae is not naturally infected by Plasmodium spp.15. Nevertheless, in this study, both species were permissive to P. vivax development in the midgut and salivary glands and transmitted the parasite during a blood meal.
It is important to note that An. benarrochi s.l. showed a lower infection rate and parasite load in the midgut and salivary glands, however, because of the low number of individuals available for experimentation, An. benarrochi s.l. was not included in the statistical analysis. This is the first time that An. benarrochi s.l. has been experimentally infected by membrane feeding assay, and these results agree with its status as “non vector.” Little is known about the epidemiological importance of An. benarrochi s.l. in Brazil. Klein et al.25 showed that this species developed P. vivax oocysts under laboratory conditions; however, sporozoites were not found in the salivary glands. In this study, we examined midgut oocyst formation and sporozoite transmission. Although we did not consider the vector species in the Brazilian Amazon, An. benarrochi s.l. is a vector for P. vivax and P. falciparum in the Peruvian Amazon33. Anopheles benarrochi s.l. is part of a species complex34,35 and different geographical populations probably have different susceptibilities to Plasmodium infection. The population density of this species is very low in the Brazilian Amazon25,36,37; therefore, the number of available individuals for experimentation was too low. Combining the infection rate, intensity of oocysts and sporozoites, and low population densities, it is reasonable to suggest that this species does not significantly participate in P. vivax transmission cycles. This type of work must be done in areas where this species is more abundant to clarify its role in Plasmodium transmission.
Despite the low number of oocysts observed in An. nuneztovari s.l., An. triannulatus s.l., An. evansae, and An. benarrochi s.l., their salivary glands in infected insects were found with sporozoites. A correlation was found between the number of oocysts in the midgut and the number of sporozoites in the salivary glands of the studied species. Santos et al.38 reported a positive correlation between the number of P. vivax oocysts and sporozoites in An. darlingi and An. deaneorum-infected mosquitoes. The presence of oocysts in the mosquito midgut is considered a parameter for determining the susceptibility of Anopheles to Plasmodium. These results indicate that in these Amazonian Anopheles species, the presence of oocysts in the midgut would lead to salivary gland invasion by sporozoites, regardless of the number of oocysts; that is, a mosquito with oocysts is potentially infectious.
Despite the low numbers of oocysts in An. nuneztovari s.l., An. triannulatus s.l., An. evansae, and An. benarrochi s.l., the resulting sporozoites were transmitted during blood meals. The salivary glands of mosquitoes are invaded by thousands of sporozoites; however, only 10–100 sporozoites are inoculated in the vertebrate host39. The smallest number of sporozoites (mean of 30 sporozoites per mosquito) was counted in the salivary glands of An. benarrochi s.l. Mosquitoes with ≤ 10 sporozoites can initiate an infection in 32% of vertebrate hosts and mosquitoes with > 1000 sporozoites in 78%40. In this study, we observed sporozoite motility in the salivary glands of all Anopheles species, demonstrating that sporozoites were viable at 14 dpi (S1 Movie). Sporozoite motility is a fundamental requirement for migration to the salivary glands, exiting the dermal tissue, and reaching the bloodstream of the vertebrate host41,42.
Studies on mosquito infectivity generally focused on oocysts and on blocking their development43,44,45, despite the fact that it is the sporozoites that are key to Plasmodium transmission. This study shows, for the first time, that in the Amazon mosquito species studied here: (a) a low number of oocysts usually translates into salivary gland invasion, and (b) if the salivary gland is invaded, the sporozoites can be transmitted during the blood meal.
The available strategies for blocking transmission only reduce the intensity of infection in mosquitoes46,47, but what is the significance of this from the point of view of Plasmodium transmission? According to our results, low numbers of oocysts are insufficient to prevent salivary gland invasion or parasite transmission. Thus, to interrupt malaria transmission, any intervention must result in the complete inhibition of oocyst formation.
We detected P. vivax DNA in the saliva of six Anopheles species, two known vector species, and four non-vector species in the Brazilian Amazon Region. These results show for the first time that P. vivax completes its development in An. triannulatus s.l., An. nuneztovari s.l., An. evansae, and An. benarrochi s.l. The parasite invades the intestinal epithelium and salivary glands and is expelled during bloodmeals. We showed that mosquitoes with low numbers of oocysts can transmit the parasite during a blood meal. From an epidemiological point of view, whether there are few or many oocysts does not necessarily impact sporozoite transmission. The question is: What is the role of these mosquito species in malaria transmission in the areas where An. darlingi is in low abundance? It could be expected that mosquito species that have infected salivary glands and are able to eject sporozoites during a blood meal could also play an important role in maintaining the number of malaria cases in the Amazon region.
Conclusion
In this study, we showed that at least three oocysts in the midgut are sufficient for successful salivary gland invasion and P. vivax transmission. We conclude that populations of Amazonian Anopheles species with low numbers of oocysts are robust transmitters of P. vivax. Thus, alternative malaria elimination strategies are needed to identify and explore mechanisms that block, rather than suppress, transmission of the parasite. Anopheles triannulatus s.l., An. nuneztovari s.l., An. evansae, and An. benarrochi s.l. have high potential as malaria vectors; therefore, our results suggest that these species may act occasionally as competent vectors of P. vivax in nature. In the area of mosquito collection, the abundance of mosquito species changes throughout the year, similar to the substitution of species according to weather. At the same time, in these localities, malaria transmission occurred throughout the year. Our hypothesis is that in the absence of An. darlingi, species such An. nuneztovari s.l., and An. triannulatus s.l. helps to maintain transmission of P. vivax.
Methods
Anopheles collections
In total, 30 field collections were made during March 2021 to April 2022 in three sites located in the Manaus municipality, Amazonas State, Brazil. Mosquito larvae were collected in breeding sites at Puraquequara neighborhood (3°02′40.1″S 59°53′47.5″W) and Brasileirinho Roads (3°01′25.0″S 59°52′55.2″W), during the daytime from 08:00 to 12:00. Adult mosquitoes were collected biting horses, using the mouth aspirator method at twilight from 16:00 to 20:00, in a ranch located at Tarumã Açu neighborhood (2°58′54.0″S 60°02′49.0″W). Adults of An. aquasalis were obtained from the colony of Laboratory of infectious Ecology Diseases of Amazon facility of Leônidas e Maria Deane Institute (ILMD-Fiocruz AM).
F1 mosquitoes
The field-collected females were blood-fed on chicken and on the fourth day, transferred to individual oviposition cups after a blood meal. Mosquito larvae collected in the field and those obtained through eclosion of hatching eggs were reared in the laboratory in plastic trays and fed Tetramin fish food. The emerged adults were maintained at 27 °C with 70% relative humidity and fed 10% sucrose solution ad libitum48. Mosquitoes were anesthetized at 4 °C and identified at the species level using the dichotomous key of Consoli and Oliveira49. They were maintained in cages at 27 °C with 70% relative humidity and fed 10% sucrose solution ad libitum.
Blood collection and membrane-feeding assay
Blood from malaria-infected patients diagnosed by the thick blood smear method at the Dr. Heitor Vieira Dourado Tropical Medicine Foundation (FMT-HVD) was used for the experiments. The inclusion criteria were adult patients over 18 years old, infected with P. vivax, with two crosses of parasitemia (501–10,000 parasites/μl)50 who agreed to participate in the project as volunteers. For each patient, 5 mL of blood was collected in heparinized Vacutainer tubes.
Adult mosquitoes were starved for 24 h prior to blood feeding. The experimental infection was carried out through a membrane feeding assay using a hose system connected to a thermal bath held at 39 °C to keep the blood warm14. Oocysts in the midgut and sporozoites in the salivary glands of An. aquasalis were used as controls, and all species were fed with blood from the same malaria-infected patients. Fully engorged females were maintained at 27 °C with 70% relative humidity, and fed 10% sucrose solution ad libitum.
Oocyst and sporozoite counts
Seven days post-infection, the midgut of mosquitoes was dissected in phosphate buffered saline (PBS; 1X) on glass slides, stained with 2% Mercurochrome (Merbromin), and observed under an optical microscope (Leica DM1000, Germany) at 40X magnification. The number of oocysts in each midgut sample was recorded. Fourteen days post-infection, the salivary glands of 20 mosquitoes were dissected in RPMI-1640, pooled, macerated, and sporozoites were counted using a hemocytometer slide under an optical light microscope at 40X magnification. The counts were performed according to the method described by Prinz et al.51 An. aquasalis oocysts and sporozoites were used as controls.
Salivation
Salivation was performed on the day 14 post-infection. Twenty females of each species were held in saliva using a membrane feeding assay in glass feeders with 100 µL of uninfected blood for 2 h. The samples (blood and saliva) were transferred to microtubes and stored at − 20 °C.
DNA extraction
Blood samples containing mosquito saliva were placed in 1.5 ml microtubes containing 1 ml distilled water, and DNA was extracted using the Chelex 100 Resin 5% (Bio-Rad) method. The samples were incubated at 27 °C for 30 min and vortexed for 15 s. After homogenization, the samples were centrifuged at 14,000 rpm for 3 min, the supernatant was removed, and 170 µl of 5% Chelex 100 Resin was added, heated in a thermoblock at 56 °C for 30 min, and vortexed for 15 s. Finally, the samples were incubated at 96 °C for 8 min, vortexed for 15 s, and centrifuged at 15,000 rpm for 3 min. The extracted DNA was stored at − 20 °C.
Detection of P. vivax
PCR was performed using the GoTaq Flexi DNA Polymerase Kit (Promega, Madison, WI, USA) targeting a sequence of the Pv18S gene. The reaction was performed in a 25 μl final volume with 1 µL of DNA. The PCR cycle used is as follows: 10 min at 95 °C, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C/1 cycle at 16 °C52. The samples were visualized via gel electrophoresis on a 2% agarose gel containing GelRed (Phenix Research). Gel was imaged with a UVP Epichemi3 Darkroom (UVP, LLC., Upland, CA).
Ethical aspects
This study was approved by the Ethical Review Committee at FMT-HVD (CAAE 39706514.2.00000.0005), all subjects included in the study, or their legal guardians were informed about the project, and those accepted to participate assigned the informed consent terms. Field collection of mosquitoes was approved by Biodiversity Authorization and Information System (No. 75496). Animal Use Ethics Committee of National Institute of Amazonian Research—INPA (No. 008/2021, SEI 01280.000118/2021-34) approved the use of horses as attractive and chickens as blood meal resources for mosquitoes. All methods were carried out in accordance with relevant guidelines and regulations. This study is reported in accordance with ARRIVE guidelines.
Statistical analysis
The infection rate (IR) was calculated by dividing the number of infected midguts by the number of dissected midguts and multiplying by 100. Oocyst infection intensity was calculated by dividing the total number of oocysts by the total number of infected mosquitoes. The mean number of sporozoites was calculated by dividing the total number of sporozoites by the total number of dissected female mosquitoes. The infection rate and intensity of all the mosquito species were compared with the infection rate and intensity of An. aquasalis (the reference species). The mean number of sporozoites in the salivary glands of all the mosquito species evaluated was compared using heatmaps. The Shapiro–Wilk test was used to test the normality of the data. The Kruskal–Wallis test was used to compare the number of oocysts and sporozoites between the evaluated species using the An. aquasalis as a reference species. Pearson’s correlation coefficient was calculated to evaluate the correlation between the infection intensity and sporozoite production. All data analyses were performed using the GraphPad Prism v.6 software (San Diego, CA, USA, https://www.graphpad.com/).
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request. All methods were carried out in accordance with relevant guidelines and regulations.
References
WHO. World Malaria Report 2021 (2021).
Cohuet, A., Harris, C., Robert, V. & Fontenille, D. Evolutionary forces on Anopheles: What makes a malaria vector?. Trends Parasitol. 26(3), 130–136. https://doi.org/10.1016/j.pt.2009.12.001 (2010).
Sinka, M. E. et al. A global map of dominant malaria vectors. Parasites Vectors 4(5), 69. https://doi.org/10.1186/1756-3305-5-69 (2012).
Klug, D. & Frischknecht, F. Motility precedes egress of malaria parasites from oocysts. Elife 24(6), e19157. https://doi.org/10.7554/eLife.19157 (2017).
Baton, L. A. & Ranford-Cartwright, L. C. Spreading the seeds of million-murdering death: Metamorphoses of malaria in the mosquito. Trends Parasitol. 21(12), 573–580. https://doi.org/10.1016/j.pt.2005.09.012 (2005).
Alavi, Y. et al. The dynamics of interactions between Plasmodium and the mosquito: A study of the infectivity of Plasmodium berghei and Plasmodium gallinaceum, and their transmission by Anopheles stephensi, Anopheles gambiae and Aedes aegypti. Int. J. Parasitol. 33(9), 933–943. https://doi.org/10.1016/S0020-7519(03)00112-7 (2003).
Carlos, B. C., Rona, L. D. P., Christophides, G. K. & Souza-Neto, J. A. A comprehensive analysis of malaria transmission in Brazil. Pathog. Glob. Health 113, 1–13. https://doi.org/10.1080/20477724.2019.1581463 (2019).
Tadei, W. P. & Thatcher, D. B. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Rev. Inst. Med. Trop. S. Paulo. 42(2), 87–94. https://doi.org/10.1590/S0036-46652000000200005 (2000).
Arruda, M. et al. Potential vectors of malaria and their different susceptibility to Plasmodium falciparum and Plasmodium vivax in northern Brazil identified by immunoassay. Am. J. Trop. Med. Hyg. 35(5), 873–881. https://doi.org/10.4269/ajtmh.1986.35.873 (1986).
Duarte, A. M. R. et al. Natural infection in anopheline species and its implications for autochthonous malaria in the Atlantic Forest in Brazil. Parasites Vectors 7(6), 58. https://doi.org/10.1186/1756-3305-6-58 (2013).
Zimmerman, R. H. et al. Nightly biting cycles of malaria vectors in a heterogeneous transmission area of eastern Amazonian Brazil. Malar. J. 26(12), 262. https://doi.org/10.1186/1475-2875-12-262 (2013).
Figueiredo, M. A. P. et al. Molecular identification of Plasmodium spp. and blood meal sources of anophelines in environmental reserves on São Luís Island, state of Maranhão Brazil. Parasites Vectors 10, 203. https://doi.org/10.1186/s13071-017-2133-5 (2017).
Oliveira, T. M. P. et al. Vector role and human biting activity of Anophelinae mosquitoes in different landscapes in the Brazilian Amazon. Parasit Vectors 14(1), 236. https://doi.org/10.1186/s13071-021-04725-2 (2021).
Rios-Velásquez, C. M. et al. Experimental Plasmodium vivax infection of key Anopheles species from the Brazilian Amazon. Malar. J. 12(1), 460. https://doi.org/10.1186/1475-2875-12-460 (2013).
Póvoa, M., Wirtz, R., Lacerda, R., Miles, M. & Warhurst, D. Malaria vectors in the municipality of Serra do Navio, State of Amapá, Amazon region Brazil. Mem Inst Oswaldo Cruz 96(2), 179–184. https://doi.org/10.1590/S0074-02762001000200008 (2001).
Hiwat, H. & Bretas, G. Ecology of Anopheles darlingi root with respect to vector importance: A review. Parasiters Vectors 16(4), 177. https://doi.org/10.1186/1756-3305-4-177 (2011).
Pimenta, P. F. et al. An overview of malaria transmission from the perspective of Amazon Anopheles vectors. Mem. Inst. Oswaldo Cruz 110(1), 23–47. https://doi.org/10.1590/0074-02760140266 (2015).
Bousema, T. et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE 7(8), e42821. https://doi.org/10.1371/journal.pone.0042821 (2012).
Churcher, T. S. et al. Measuring the blockade of malaria transmission-an analysis of the standard membrane feeding assay. Int. J. Parasitol. 42(11), 1037–1044. https://doi.org/10.1016/j.ijpara.2012.09.002 (2012).
Pereira-Silva, J. W. et al. Long-lasting infectivity of Plasmodium vivax present in malarial patient blood to Anopheles aquasalis. Exp. Parasitol. 222, 108064. https://doi.org/10.1016/j.exppara.2021.108064 (2021).
Moreno, M. et al. Continuous supply of Plasmodium vivax Sporozoites from colonized Anopheles darlingi in the Peruvian Amazon. ACS Infect. Dis. 4(4), 541–548. https://doi.org/10.1021/acsinfecdis.7b00195 (2018).
Mitri, C. & Vernick, K. D. Anopheles gambiae pathogen susceptibility: The intersection of genetics, immunity, and ecology. Curr. Opin. Microbiol. 15(3), 285–291. https://doi.org/10.1016/j.mib.2012.04.001 (2012).
Bousema, T. & Drakeley, C. Determinants of Malaria transmission at the population level. Cold Spring Harb. Perspect. Med. 7(12), a025510. https://doi.org/10.1101/cshperspect.a025510 (2017).
Galardo, A. K. R. et al. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 76, 461–469. https://doi.org/10.4269/ajtmh.2007.76.461 (2007).
Klein, T. A., Lima, J. B., Tada, M. S. & Miller, R. Comparative susceptibility of anopheline mosquitoes in Rondônia, Brazil to infection by Plasmodium vivax. Am. J. Trop. Med. Hyg. 45(4), 463–470. https://doi.org/10.4269/ajtmh.1991.45.463 (1991).
Rubio-Palis, Y. Variation of the vectorial capacity of some anophelines in western Venezuela. Am. J. Trop. Med. Hyg. 50(4), 420–424. https://doi.org/10.4269/ajtmh.1994.50.420 (1994).
Aramburú, G. J., Asayag, R. C. & Witzig, R. Malaria reemergence in the Peruvian Amazon region. Emerg. Infect. Dis. 5(2), 209–215. https://doi.org/10.3201/eid0502.990204 (1999).
Rosero, D. A. et al. Colombian Anopheles triannulatus (Diptera: Culicidae) naturally infected with Plasmodium spp. ISRN Parasitol. 8, 927453. https://doi.org/10.5402/2013/927453 (2013).
Pereira-Silva, J. W. et al. Distribution and diversity of mosquitoes and Oropouche-like virus infection rates in an Amazonian rural settlement. PLoS ONE 16(2), e0246932. https://doi.org/10.1371/journal.pone.0246932 (2021).
Meireles, A. C. A. et al. Anopheline diversity in urban and peri-urban malaria foci: Comparison between alternative traps and seasonal effects in a city in the Western Brazilian Amazon. Malar. J. 21(1), 258. https://doi.org/10.1186/s12936-022-04274-8 (2022).
Olano, V., Brochero, H. L., Sáenz, R., Quiñones, M. L. & Molina, J. A. Mapas preliminares de la distribución de especies de Anopheles vectores de malaria en Colombia. Biomedica 21, 402–408 (2001).
Montoya, C., Bascuñán, P., Rodríguez-Zabala, J. & Correa, M. M. Abundancia, composición e infección natural de mosquitos Anopheles en dos regiones endémicas para malaria en Colombia. Biomedica 37, 98–105. https://doi.org/10.7705/biomedica.v37i0.3553 (2017).
Flores-Mendoza, C., Fernández, R., Escobedo-Vargas, K. S., Vela-Perez, Q. & Schoeler, G. B. Natural Plasmodium infections in Anopheles darlingi and Anopheles benarrochi (Diptera: Culicidae) from Eastern Peru. J. Med. Entomol. 41(3), 489–494. https://doi.org/10.1603/0022-2585-41.3.489 (2004).
Ruiz, F. et al. Molecular differentiation of Anopheles (Nyssorhynchus) benarrochi and An. (N) oswaldoi from Southern Colombia. Mem. Inst. Oswaldo Cruz 100, 155–160. https://doi.org/10.1590/S0074-02762005000200008 (2005).
Sallum, M. A., Marrelli, M. T., Nagaki, S. S., Laporta, G. Z. & Santos, C. L. Insight into Anopheles (Nyssorhynchus) (Diptera: Culicidae) species from Brazil. J. Med. Entomol. 45(6), 970–981. https://doi.org/10.1603/00222585(2008)45[970:iiandc]2.0.co;2 (2008).
Maciel, G. B. M. L. & Missawa, N. A. Descrição de fauna anofélica em área endêmica de malária no Município de Colniza, Estado de Mato Grosso Brasil. Epidemiologia e Serviços de Saúde 21(1), 141–148. https://doi.org/10.5123/S1679-49742012000100014 (2012).
Gil, L. H. S., Rodrigues, M. S., Lima, A. A. & Katsuragawa, T. H. Seasonal distribution of malaria vectors (Diptera: Culicidae) in rural localities of Porto Velho, Rondônia, Brazilian amazon. Rev. Inst. Med. Trop. Sao Paulo. 57, 263–267. https://doi.org/10.1590/S0036-46652015000300014 (2015).
Santos, N. A. C. et al. Evaluation of sustainable susceptibility to Plasmodium vivax infection among colonized Anopheles darlingi and Anopheles deaneorum. Malar. J. 21(1), 163. https://doi.org/10.1186/s12936-022-04204-8 (2022).
Medica, D. L. & Sinnis, P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect. Immun. 73(7), 4363–4369. https://doi.org/10.1128/IAI.73.7.4363-4369.2005 (2005).
Aleshnick, M., Ganusov, V. V., Nasir, G., Yenokyan, G. & Sinnis, P. Experimental determination of the force of malaria infection reveals a non-linear relationship to mosquito sporozoite loads. PLoS Pathog. 16(5), e1008181. https://doi.org/10.1371/journal.ppat.1008181 (2020).
Korne, C. M. et al. Regulation of Plasmodium sporozoite motility by formulation components. Malar J. 18(1), 155. https://doi.org/10.1186/s12936-019-2794-y (2019).
Beyer, K. et al. Limited Plasmodium sporozoite gliding motility in the absence of TRAP family adhesins. Malar. J. 20(1), 430. https://doi.org/10.1186/s12936-021-03960-3 (2021).
Bahia, A. C. et al. The JAK-STAT pathway controls Plasmodium vivax load in early stages of Anopheles aquasalis infection. PLoS Negl. Trop. Dis. 5(11), e1317. https://doi.org/10.1371/journal.pntd.0001317 (2011).
Gomes, F. M. & Barillas-Mury, C. Infection of anopheline mosquitoes with Wolbachia: Implications for malaria control. PLoS Pathog. 14(11), e100733. https://doi.org/10.1371/journal.ppat.1007333 (2018).
Dong, S., Dong, Y., Simões, M. L. & Dimopoulos, G. Mosquito transgenesis for malaria control. Trends Parasitol. 38(1), 54–66. https://doi.org/10.1016/j.pt.2021.08.001 (2022).
Pinilla, Y. T. et al. Promising approach to reducing malaria transmission by ivermectin: Sporontocidal effect against Plasmodium vivax in the South American vectors Anopheles aquasalis and Anopheles darlingi. PLoS Negl. Trop. Dis. 12(2), e0006221. https://doi.org/10.1371/journal.pntd.0006221 (2018).
Tachibana, M. et al. Plasmodium vivax transmission-blocking vaccines: Progress, challenges, and innovation. Parasitol. Int. 87, 102525. https://doi.org/10.1016/j.parint.2021.102525 (2022).
Silva, A. N. et al. Laboratory colonization of Anopheles aquasalis (Diptera: Culicidae) in Belém, Pará Brazil. J. Med. Entomol. 43(1), 107–109 (2006).
Consoli, R.A.G.B.; Oliveira, R.L. Principais Mosquitos de Importância Sanitária no Brasil. Rio de Janeiro: Editora Fiocruz, 228p (1994).
Ministério da Saúde. Manual do diagnóstico laboratorial de Malária. https://bvsms.saude.gov.br/bvs/publicacoes/malaria_diag_manual_final.pdf (2005).
Prinz, H. L., Sattler, J. M. & Frischknecht, F. Plasmodium sporozoite motility on flat substrates. Bio Protocol 7(14), e2395. https://doi.org/10.21769/BioProtoc.2395 (2017).
Bahia, A. C. et al. Anopheles aquasalis Infected by Plasmodium vivax displays unique gene expression profiles when compared to other Malaria vectors and Plasmodia. PLoS ONE 5(3), e9795. https://doi.org/10.1371/journal.pone.0009795 (2010).
Acknowledgements
We are grateful to CAPES (Coordination for the Improvement of Higher Education Personnel) for the doctorate and master scholarships to Jordam William Pereira-Silva and José Vicente Ferreira-Neto, respectively; to CNPq (Brazilian National Council for Scientific and Technological Development) for productivity fellowship to FACP. We also thank the insectary team from EDTA (Laboratório de Ecologia de Doenças Transmissíveis na Amazônia) for mosquito colonization. To farmers who allowed the mosquito collection in their sites.
Funding
This work was supported by the Programa de Excelência em Pesquisa Básica e Aplicada em Saúde (PROEP-LABS/ILMD FIOCRUZ AMAZÔNIA-Nº 001/2020), FAPEAM—Fundação de Amparo à Pesquisa do Estado do Amazonas, CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, ILMD—Instituto Leônidas e Maria Deane—Fiocruz-Amazônia, and CNPq (Universal MCTI/CNPq Nº 14/2014).
Author information
Authors and Affiliations
Contributions
J.W.P.S., K.M.M.C., C.M.R.V. Experiment conception and design; J.W.P.S., K.M.M.C., F.A.C.P., J.V.F.N., C.M.R.V. Fieldwork; C.M.R.V., J.W.P.S. Data analysis; C.M.R.V., M.V.G.L., F.A.C.P. Review, editing and supervision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira-Silva, J.W., Martins-Campos, K.M., Ferreira-Neto, J.V. et al. Amazonian Anopheles with low numbers of oocysts transmit Plasmodium vivax sporozoites during a blood meal. Sci Rep 12, 19442 (2022). https://doi.org/10.1038/s41598-022-24058-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24058-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.