Abstract
Stunting is a public health issue of global concern. Despite, poor sanitation, diarrhea, parasitic infections, and environmental enteric dysfunction (EED) are associated with stunting, their link is poorly understood and has not been investigated in Ethiopia. This study was conducted to assess the associations of stunting with sanitation, enteric infections, and EED among children aged 24–59 months in rural northwest Ethiopia. A community-based cross-sectional study was conducted among 224 randomly selected children aged 24–59 months in rural areas of the east Dembiya district. We collected information on household food insecurity and dietary diversity using pre-tested questionnaires adopted from the food and nutrition technical assistance (FANTA) project. We used height-for-age-z score (HAZ) to define stunting. We also used the data collected to measure the environmental exposures of children to intestinal parasitic infections and fecal biomarkers of EED. A multivariable binary logistic regression model was used to assess the association of stunting with sanitation, enteric infections, and EED. Of the 224 children, 33% (95% CI 27, 39%) were stunted. Stunting in children was significantly associated with poor dietary intake (AOR 3.0, 95% CI 1.2, 7.3), open defecation practice (AOR 3.0, 95% CI 1.2, 7.9), presence of animal excreta in the living environment (AOR 3.4, 95% CI 1.2, 9.9), E. coli contamination of drinking water (AOR 4.2, 95% CI 1.1, 15.3), diarrheal disease incidence (AOR 3.4, 95% CI 1.5, 7.7), intestinal parasites in children (AOR 3.3, 95% CI 1.3, 8.8), and higher EED disease activity scores (AOR 2.9, 95% CI 1.2, 6.7). One-third of the children in the study area were stunted and this high prevalence of stunting was associated with poor dietary intake, poor hygiene and sanitation conditions, enteric infections, and EED. Thus, stunting can be prevented by improving sanitation and hygienic conditions to prevent repeated enteric infections in children and by promoting dietary diversity of children.
Similar content being viewed by others
Introduction
Poor sanitation, enteric infections, environmental enteric dysfunction, and stunting are public health issues of global concern and failure to provide sanitation and nutrition services in the twenty-first century is the greatest development failure1,2,3 that leads to diseases4,5,6 and stifles social and economic development by negatively impacting health, education, and livelihoods and results stunted growth7,8,9. Stunting is defined as a deficit in height relative to a child’s age10. Children in the developing world are the most impacted group by stunting5. According to the 2018 United Nations Children’s Fund (UNICEF) report, 149 million (21.9%) children under 5 years of age across the globe and 58.8 million (30%) in Africa are stunted11 and in Ethiopia 38% children under 5 years of age were stunted in 201912. Stunting in children could lead to impaired physical development and have a long-term effect on cognitive development, educational performance, and economic productivity in adulthood and on maternal reproductive outcomes13,14. Maternal stunting could result high risks to the survival, health and development of her offspring. Decreased maternal stature is associated with an increased risk of underweight and stunting among offspring. Maternal stunting can restrict uterine blood flow and growth of the uterus, placenta, and fetus. Intrauterine growth restriction (IUGR) is associated with many adverse fetal and neonatal outcomes15.
Traditionally, stunting was believed to be caused by lack of food16. Adequate and nutritious foods are necessary to nourish children but not enough. Only a small portion of stunting can be solved by nutrition interventions17. Research findings indicated that stunting occurs even among well-fed children18, which showed that there are other factors, such as poor WASH conditions which are linked to stunting19,20. Children who live without access to basic WASH services do not grow as children with access to adequate sanitation21,22. Unhygienic disposal of human and animal excrement causes fecal contamination of the living environment23,24,25,26. This results a continuous exposure of children to enteropathogens through the ingestion of contaminated food, water, and even soil27,28,29. Microorganisms like rotavirus, adenovirus, and astrovirus cause limited mucosal disturbances. Enterotoxigenic E. coli (ETEC) causes secretory diarrhea with only limited mucosal changes. Others, such as Campylobacter, Shigella, Salmonella, enteroaggregative E. coli (EAEC), enteropathogenic E. coli (EPEC), and enteroinvasive E. coli (EIEC) are enteroinvasive or cause extensive mucosal disruption30. Generally, enteropathogens can cause chronic gut inflammation and morphological abnormalities in the small intestine such as blunted villi and crypt hyperplasia27,31, which can cause bacterial translocation, systemic inflammation, metabolic changes, increased permeability, and nutrient malabsorption, growth faltering. This process is known as EED, is a poorly understood condition that has the potential to affect child growth, health, and development in low and middle-income countries (LMICs)32. The pathogenic pathways in EED is characterized by (i) gut inflammation, (ii) gut leakiness/permeability, (iii) microbial translocation, (iv) dysbiosis, (v) systemic inflammation, and (vi) nutrient malabsorption33,34.
Enteric infections and EED tie poor WASH to stunting35,36. However, the link between poor WASH, enteric infections, EED, and stunting is poorly understood37 and it has not been investigated in Ethiopia. Accordingly, this study was conducted to assess the associations of stunting with sanitation, enteric infections, and EED among children aged 24–59 months in rural northwest Ethiopia.
Methods
This community-based cross-sectional study is part of a large-scale study conducted in rural northwest Ethiopia during May–June 2021 to assess environmental exposures of children to enteric infections and fecal biomarkers of EED in children aged 24–59 months. The method has been described in more detail elsewhere38,39. The sample size was determined using double proportion population formula with the following assumptions: proportion of stunting with enteric infection (p1) = 38.5%, proportion of stunting with no enteric infection (p2) = 61.5%40, Zα/2 at type I error of 5% = 1.96, Zβ at 80% power = 0.842, and an allocation ratio of 1:1. Therefore, the sample size was determined to be 71 in each group. After considering a design effect of 1.5 and 5% non-response rate, the final sample size in each group was found to be 112, leading to a total of 224 study subjects. In the current study, we randomly selected 224 out of 235 children included in a study done to measure fecal biomarkers of EED39 using a simple random sampling technique, i.e., computer-based random number generator to further analyze the data for nutritional assessments.
Food access and nutrition assessment
Household food insecurity was assessed using a pre-tested food insecurity assessment questionnaire adopted from the FANTA project, version 3, and households were classified as food secure, mildly food insecure, moderately food insecure, and severely food insecure41. The FANTA household food insecurity access scale generic questions that have been used in several countries and appear to distinguish the food secure from the insecure households across different cultural contexts41.
Dietary intake of the children over a period of 24 h was assessed using a pre-tested dietary diversity questionnaire adopted from the FANTA project42. The caregivers were asked whether the children had eaten foods from the seven main food groups in a 24-h period. The food groups assessed were: (a) grains, roots, or tubers; (b) vitamin A-rich plant foods; (c) other fruits and vegetables; (d) flesh foods (meat, poultry, fish, and seafood); (e) eggs; (f) pulses, legumes, or nuts; and (g) milk and milk products. Dietary diversity score (DDS) was used to qualitatively assess the dietary intake of children. Poor dietary diversity was defined as a child with a DDS of less than four43. Height was measured using the seca vertical height scale (German, serial No. 0123) standing upright in the middle of the board. The child’s head, shoulders, buttocks, knees, and heels touch the vertical board and the height-for-age-z score (HAZ) was calculated using WHO Anthro software, and the values of each child were compared with the WHO Multicenter Growth Reference Standard. Trained data collectors who have BSc degree in Public Health, Nursing, and Environmental Health performed the measurement and data collection.
Measurement of study variables
Stunting among children is the primary outcome variable of this study and a child was categorized as stunted if his or her HAZ was less than − 2SD from the reference population. In addition, a child with HAZ less than − 3SD and between − 3 and − 2SD was considered severely and moderately stunted, respectively44.
Mouthing of soil-contaminated materials, fecal contamination of drinking water, fecal contamination of ready-to-eat foods, fecal contamination of courtyard soil, diarrheal disease, and intestinal parasites were the exposure variables for this study. Childhood diarrheal disease was defined as having three or more loose or watery stools within 24 h period45. A 2-week period of diarrheal disease in children was determined based on history from mothers or caregivers. Fecal contamination of the living environment (drinking water, ready-to-eat foods, and courtyard soil) was measured by detection of fecal indicator bacteria, i.e., Escherichia coli (E. coli) using membrane filter technique46. Sampling procedures of environmental samples and E. coli detection procedures are described elsewhere in more detail38. Intestinal parasites in children were measured by detecting ova of one or more intestinal parasites in stool samples of children using wet mount and Kato-Katz techniques47. The detailed procedures of stool sample collection and investigation of ova of parasites are described elsewhere in more detail38. Geophagy or mouthing of soil-contaminated materials is a repeated ingestion of nonfood substances (such as clays, yard soil), or large quantities of certain particular foods contaminated with soil48. Environmental enteric dysfunction was measured by three fecal biomarkers: Alpha-1-antitrypsin (AAT), myeloperoxidase (MPO), and neopterin (NEO). We used commercial ELISA kits to measure stool concentrations of MPO (Immundiagnostik AG, Germany), AAT (BioVendor, ImmuChorm, Germany), and NEO (GenWay Biotech Inc., USA). The use of a combination of biomarkers (AAT, MPO, and NEO) to measure EED is standardized in developing countries and the sensitivity and specificity of these biomarkers in predicting poor linear growth is well documented49,50,51. The measurement of fecal biomarkers of EED is described elsewhere in more detail39. Fecal concentrations of AAT, MPO, and NEO were categorized in to low (concentrations in first quartile), medium (concentrations in the interquartile range), or high (concentrations in fourth quartile). For each of the three biomarkers, 0 point was given for low concentrations, 1 point for medium concentrations, and 2 points for high concentrations and EED disease activity score was calculated as 2 × (AAT category) + 2 × (MPO category) + 1 × (NEO category)49,52,53.
Statistical analysis
We used STATA version 14 (Stata Corp, College Station, TX, USA) to analyze the data. Multivariable binary logistic regression analysis was done to identify factors associated with stunting. Covariates for the adjusted model were selected using bivariate analysis on the basis of p-values < 0.2. In the adjusted model, statistically significant associations were declared based on adjusted odds ratio (AOR) with the corresponding 95% confidence interval (CI) and p-value < 0.05. Model fitness was checked using the Hosmer–Lemeshow model fitness test and we checked multicollinearity using variance inflation factors and we found no collinearity.
Ethics approval and consent to participate
Ethical clearance was obtained from the Institutional Review Board of the University of Gondar (reference number: V/P/RCS/05/1933/2020). There were no risks due to participation and the collected data were used only for this research purpose with complete confidentiality. Written informed consent was obtained from mothers or caregivers. All the methods were carried out in accordance with relevant guidelines and regulations.
Results
Socio-demographic characteristics
Among the total 224 children included in this study, 50.9% of the participants in the study were females. The youngest and oldest children were 24 and 59 months old, respectively, with a mean age of 43 (± 13 SD) months. The highest proportion of children, 42%, were between the ages of 49 and 59 months. About one-tenth (11%) of mothers or caregivers attended post-secondary education. Moreover, 60% of the households had a family size of below five and 69% of the households owned livestock (Table 1).
Hygiene and sanitation conditions
The results showed that 69% of the households defecated in the open field. We found animal excreta in the courtyards in 76% of the households during the spot-check observation. Furthermore, E. coli was detected in 81%, 70%, and 67% of courtyard soil, drinking water, and ready-to-eat food samples, respectively. We also observed that 71% of the children mouthed soil-contaminated materials (Table 2).
Enteric infections and environmental enteric dysfunction in children
In the current study, 61% of the children had one or more intestinal parasitic infections and 31% of the mothers or caregivers reported that their children had diarrhea in a 2 week period prior to the survey and 9% of the children had both diarrheal disease and intestinal parasitic infections. Moreover, the concentration of Alpha-1-antitrypsin (AAT) was elevated among 67% of the children compared with the normal concentration, i.e., < 270 μg/ml. Similarly, the concentrations of Myeloperoxidase (MPO) and Neopterin (NEO), respectively were elevated in 72% and 79% of the children compared with concentrations considered normal, i.e., < 2000 ng/ml for MPO and < 70 nmol/l for NEO. Moreover, 42% of the children had high EED disease activity scores (above the median score of 5), indicating that the concentrations of fecal biomarkers in these children are elevated (Table 3).
Food access and nutrition status of children
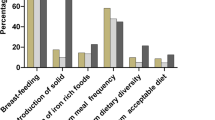
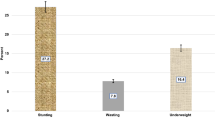
In the study area, 47% of the households were food insecure, out of which 19% were severely insecure. The minimum dietary score (children who consumed foods from four or more food groups) was 69%. Grains, roots, or tubers (97%); pulses, legumes, or nuts (90%); and milk and milk products (74%) were the most commonly consumed food groups. Of the 224 children, 73 had HAZ less than − 2SD. The prevalence of stunting was, therefore, found to be 33% (95% CI 27, 39%), out of which 5% and 27% were severely and moderately stunted (Table 4).
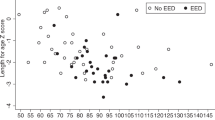
The prevalence of stunting in various groups of children is illustrated in Fig. 1. Children with intestinal parasitic infections, for example, had a higher prevalence of stunting than children without such infections. Similarly, when comparing children with elevated fecal AAT, MPO, and NEO concentrations to children with normal concentrations, the prevalence of stunting was very high.
Factors associated with stunting
Child age, sex of a child, education status of mothers, household food security, dietary diversity, defecation practice of the household, animal excreta in the living environment, mouthing of soil-contaminated materials, E. coli contamination of drinking water, E. coli contamination of courtyard soil, E. coli contamination of ready-to-eat foods, childhood diarrhea, intestinal parasites in children, and EED disease activity scores were entered in to the bivariate analysis. In the final model, only dietary diversity, defecation practice of the household, animal excreta in the living environment, E. coli contamination of drinking water, childhood diarrhea, intestinal parasites in children, and EED disease activity scores were significantly associated with stunting among children.
This study depicted that children who had poor dietary intake had higher odds of stunting compared with children who had good dietary intake (AOR 3.0, 95% CI 1.2, 7.3). Stunting among children in the study area was also significantly associated with WASH conditions. For instance, open defecation practice (AOR 3.0, 95% CI 1.2, 7.9) and presence of animal excreta in the living environment (AOR 3.4, 95% CI 1.2, 9.9) was associated with higher odds of stunting among children. Similarly, E. coli contamination of drinking water was significantly associated with 4.2 times higher odds of stunting among children (AOR 4.2, 95% CI 1.1, 15.3). Moreover, enteric infections and EED were statistically associated with stunting among children in the study area. The odds of stunting was 3.4 times higher among children who had diarrhea compared with children who had no diarrhea (AOR 3.4, 95% CI 1.5, 7.7). Similarly, the odds of stunting was 3.3 times higher among children who had one or more intestinal parasites compared with children who had no parasites (AOR 3.3, 95% CI 1.3, 8.8). Higher EED disease activity scores was also significantly associated with higher odds of stunting among children compared with low EED disease activity scores (AOR 2.9, 95% CI 1.2, 6.7) (Table 5).
Discussion
This community-based cross-sectional study was conducted to assess stunting and its associations with environmental factors (hygiene and sanitation), and its related host factors (enteric infections and EED) among children aged 24–59 months in rural northwest Ethiopia. We found that E. coli was detected in 81%, 70%, and 67% of courtyard soil, drinking water, and ready-to-eat food samples, respectively. Ova of one or more of intestinal parasites were detected in 61% of the children and 31% of the children had diarrhea in a 2 week period prior to the survey. The concentration of AAT among 67%, myeloperoxidase among 72%, and neopterin among 79% of the stool samples were above the normal values and 42% of the children had high EED disease activity scores. Moreover, 33% (95% CI 27, 39%) of the children were stunted.
The prevalence of stunting reported in this study is comparable with findings of similar studies conducted in different parts of Ethiopia, such as in west Guji zone (32%)54, southwest Ethiopia (33%)55, Mizan Aman town (35%)56, and Hosanna (35%)57. Similarly, this finding is comparable with findings of studies in rural Sierra Leone (32%)58, rural Bangladesh (27%)59, and rural Zambia (35%)60. Prevalence of stunting in the current study is also lower than findings of other studies in different parts of Ethiopia, such as in Albuko district (39%)61, Dembia district (46%)62, Angolela Tera district (39%)63, Boricha district (49%)64, Arba Minch (48%)65, Bule Hora district (48%)66, Lalibela town (47%)67, and Enticho town (47%)68. Compared with other developing countries, the prevalence reported in this study was lower studies conducted among children under 5 years of age in a rural area of Maharashtra, India (42%)69, rural Rwenzori Sub-Region of Western Uganda (45%)70, Manyovu, Buhigwe District of Tanzania (43%)71, and rural Community in Kaduna State of North Western Nigeria (60%)72. Moreover, the current study reported higher prevalence of stunting compared with findings of studies in Hawassa Zuria district (27%)73, Sodo Zuria district (25%)74, Debretabor town (23%)75, and Wolayta Sodo (22%)76. The prevalence of stunting reported in the current study is also higher than reports of a study in Bandja village of Cameroon (16%)77.
The high prevalence of stunting among children in the studied region may be explained by poor hygiene and sanitation conditions, a high burden of enteric infections, and gut inflammation of children in the area. The association between poor WASH and stunting is due to the microbial contamination of soil, water, and food and subsequent acquisition of enteric infections such as helminthiases and diarrheal disease by children78,79,80, which have been reported to be associated with stunting81,82. Intestinal worms and other enteropathogens can cause chronic gut inflammation and morphological abnormalities in intestine lead to increased intestinal permeability with subsequent bacterial translocation and immune stimulation27,31, increased energy expenditure due to systemic inflammation27,31, metabolic alterations27,31, reduced absorption capacity of the intestine and altered nutrient absorption27,31, and nutrient malabsorption or decreased nutrient intake27,31.
Moreover, the high prevalence of stunting among children in the study area may be associated with poor dietary intake, which is in line with findings of other studies83,84,85. Insufficient dietary intake of nutrients can irreversibly harm children’s rapidly growing bodies and brains, limiting their potential to grow86. Receiving an inadequately diversified diet may predispose children to opportunistic infections and severe illnesses87,88. As a result, the world health organization has recommended that an infant should receive at least four food groups out of seven in order to maintain proper growth and development during this critical period89.
As a strength, we assessed EED using fecal AAT, MPO, and NEO since the use of these three fecal biomarkers in combination can better explain EED than any single biomarker49,50. Moreover, to effectively answer the research question, we used a combination of methods, including observation of environmental sanitation and child behaviors that result in a high risk of infection; household survey; laboratory investigation of water, food, soil, and stool samples; food security and dietary assessment; and anthropometric assessment. However, although the use of fecal biomarkers permits assessment of intestinal/systemic inflammation and/or intestinal epithelial barrier dysfunction, the main limitation to their use is that they are not specific for EED because they correlate with prevalence, activity, and/or severity of various other gastrointestinal diseases and the fecal biomarkers may not be reflective for small bowel activity90. Furthermore, HAZ is inappropriate to measure changes in linear growth over time because they are constructed using standard deviations from cross-sectional data. The self-reported nature of food insecurity and dietary diversity data, as well as a 4-week and a 24-h recall period, make the data prone to social desirability and recall biases. In addition to social desirability and recall biases, there was another significant limitation to this study. A 24-h food consumption assessment is likely to be subjected to normal day-to-day variability, and it also ignores the amount of food consumed. Despite this, the methods used in this study have been widely implemented and validated. This study's limitations are not unique. Others have noted the limitations of self-reported food insecurity and dietary diversity data collection methods91,92. We attempted to reduce bias by assuring respondents of confidentiality and privacy, as well as informing them that their responses to the questions would have no bearing on their eligibility for aid. Moreover, the use of bivariate p-values to select candidate variables for the adjusted model could lead to the incorrect exclusion of a potential confounder and hence led to an inadequate adjustment for confounding.
Conclusion
One-third of the children in the study area had stunted growth and stunting in the study area was associated with poor dietary intake, poor hygiene and sanitation conditions, enteric infections, and EED. This suggests that lack of access to adequate and nutritious foods is not the only cause of stunting among children in the studied region. Thus, stunting can be prevented by improving sanitation and hygienic conditions to prevent repeated enteric infections in children and by promoting dietary diversity of children.
Data availability
Data will be made available upon requesting the primary author, i.e., ZG.
Abbreviations
- μg/ml:
-
Microgram per milliliter
- AAT:
-
Alpha-1-antitrypsin
- CI:
-
Confidence interval
- DDS:
-
Dietary diversity score
- EED:
-
Environmental enteric dysfunction
- E. coli :
-
Escherichia coli
- FANTA:
-
Food and nutrition technical assistance
- HAZ:
-
Height-for-age-z score
- MPO:
-
Myeloperoxidase
- NEO:
-
Neopterin
- ng/ml:
-
Nanogram per milliliter
- nmol/l:
-
Nanomoles per milliliter
- SD:
-
Standard deviation
- UNICEF:
-
United Nations Children’s Fund
- WASH:
-
Water, sanitation and hygiene
- WHO:
-
World Health Organization
References
Webb, P. et al. Hunger and malnutrition in the 21st century. BMJ 361, k2238 (2018).
Reinhardt, K. & Fanzo, J. Addressing chronic malnutrition through multi-sectoral, sustainable approaches: A review of the causes and consequences. Front. Nutr. 1, 13 (2014).
Moszynski, P. Drive Against Childhood Illness is Jeopardised by Failure to Invest in Sanitation, Warns Charity (British Medical Journal Publishing Group, 2011).
Martins, V. J. et al. Long-lasting effects of undernutrition. Int. J. Environ. Res. Public Health 8(6), 1817–1846 (2011).
Walson, J. L. & Berkley, J. A. The impact of malnutrition on childhood infections. Curr. Opin. Infect. Dis. 31(3), 231 (2018).
Mara, D., Lane, J., Scott, B. & Trouba, D. Sanitation and health. PLoS Med. 7(11), e1000363 (2010).
Lenoir-Wijnkoop, I. et al. Nutrition economics—Characterising the economic and health impact of nutrition. Br. J. Nutr. 105(1), 157–166 (2011).
Joffe, M. Health, livelihoods, and nutrition in low-income rural systems. Food Nutr. Bull. 28(Suppl 2), S227–S236 (2007).
World Health Organization (WHO). Sanitation, March 2022 key facts. https://www.who.int/news-room/fact-sheets/detail/sanitation. Accessed 19 Sept 2022.
World Health Organization (WHO). Malnutrition. https://www.who.int/health-topics/malnutrition#tab=tab_1. Accessed 29 July 2022.
UNICEF. Malnutrition rates remain alarming: Stunting is declining too slowly while wasting still impacts the lives of far too many young children. UNICEF Data: Monitoring the situation of children and women. https://data.unicef.org/topic/nutrition/malnutrition/. Accessed 03 Oct 2019.
Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. Ethiopia Mini Demographic and Health Survey 2019: Final Report (EPHI and ICF, 2021). https://dhsprogram.com/publications/publication-FR363-DHS-Final-Reports.cfm. Accessed 25 July 2022.
Stewart, C. P., Iannotti, L., Dewey, K. G., Michaelsen, K. F. & Onyango, A. W. Contextualising complementary feeding in a broader framework for stunting prevention. Matern. Child Nutr. 9, 27–45 (2013).
World Health Organization (WHO). Stunting in a nutshell. https://www.who.int/news/item/19-11-2015-stunting-in-a-nutshell. Accessed 29 July 2022.
Dewey, K. G. & Begum, K. Long-term consequences of stunting in early life. Matern. Child Nutr. 7, 5–18 (2011).
Raiten, D. J. & Bremer, A. A. Exploring the nutritional ecology of stunting: New approaches to an old problem. Nutrients 12(2), 371 (2020).
Schmidt, C. W. Beyond malnutrition: The role of sanitation in stunted growth. Environ. Health Perspect. 122(11), A298-303 (2014).
Prendergast, A. J. & Humphrey, J. H. Stunting persists despite optimal feeding: Are toilets part of the solution?. Nestle Nutr. Inst. Workshop Ser. 81, 99–110 (2015).
Cumming, O. & Cairncross, S. Can water, sanitation and hygiene help eliminate stunting? Current evidence and policy implications. Matern. Child Nutr. 12, 91–105 (2016).
Dodos, J., Mattern, B., Lapegue, J., Altmann, M. & Aissa, M. A. Relationship between water, sanitation, hygiene, and nutrition: What do Link NCA nutrition causal analyses say?. Waterlines 36, 284–304 (2017).
Johri, M., Sylvestre, M.-P., Koné, G. K., Chandra, D. & Subramanian, S. Effects of improved drinking water quality on early childhood growth in rural Uttar Pradesh, India: A propensity-score analysis. PLoS One 14(1), e0209054 (2019).
Dearden, K. A. et al. Children with access to improved sanitation but not improved water are at lower risk of stunting compared to children without access: A cohort study in Ethiopia, India, Peru, and Vietnam. BMC Public Health 17(1), 1–19 (2017).
Sclar, G. D. et al. Assessing the impact of sanitation on indicators of fecal exposure along principal transmission pathways: A systematic review. Int. J. Hyg. Environ. Health 219(8), 709–723 (2016).
Fuhrmeister, E. R. et al. Effect of sanitation improvements on pathogens and microbial source tracking markers in the rural Bangladeshi household environment. Environ. Sci. Technol. 54(7), 4316–4326 (2020).
Steinbaum, L. et al. Effect of a sanitation intervention on soil-transmitted helminth prevalence and concentration in household soil: A cluster-randomized controlled trial and risk factor analysis. PLoS Negl. Trop. Dis. 13(2), e0007180 (2019).
Goddard, F. G. et al. Faecal contamination of the environment and child health: A systematic review and individual participant data meta-analysis. Lancet Planet. Health 4(9), e405–e415 (2020).
Watanabe, K. & Petri, W. A. Jr. Environmental enteropathy: Elusive but significant subclinical abnormalities in developing countries. EBioMedicine 10, 25–32 (2016).
Bloomfield, S., Stanwell-Smith, R., Crevel, R. & Pickup, J. Too clean, or not too clean: The hygiene hypothesis and home hygiene. Clin. Exp. Allergy 36(4), 402–425 (2006).
Gerba, C. P. & Pepper, I. L. Microbial contaminants. Environ. Pollut. Sci., 191–217 (2019).
Kosek, M. N. et al. Causal pathways from enteropathogens to environmental enteropathy: Findings from the MAL-ED birth cohort study. EBioMedicine 18, 109–117 (2017).
Kelly, P. et al. Endomicroscopic and transcriptomic analysis of impaired barrier function and malabsorption in environmental enteropathy. PLoS Negl. Trop. Dis. 10(4), e0004600 (2016).
Cook, G., Kajubi, S. & Lee, F. Jejunal morphology of the African in Uganda. J. Pathol. 98(3), 157–169 (1969).
Harper, K. M., Mutasa, M., Prendergast, A. J., Humphrey, J. & Manges, A. R. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Negl. Trop. Dis. 12(1), e0006205 (2018).
Prendergast, A. J. et al. Assessment of environmental enteric dysfunction in the SHINE trial: Methods and challenges. Clin. Infect. Dis. 61(suppl 7), S726–S732 (2015).
Lin, A. et al. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am. J. Trop. Med. Hyg. 89(1), 130 (2013).
Bhutta, Z. A. et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 371(9610), 417–440 (2008).
Ngure, F. M. et al. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: Making the links. Ann. N. Y. Acad. Sci. 1308, 118–128 (2014).
Gizaw, Z., Yalew, A. W., Bitew, B. D., Lee, J. & Bisesi, M. Fecal indicator bacteria along multiple environmental exposure pathways (water, food, and soil) and intestinal parasites among children in the rural northwest Ethiopia. BMC Gastroenterol. 22, 84 (2022).
Gizaw, Z., Yalew, A. W., Bitew, B. D., Lee, J. & Bisesi, M. Fecal biomarkers of environmental enteric dysfunction and associated factors among children aged 24–59 months in east Dembiya district, northwest Ethiopia. BMC Gastroenterol. 22, 172 (2022).
Alemu, A., Geta, M., Taye, S., Eshetie, S. & Engda, T. Prevalence, associated risk factors and antimicrobial susceptibility patterns of Shigella infections among diarrheic pediatric population attending at Gondar town healthcare institutions, Northwest Ethiopia. Trop. Dis. Travel Med. Vaccines 5(1), 7 (2019).
Coates, J., Swindale, A. & Bilinsky, P. Household Food Insecurity Access Scale (HFIAS) for measurement of household food access: Indicator guide (v. 3) (Food and Nutrition Technical Assistance Project, Academy for Educational Development, 2007).
Swindale, A. & Bilinsky, P. Household Dietary Diversity Score (HDDS) for Measurement of Household Food Access: Indicator Guide (Food and Nutrition Technical Assistance Project, Academy for Educational Development, 2006).
World Health Organization. Indicators for assessing infant and young child feeding practices part 3 (2010). https://apps.who.int/iris/bitstream/handle/10665/44368/9789241599757_eng.pdf;jsessionid=B95820985FEAD45C2560FEEAE6B11F1F?sequence=1. Accessed 18 Sept 2021.
World Health Organization and United Nations Children's Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children: Joint statement by the World Health Organization and the United Nations Children's fund (2009). https://apps.who.int/iris/bitstream/handle/10665/44129/9789241598163_eng.pdf?sequence=1. Accessed 19 Sept 2021.
Levine, G. A., Walson, J. L., Atlas, H. E., Lamberti, L. M. & Pavlinac, P. B. Defining pediatric diarrhea in low-resource settings. J. Pediatr. Infect. Dis. Soc. 6(3), 289–293 (2017).
WHO. Guidelines for drinking-water quality. Surveillance and control of community supplies, vol. 3, 2nd edn. (1997). http://www.who.int/water_sanitation_health/dwq/gdwqvol32ed.pdf. Accessed 04 July 2021.
World Health Organization (WHO). Training manual on diagnosis of intestinal parasites based on the WHO bench aids for the diagnosis of intestinal parasites, district laboratory practice in tropical countries. WHO/CTD/SIP/98.2 CD-Rom (2004). http://usaf.phsource.us/PH/PDF/HELM/trainingmanual_sip98-2.pdf. Accessed 27 May 2021.
Abrahams, P. W. Geophagy and the involuntary ingestion of soil. In Essentials of Medical Geology 433–454 (Springer, 2013).
Kosek, M. et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am. J. Trop. Med. Hyg. 88(2), 390 (2013).
George, C. M. et al. Fecal markers of environmental enteropathy are associated with animal exposure and caregiver hygiene in Bangladesh. Am. J. Trop. Med. Hyg. 93(2), 269 (2015).
Iqbal, N. T. et al. Promising biomarkers of environmental enteric dysfunction: A prospective cohort study in Pakistani children. Sci. Rep. 8(1), 1–10 (2018).
Arndt, M. B. et al. Fecal markers of environmental enteropathy and subsequent growth in Bangladeshi children. Am. J. Trop. Med. Hyg. 95(3), 694 (2016).
George, C. M. et al. Geophagy is associated with environmental enteropathy and stunting in children in rural Bangladesh. Am. J. Trop. Med. Hyg. 92(6), 1117 (2015).
Afework, E., Mengesha, S. & Wachamo, D. Stunting and associated factors among under-five-age children in West Guji Zone, Oromia, Ethiopia. J. Nutr. Metab. 2021, 8890725 (2021).
Teferi, M. et al. Prevalence of stunting and associated factors among children aged 06–59 months in Southwest Ethiopia: A cross-sectional study. J. Nutr. Health Food Sci. 2016, 1–6 (2016).
Ermias, A. The prevalence of stunting and associated factors among children age 6–59 months at Mizan-Aman Town, Bench Maji zone, SNNPR region, Ethiopia, 2015 (Addis Ababa University, 2015).
Moges, B., Feleke, A., Meseret, S. & Doyore, F. Magnitude of stunting and associated factors among 6–59 months old children in Hossana Town, Southern Ethiopia. J. Clin. Res. Bioeth. 6(1), 1 (2015).
Sserwanja, Q., Kamara, K., Mutisya, L. M., Musaba, M. W. & Ziaei, S. Rural and urban correlates of stunting among under-five children in Sierra Leone: A 2019 nationwide cross-sectional survey. Nutr. Metab. Insights 14, 11786388211047056 (2021).
Sen, L. C. et al. Nutritional status of under-five children in rural Bangladesh. Int. J. Public Health 9(3), 205–210 (2020).
Nkhoma, B., Ngambi, W. F., Chipimo, P. J. & Zambwe, M. Determinants of stunting among children< 5 years of age: Evidence from 2018–2019 Zambia Demographic and Health Survey. medRxiv. https://doi.org/10.1101/2021.05.19.21257389 (2021).
Berhanu, G., Mekonnen, S. & Sisay, M. Prevalence of stunting and associated factors among preschool children: A community based comparative cross sectional study in Ethiopia. BMC Nutr. 4(1), 1–15 (2018).
Tariku, A. et al. Nearly half of preschool children are stunted in Dembia district, Northwest Ethiopia: A community based cross-sectional study. Arch. Public Health 74(1), 1–9 (2016).
Mengiste, L. A., Worku, Y., Aynalem, Y. A. & Shiferaw, W. S. Prevalence of stunting and its associated factors among children aged 6–59 months in Angolela Tera District, Northeast Ethiopia. Nutr. Diet. Suppl. 12, 311–319 (2020).
Yoseph, A. & Beyene, H. The high prevalence of intestinal parasitic infections is associated with stunting among children aged 6–59 months in Boricha Woreda, Southern Ethiopia: A cross-sectional study. BMC Public Health 20(1), 1–13 (2020).
Bogale, B., Gutema, B. T. & Chisha, Y. Prevalence of stunting and its associated factors among children of 6–59 months in Arba Minch Health and Demographic Surveillance Site (HDSS), southern Ethiopia: A community-based cross-sectional study. J. Environ. Public Health 2020, 9520973 (2020).
Asfaw, M., Wondaferash, M., Taha, M. & Dube, L. Prevalence of undernutrition and associated factors among children aged between six to fifty nine months in Bule Hora district, South Ethiopia. BMC Public Health 15(1), 1–9 (2015).
Yalew, B. M. Prevalence of malnutrition and associated factors among children age 6–59 months at lalibela town administration, North WolloZone, Anrs, Northern Ethiopia. J. Nutr. Disord. Ther. 4(132), 2161 (2014).
Begum, F. et al. Clostridium difficile associated diarrhea in children with hematological malignancy-experience from a pediatric oncologic centre, Bangladesh. Int. J. Child Health Nutr. 8(4), 154–161 (2019).
Murarkar, S. et al. Prevalence and determinants of undernutrition among under-five children residing in urban slums and rural area, Maharashtra, India: A community-based cross-sectional study. BMC Public Health 20(1), 1–9 (2020).
Enos Mirembe, M., Arthur, K., Edson, K. & Clement, M. The prevalence and determinants of stunting among children 6–59 months of age in one of the sub-counties in the Rwenzori sub-region, Western Uganda (2020).
Tshiya, Y. & Magoha, H. Prevalence and risk factors of malnutrition among children of ages 6 to 59 months in Manyovu, Buhigwe District Kigoma-Tanzania. J. Food Nutr. Res. 8, 320–328 (2020).
Usman, N., Kene-Ibeagha, N., Nmadu, A., Omole, V. & Adiri, F. Socio-demographic determinants of malnutrition among under-fives in Mil-Goma: A rural community in Kaduna State, North Western Nigeria. Trop. J. Health Sci. 25(4), 23–27 (2018).
Desalegn, B. B., Kinfe, E., Fikre, K. & Bosha, T. Stunting and its associated factors in under five years old children: The case of Hawassa University Technology villages, Southern Ethiopia. J. Environ. Sci. Toxicol. Food Technol. 10(11), 25–31 (2016).
Dake, S. K., Solomon, F. B., Bobe, T. M., Tekle, H. A. & Tufa, E. G. Predictors of stunting among children 6–59 months of age in Sodo Zuria District, South Ethiopia: A community based cross-sectional study. BMC Nutr. 5(1), 1–7 (2019).
Yisak, H. & Ewunetei, A. Prevalence of malnutrition and its associated factors among under five children at Debretabor town north-west Ethiopia. Arch. Community Med. Public Health 6(2), 213–222 (2020).
Eshete, H., Abebe, Y., Loha, E., Gebru, T. & Tesheme, T. Nutritional status and effect of maternal employment among children aged 6–59 months in Wolayta Sodo Town, Southern Ethiopia: A cross-sectional study. Ethiop. J. Health Sci. 27(2), 155–162 (2017).
DapiNzefa, L., Monebenimp, F. & Äng, C. Undernutrition among children under five in the Bandja village of Cameroon, Africa. South Afr. J. Clin. Nutr. 32(2), 46–50 (2019).
Julian, T. R., Canales, R. A., Leckie, J. O. & Boehm, A. B. A model of exposure to rotavirus from nondietary ingestion iterated by simulated intermittent contacts. Risk Anal. 29(5), 617–632 (2009).
Saathoff, E., Olsen, A., Kvalsvig, J. D. & Geissler, P. W. Geophagy and its association with geohelminth infection in rural schoolchildren from northern KwaZulu-Natal, South Africa. Trans. R. Soc. Trop. Med. Hyg. 96(5), 485–490 (2002).
Shivoga, W. A. & Moturi, W. N. Geophagia as a risk factor for diarrhoea. J. Infect. Dev. Ctries. 3(02), 094–098 (2009).
Petri, W. A. et al. Enteric infections, diarrhea, and their impact on function and development. J. Clin. Investig. 118(4), 1277–1290 (2008).
Stephensen, C. B. Burden of infection on growth failure. J. Nutr. 129(2), 534S-538S (1999).
Schmeer, K. K. & Piperata, B. A. Household food insecurity and child health. Matern. Child Nutr. 13(2), e12301 (2017).
Motbainor, A., Worku, A. & Kumie, A. Stunting is associated with food diversity while wasting with food insecurity among underfive children in East and West Gojjam Zones of Amhara Region, Ethiopia. PLoS One 10(8), e0133542 (2015).
Ali, D. et al. Household food insecurity is associated with higher child undernutrition in Bangladesh, Ethiopia, and Vietnam, but the effect is not mediated by child dietary diversity. J. Nutr. 143(12), 2015–2021 (2013).
Black, R. E. et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet (London, England) 382(9890), 427–451 (2013).
Caulfield, L. E., de Onis, M., Blössner, M. & Black, R. E. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am. J. Clin. Nutr. 80(1), 193–198 (2004).
Khamis, A. G., Mwanri, A. W., Ntwenya, J. E. & Kreppel, K. The influence of dietary diversity on the nutritional status of children between 6 and 23 months of age in Tanzania. BMC Pediatr. 19(1), 518 (2019).
World Health Organization (WHO). Indicators for assessing infant and young child feeding practices: Part 1 Definitions. Conclusions of a consensus meeting held 6–8 November 2007 in Washington D.C., USA (World Health Organization, 2008). http://whqlibdoc.who.int/publications/2008/9789241596664_eng.pdf. Accessed 25 July 2022.
Owino, V. et al. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics 138(6), e20160641 (2016).
Hebert, J. R., Clemow, L., Pbert, L., Ockene, I. S. & Ockene, J. K. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int. J. Epidemiol. 24(2), 389–398 (1995).
Tadesse, G., Abate, G. T. & Zewdie, T. Biases in self-reported food insecurity measurement: A list experiment approach. Food Policy 92, 101862 (2020).
Acknowledgements
The authors are pleased to acknowledge the University of Gondar and One Health Eastern Africa Research Training (OHEART) program at the Ohio State University, Global One Health Initiative (GOHi) and National Institutes of Health (NIH) Fogarty International Center for their support.
Funding
This study is funded by One Health Eastern Africa Research Training (OHEART) programme at the Ohio State University, Global One Health Initiative (GOHi) through National Institutes of Health (NIH) Fogarty International Center (grant number TW008650) and the University of Gondar (grand number R/T/T/C/Eng./300/08/2019).
Author information
Authors and Affiliations
Contributions
Z.G. designed the study, conducted data analysis and produced the initial draft of the manuscript. B.D.B. supervised data collection. A.W.Y., J.L. and M.B. contributed to conceptualizing the study. All authors approved the final version of the manuscript. This manuscript does not contain any individual person’s data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gizaw, Z., Yalew, A.W., Bitew, B.D. et al. Stunting among children aged 24–59 months and associations with sanitation, enteric infections, and environmental enteric dysfunction in rural northwest Ethiopia. Sci Rep 12, 19293 (2022). https://doi.org/10.1038/s41598-022-23981-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23981-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.