Abstract
Deep neuromuscular block (NMB) has been increasingly utilized, but its role in reducing intraoperative opioid requirement has yet to be investigated. Surgical pleth index (SPI) quantifies nociception. We investigated the effects of deep NMB on SPI-guided remifentanil administration in laparoscopic herniorrhaphy. Total 128 patients undergoing laparoscopic inguinal herniorrhaphy were randomly allocated to two groups of NMB: deep (n = 64) and moderate (n = 64). The remifentanil dose was assessed during intubation, from skin incision until CO2 insertion, and pneumoperitoneum. Mean infusion rate of remifentanil during pneumoperitoneum was higher in moderate NMB group than in deep NMB group (0.103 [0.075–0.143] µg/kg/min vs. 0.073 [0.056–0.097] µg/kg/min, p < 0.001). Consequently, median infusion rate of remifentanil during anesthesia was higher in moderate NMB group (0.076 [0.096–0.067] µg/kg/min vs. 0.067 [0.084–0.058] µg/kg/min, p = 0.016). The duration of post-anesthesia care unit stay was longer in the moderate NMB group (40 [30–40] min vs. 30 [30–40] min, p = 0.045). In conclusion, deep NMB reduced the remifentanil requirement compared with moderate NMB in SPI-guided anesthesia for laparoscopic herniorrhaphy.
Similar content being viewed by others
Introduction
Laparoscopy is routinely used in general surgery due to less inflammation, immunosuppression, bleeding, postoperative pain, and rapid recovery compared with open laparotomy1,2. However, laparoscopic surgery requires elevated intra-abdominal pressure (IAP) and steep changes in position, which lead to pathophysiological aberrations, which are a challenge to anesthesiologists3. To reduce the risk of these complications, the international guideline recommends the use of the lowest IAP possible; however, it can impair the quality of the surgical field4.
Since the introduction of sugammadex, the potential advantages of deep NMB in laparoscopic surgery have been extensively investigated. Deep neuromuscular block (NMB) lowers IAP and improves surgical space at a time, thus enhancing the surgical outcome5. Besides, deep NMB reduces laparoscopy-related lung injury and postoperative pain5,6,7.
Surgical pleth index (SPI) can be used to detect cardiovascular changes resulting from nociception-induced sympatho-vagal imbalance and quantify the nociception for titration of analgesic administration8. SPI-guided anesthesia is associated with clinical advantages compared with standard analgesic practice based on clinical parameters; including earlier injection of sufentanil bolus, stabler modulation of CO2 insufflation-induced sympathetic activation, faster recovery and reduced remifentanil dose9,10,11,12. These advantages reinforce the role of SPI in anesthetic management13.
Studies comparing deep NMB with moderate NMB during laparoscopic surgery utilized similar opioid doses6,14,15,16. In there, all opioids were administered via standard analgesic practice. Currently, the superior effects of deep NMB during laparoscopic surgery are disputed, while the benefits of deep NMB in intraoperative opioid treatment have rarely been investigated clinically17,18. Therefore, we hypothesized that deep NMB reduces the need for remifentanil compared with moderate NMB, when guided by SPI. The aim of this study was to investigate the effects of deep NMB on remifentanil requirements in patients undergoing SPI-guided anesthesia for laparoscopic inguinal herniorrhaphy.
Methods
Patients
This prospective, double-blind, randomized controlled trial was approved by institutional review board of Ajou University Hospital (AJIRB-MED-THE-19-056, 9 April 2019) and registered with ClinicalTrials.gov (NCT04022733, 17 July 2019). Written informed consent was obtained from eligible patients with American Society of Anesthesiologists (ASA) physical status classification I–III, aged 19–85 years, and undergoing elective laparoscopic inguinal herniorrhaphy from October 2019 to August 2021. We excluded patients with arrythmia, hyperbilirubinemia, chronic pain, opioid abuse, infection, and the possibility of conversion to open herniorrhaphy. Peripheral vascular disease was excluded but diabetes was included.
Interventions
A total of 134 participants were randomly assigned to 2 groups (deep and moderate) depending on the depth of NMB using a computer-generated random table (http://www.random.org). In the deep NMB group (n = 67), NMB was maintained as post-tetanic count (PTC) 1–2 during surgery and reversed using sugammadex 4 mg/kg based on actual body weight after completion of surgery. In the moderate NMB group (n = 67), NMB was maintained as a train-of-four (TOF) count 1–2 and reversed with neostigmine neostigmine 50 μg/kg and glycopyrrolate 10 μg/kg. NMB was monitored using kinemyography (MechanoSensor™; GE healthcare, Chicago, IL, USA) administered to the adductor pollicis muscle. An assistant who was not involved in the trial performed the randomization in a 1:1 ratio and concealed the allocation sequence in opaque, sealed envelopes. The study interventions including NMB titration and administration of reversal agent were carried out by an independent investigator (J.E.K.) who did not participate in the outcome assessment. The other investigators and the patients were blinded to the group assignment.
Trendelenburg position was set to 30°. Pneumoperitoneum was controlled by limiting CO2 insufflator, and IAP was maintained at 12 mmHg in both groups.
Anesthesia
Two investigators (I.K.Y. and D.-G.H) managed the anesthesia according to a protocol. Without premedication, patients were monitored via electrocardiography, non-invasive arterial pressure measurement, pulse oximetry, and SedLine® (Masimo, Irvine, CA, USA). A pulse oximeter sensor for SPI (GE healthcare, Chicago, IL, USA) was applied to index finger of contralateral side to the arm with an arterial pressure cuff. SPI is computed using an algorithm (1) that combines normalized heartbeat interval (HBInorm) and normalized photoplethysmographic pulse wave amplitude (PPGAnorm)19:
A balanced anesthesia was implemented with sevoflurane and remifentanil (Ultiva; GlaxoSmithKline, Brentford, UK) via target-controlled infusion based on Minto’s pharmacokinetics. Following pre-oxygenation, anesthesia was induced with intravenous (IV) propofol 2 mg/kg and remifentanil of 3–5 ng/mL as target concentration. MechanoSensor™ was calibrated and stabilized (< 5% variation in the TOF ratios) after the loss of consciousness. Subsequently, IV rocuronium bromide 0.6 mg/kg was administered. Following confirmation of relaxation, patients were intubated with a videolaryngoscope. Mechanical ventilation was achieved with a tidal volume of 6–8 mL/kg, positive end-expiratory pressure of 5 cmH2O and an inspired oxygen fraction 0.5. Respiratory rate was changed for an end-tidal carbon dioxide tension of 30–40 mmHg. Normal saline or plasmalyte was infused at a rate of 6 mL/kg/hr.
Anesthetic depth was maintained at a Sedline® patient state index (PSi) of 25–50 by adjusting the end-tidal concentration of sevoflurane. Rocuronium 0.3–0.4 mg/kg/h was continuously infused and titrated according to the group assignment until the end of the fascial suturing (by J.E.K.). The remifentanil dose was adjusted to maintain an SPI range of 20–50. To manage the hypotension associated with a mean arterial pressure (MAP) < 60 mmHg, a bolus of ephedrine 4 mg was primarily administered along with an infusion of norepinephrine as needed. An IV propacetamol 1 g was given at the end of the surgery. NMB was reversed according to the group assignment (by J.E.K.), and extubation was performed after confirming the TOF ratio > 0.9. Patients were then transferred to a post-anesthesia care unit (PACU).
Data collection
The investigators (I.K.Y. and D.-G.H) recorded the time and remifentanil dose during the surgery. Since the dynamic conditions such as endotracheal intubation or skin incision possibly affect the remifentanil dose requirement during deep NMB, each remifentanil dose was assessed three times: during intubation, time from skin incision until CO2 insertion, and time from CO2 insertion until the removal of laparoscope (pneumoperitoneum). In addition, the remifentanil concentration was maintained at 0 ng/mL after intubation and then increased to 3.0 ng/mL immediately before skin incision. Infusion rate of remifentanil (µg/kg/min) was calculated by adjusting total doses of remifentanil using body weight and infusion time.
During surgery, SPI, PSi, and hemodynamic parameters such as heart rate (HR) and MAP were recorded at the following four time points: before induction (T0), 10 min after induction (T1), 20 min after CO2 insertion (T2), and the removal of laparoscopy (T3). In the PACU, the investigator (D.-G.H) evaluated the recovery data at 30 min after the end of surgery. Nausea (1 = none, 2 = mild, 3 = moderate, and 4 = severe), vomiting, and pain scores (11-point numerical rating scale, 0 = no pain, 10 = worst pain) were assessed. IV ramosetron 3 mg was administered as a rescue antiemetics in the event of nausea ≥ 3, and fentanyl 0.5 μg/kg was used as a rescue analgesia when the pain score was ≥ 5.
Sample size calculation
The primary outcome was the infusion rate of remifentanil during pneumoperitoneum. Based on the findings of a previous study20, we considered a difference in remifentanil infusion rate > 0.032 µg/kg/min (16% of mean infusion rate of remifentanil in moderate NMB under SPI-guided anesthesia for laparoscopic surgery [mean infusion rate of 0.192 (SD 0.064) µg/kg/min]) as clinically relevant. Based on a significance level of 5% and statistical power of 80%, each group required 63 subjects for analysis. We enrolled a total of 134 patients to compensate for dropouts and observational variation.
Statistical analysis
Statistical analyses were conducted using SPSS software version 25.0 (IBM Corp., Armonk, NY, USA). Data are presented as mean ± SD, median (interquartile range), and number of patients. The normality of distribution was tested using the Kolmogorov–Smirnov. Continuous data were compared using a two-tailed t test when normally distributed; however, Mann–Whitney U test was used for non-normally distributed data. Categorical data were compared using the Chi-square test or Fisher’s exact test, as appropriate. Intergroup comparisons for repetitively measured data were performed by unpaired t-test with Bonferroni correction. There was no multiplicity adjustment made for multiple comparisons. All tests were two-sided, and p values < 0.05 were considered statistically significant.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Ajou Hospital (AJIRB-MED-THE-19-056, April 2019).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Results
Study population
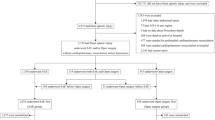
A total of 134 participants were enrolled and randomized. However, 6 participants dropped out of the study due to conversion to open herniorrhaphy (n = 5) and bradycardia during anesthetic induction (n = 1) (Fig. 1).
Baseline characteristics
Patient characteristics and intraoperative data were comparable between two groups, except for the total dose of rocuronium used during the anesthesia (Table 1). Hemodynamic parameters, SPI and PSi were adequately maintained throughout the study period (Table 2). Although MAP, SPI and PSi did not differ between two groups, HR in the deep NMB group was higher than that in the moderate NMB group at T2 (padjusted = 0.040). Pneumoperitoneum was adequately maintained in all patients without changing the IAP.
Remifentanil doses
Remifentanil doses administered at each time point are presented in Table 3. No significant differences were detected in total doses or infusion rate of remifentanil during intubation and during time from skin incision until CO2 insertion (p = 0.770, p = 0.708 and p = 0.571, respectively). In addition, the total dose of remifentanil during pneumoperitoneum was comparable between the two groups (p = 0.074). However, when adjusted for body weight and infusion time, the median infusion rate of remifentanil during pneumoperitoneum was significantly higher in the moderate NMB group than in the deep NMB group (0.103 [0.075–0.143] µg/kg/min vs. 0.073 [0.056–0.097] µg/kg/min, p < 0.001). Consequently, the median infusion rate of remifentanil during anesthesia also was significantly higher in the moderate NMB group than in the deep NMB group (0.076 [0.096–0.067] µg/kg/min vs. 0.067 [0.084–0.058] µg/kg/min, p = 0.016).
Recovery data
Recovery data at PACU are presented in Table 4. Although no differences were found in pain score, nausea, vomiting, the numbers of patients receiving analgesics or antiemetics, and the duration of hospital stay, the duration of PACU stay was significantly longer in the moderate NMB group than in the deep NMB group (40 [30–40] min vs. 30 [30–40] min, p = 0.045). Postoperatively, none of the patients exhibited any complications regarding deep NMB or sugammadex use.
Discussion
This study is the first that SPI-guided anesthesia was used to evaluate the effects of deep NMB on remifentanil required during surgery. The deep NMB significantly reduced the intraoperative remifentanil requirements compared with the moderate NMB in patients undergoing SPI-guided anesthesia for laparoscopic inguinal herniorrhaphy. In addition, the duration of PACU stay was significantly shorter in the deep NMB group, despite similar pain scores and rescue analgesic treatment.
A high degree of muscle relaxation is needed for more complex laparoscopic procedures. Sugammadex, which antagonizes rocuronium at any level of NMB, can be used to prolong deep NMB right until the very end of the surgery21. Evidences support the routine use of deep NMB during laparoscopic surgery5,22. However, it is difficult to establish whether specific outcomes can be attributed to the effects of deep NMB or low IAP (< 12 mmHg). Also, there is a possibility that the presence of a deep NMB could influence on lowering IAP, thus different IAPs across the enrolled studies constituted a limitation22. Given the detrimental effects of pneumoperitoneum on intra-abdominal organ circulation and cardiopulmonary function, a low IAP is clinically advantageous compared with standard IAP (12 mmHg)23,24. Therefore, a distinction between the effects of low IAP and deep NMB is essential. Martini et al. reported that deep NMB improves the quality of surgical conditions compared with moderate NMB during laparoscopy without a cardiorespiratory compromise under identical retroperitoneal pressure conditions16. A subsequent meta-analysis showed that deep NMB improved the surgical space at low and high IAP5. Therefore, our study was designed to analyze the effects of NMB (deep vs. moderate) on surgical conditions under identical and standard IAP levels (12 mmHg). In addition, SPI reduced the levels of intraoperative analgesia compared with standard clinical practice, which was found in sevoflurane anesthesia but not in propofol anesthesia12. In this regard, we used sevoflurane and remifentanil anesthesia in our study.
The reduced need for remifentanil in our study can be explained as follows. First, deep NMB might reduce surgical stress compared with moderate NMB in our study. Stress response to surgical trauma activates the sympathoadrenal, endocrine and immunologic response25. Laparoscopic hernia repair is associated with less tissue injury than open approach, and thus decreases the inflammatory stress response26. However, hormonal stress response (catecholamines and cortisol) might not be altered significantly than in open hernia repair, because the stimuli for stress response originate in the visceral and peritoneal afferent nerve as well as in the abdominal wall25,27. Further, the pneumoperitoneum significantly decreased the oxygenation and perfusion in abdominal organ, which is associated with the increased stress response24,27,28,29,30. Consequently, the sympathetic activation results in cardiovascular effects such as tachycardia and hypertension, thereby increasing the SPI values. In addition, remifentanil was found to suppress the stress response notably in various conditions31,32,33. Tools such as SPI correlate effect-site concentrations of remifentanil better than other clinical parameters13. Since both groups were operated under an identical and standard IAP, deep NMB appears to reduce the surgical stress in our study entirely. In a previous study, Koo et al.33 investigated the inflammatory stress response (e.g., interleukins, tumor necrosis factor-α, and C-reactive protein) in relation to the depth of NMB in laparoscopic gastrectomy, and found no differences between the groups. Although the incidence of unwanted events such as spontaneous breathing was lower in the deep NMB group, they infused the remifentanil using vital signs as a guide, not SPI guide, and did not even measure a remifentanil consumption34. A further study is needed to evaluate hormonal stress response in deep and moderate NMB under SPI guidance.
Second, it might be explained by the effects of rocuronium on vascular tone partly. Non-depolarizing NMB agents act as antagonists in nicotinic receptors at the neuromuscular junction, but also bind to muscarinic cholinergic receptors on vascular smooth muscle35. In studies investigating the direct effects of muscle relaxants on vascular smooth muscle contraction and relaxation, the relaxation effects increased in the following order: pancuronium < rocuronium < vecuronium36,37. Clinically, compared with pancuronium, rocuronium decreased the HR at 5 min after injection in patients undergoing cardiac surgery, combined with morphine treatment38. However, rocuronium induced a mild increase in HR and decreased MAP in a study enrolled the various surgeries39. Based on a mild vagolytic action, the higher dose of rocuronium used to maintain the deep NMB would reduce the need for remifentanil in our study. Although there were no significant decreases in MAP despite higher HR at T2, the vasodilative property of rocuronium might be manifested because the coefficient of PPGA is about twice as large as that of HBI, indicating PPGA is more important than HBI when calculating the SPI value19.
Theoretically, deep NMB enables maximum stretching of abdominal wall muscle during laparoscopy, and could reduce pressure-induced postoperative pain. Studies investigating the use of deep NMB in laparoscopy-related pain show conflicting results. In 2017, a meta-analysis reported a significant reduction of early postoperative pain after deep NMB, including 5 studies using low or standard IAPs or altered pressure, underscoring the need for separation between the effects of deep NMB and low IAP5. Other studies found that deep NMB did not reduce the intensity of pain at PACU compared with moderate NMB under identical and standard IAP in laparoscopic cholecystectomy, laparoscopic and robotic gastrectomy40,41,42. Similarly, the pain scores at PACU did not differ in our study under identical IAPs (12 mmHg). In contrast, deep NMB reduced postoperative pain compared to moderate NMB in bariatric surgery under identical but elevated IAP (18 mmHg)6. A further study investigating the effects of deep NMB on postoperative pain in invasive surgery (e.g., laparotomy) is needed.
Remifentanil, an ultra-short acting opioid, has been widely used as an infusion for induction of general anesthesia. However, excessive intraoperative administration of remifentanil delays recovery from anesthesia and is associated with acute opioid tolerance and opioid-induced hyperalgesia43,44. Remifentanil infusion rates of > 0.25 µg/kg/min and > 0.2 µg/kg/min were associated with tolerance and hyperalgesia, respectively44. The infusion rates of 0.076 µg/kg/min and 0.067 µg/kg/min in moderate and deep NMB groups in our study might not affect pain score and analgesic use at PACU due to remifentanil-related tolerance or hyperalgesia. The low infusion rates might be attributed not only to minimal invasiveness of laparoscopy, but also the advantages of SPI over standard clinical practice in intraoperative analgesia12.
The duration of PACU stay was significantly shorter in the deep NMB group in our study. In the absence of differences in other outcomes at PACU, the shorter duration and early postoperative discharge may be attributed to sugammadex45. Rapid discharge to the surgical ward may be explained by sugammadex-related reduced respiratory events, complete reversal from NMB, faster arousal and neostigmine-related adverse effects associated with muscarinic antagonists45. Despite the higher dose of rocuronium in deep NMB, the reduced time of PACU stay in our study suggests the superiority of sugammadex compared with conventional agents in terms of recovery.
The study has several limitations. First, the effects of opioid vary in quality and quantity according to age. Therefore, future studies involving adults and elderly populations are needed. Second, the comparison between deep NMB plus low IAP and moderate NMB plus standard IAP can highlight the enhanced postoperative outcomes as suggested in an editorial46. Third, the depth of NMB could have been seen unintentionally by the two investigators participating in the anesthesia and outcome assessment, although all interventions were conducted only by an independent investigator (J.E.K.). Fourth, it is better to administer sugammadex 2 mg/kg as a reversal agent to patients in the moderate NMB group on par with those in the deep NMB group, instead of neostigmine and glycopyrrolate, to confirm the unique effect of NMB depth on recovery in PACU and to overcome the ethical concerns associated with the best treatment options available for the patient. Lastly, the updated 2021 guidelines of Standards of Monitoring during Anesthesia and Recovery recommend the use of “automated electronic anesthetic record systems” for accuracy of information47,48. A further study based on intraoperative electronic hemodynamic data, not a specific time points, is warranted to demonstrate the vasodilative effects of rocuronium.
Conclusions
Deep NMB significantly reduced the remifentanil requirements compared with moderate NMB in patients undergoing SPI-guided anesthesia for laparoscopic inguinal herniorrhaphy. In addition, the deep NMB accelerated the PACU discharge.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
References
Novitsky, Y. W., Litwin, D. E. & Callery, M. P. The net immunologic advantage of laparoscopic surgery. Surg. Endosc. 18, 1411–1419. https://doi.org/10.1007/s00464-003-8275-x (2004).
Xu, Q. et al. Postoperative comparison of laparoscopic radical resection and open abdominal radical hysterectomy for cervical cancer patient. Arch. Gynecol. Obstet. 302, 473–479. https://doi.org/10.1007/s00404-020-05606-2 (2020).
Henny, C. P. & Hofland, J. Laparoscopic surgery: Pitfalls due to anesthesia, positioning, and pneumoperitoneum. Surg. Endosc. 19, 1163–1171. https://doi.org/10.1007/s00464-004-2250-z (2005).
Neudecker, J. et al. The European association for endoscopic surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg. Endosc. 16, 1121–1143. https://doi.org/10.1007/s00464-001-9166-7 (2002).
Bruintjes, M. H. et al. Deep neuromuscular block to optimize surgical space conditions during laparoscopic surgery: A systematic review and meta-analysis. Br. J. Anaesth. 118, 834–842. https://doi.org/10.1093/bja/aex116 (2017).
Torensma, B. et al. Deep neuromuscular block improves surgical conditions during bariatric surgery and reduces postoperative pain: A randomized double blind controlled trial. PLoS ONE 11, e0167907. https://doi.org/10.1371/journal.pone.0167907 (2016).
Kim, J. E. et al. Effects of deep neuromuscular block with low-pressure pneumoperitoneum on respiratory mechanics and biotrauma in a steep Trendelenburg position. Sci. Rep. 11, 1935. https://doi.org/10.1038/s41598-021-81582-0 (2021).
Ledowski, T. Monitoring nociception-getting “there yet” might be easier with a road map. Br. J. Anaesth. 119, 716–717. https://doi.org/10.1093/bja/aex277 (2017).
Dostalova, V., Schreiberova, J., Bartos, M., Kukralova, L. & Dostal, P. Surgical Pleth Index and Analgesia Nociception Index for intraoperative analgesia in patients undergoing neurosurgical spinal procedures: a comparative randomized study. Minerva Anestesiol. 85, 1265–1272. https://doi.org/10.23736/s0375-9393.19.13765-0 (2019).
Colombo, R. et al. Surgical Pleth Index guided analgesia blunts the intraoperative sympathetic response to laparoscopic cholecystectomy. Minerva Anestesiol. 81, 837–845 (2015).
Kim, J. H. et al. Comparison of pupillometry with surgical pleth index monitoring on perioperative opioid consumption and nociception during propofol-remifentanil anesthesia: A prospective randomized controlled trial. Anesth. Analg. 131, 1589–1598. https://doi.org/10.1213/ane.0000000000004958 (2020).
Jiao, Y. et al. Intraoperative monitoring of nociception for opioid administration: a meta-analysis of randomized controlled trials. Minerva Anestesiol. 85, 522–530. https://doi.org/10.23736/s0375-9393.19.13151-3 (2019).
Struys, M. M. et al. Changes in a surgical stress index in response to standardized pain stimuli during propofol-remifentanil infusion. Br. J. Anaesth. 99, 359–367. https://doi.org/10.1093/bja/aem173 (2007).
Kim, M. H., Lee, K. Y., Lee, K. Y., Min, B. S. & Yoo, Y. C. Maintaining optimal surgical conditions with low insufflation pressures is possible with deep neuromuscular blockade during laparoscopic colorectal surgery: A prospective, randomized, double-blind, parallel-group clinical trial. Medicine (Baltimore) 95, e2920. https://doi.org/10.1097/md.0000000000002920 (2016).
Koo, B. W. et al. Randomized clinical trial of moderate versus deep neuromuscular block for low-pressure pneumoperitoneum during laparoscopic cholecystectomy. World J. Surg. 40, 2898–2903. https://doi.org/10.1007/s00268-016-3633-8 (2016).
Martini, C. H., Boon, M., Bevers, R. F., Aarts, L. P. & Dahan, A. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. Br. J. Anaesth. 112, 498–505. https://doi.org/10.1093/bja/aet377 (2014).
Madsen, M. V., Staehr-Rye, A. K., Claudius, C. & Gätke, M. R. Is deep neuromuscular blockade beneficial in laparoscopic surgery? Yes, probably. Acta Anaesthesiol. Scand. 60, 710–716. https://doi.org/10.1111/aas.12698 (2016).
Kopman, A. F. & Naguib, M. Is deep neuromuscular block beneficial in laparoscopic surgery? No, probably not. Acta Anaesthesiol. Scand. 60, 717–722. https://doi.org/10.1111/aas.12699 (2016).
Ryu, K. H., Kim, J. A., Ko, D. C., Lee, S. H. & Choi, W. J. Desflurane reduces intraoperative remifentanil requirements more than sevoflurane: Comparison using surgical pleth index-guided analgesia. Br. J. Anaesth. 121, 1115–1122. https://doi.org/10.1016/j.bja.2018.05.064 (2018).
de Boer, H. D. et al. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: A multicenter, dose-finding and safety study. Anesthesiology 107, 239–244. https://doi.org/10.1097/01.anes.0000270722.95764.37 (2007).
Özdemir-van Brunschot, D. M. et al. What is the evidence for the use of low-pressure pneumoperitoneum? A systematic review. Surg. Endosc. 30, 2049–2065. https://doi.org/10.1007/s00464-015-4454-9 (2016).
Aceto, P. et al. Effects of deep neuromuscular block on surgical workspace conditions in laparoscopic bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Minerva Anestesiol. 86, 957–964. https://doi.org/10.23736/s0375-9393.20.14283-4 (2020).
Desborough, J. P. The stress response to trauma and surgery. Br. J. Anaesth. 85, 109–117. https://doi.org/10.1093/bja/85.1.109 (2000).
Jukić, M., Pogorelić, Z., Šupe-Domić, D. & Jerončić, A. Comparison of inflammatory stress response between laparoscopic and open approach for pediatric inguinal hernia repair in children. Surg. Endosc. 33, 3243–3250. https://doi.org/10.1007/s00464-018-06611-y (2019).
Niu, X., Song, X., Su, A., Zhao, S. & Li, Q. Low-pressure capnoperitoneum reduces stress responses during pediatric laparoscopic high ligation of indirect inguinal hernia sac: A randomized controlled study. Medicine (Baltimore), 96, e6563. https://doi.org/10.1097/md.0000000000006563 (2017).
Schwarte, L. A., Scheeren, T. W., Lorenz, C., De Bruyne, F. & Fournell, A. Moderate increase in intraabdominal pressure attenuates gastric mucosal oxygen saturation in patients undergoing laparoscopy. Anesthesiology 100, 1081–1087. https://doi.org/10.1097/00000542-200405000-00009 (2004).
Matovic, E. & Delibegovic, S. Adrenocorticotropic hormone (ACTH) and cortisol monitoring as stress markers during laparoscopic cholecystectomy: Standard and low intraabdominal pressure and open cholecystectomy. Med. Arch 73, 257–261. https://doi.org/10.5455/medarh.2019.73.257-261 (2019).
de Lacy, F. B. et al. Impact of pneumoperitoneum on intra-abdominal microcirculation blood flow: An experimental randomized controlled study of two insufflator models during transanal total mesorectal excision. Surg. Endosc. 34, 4494–4503. https://doi.org/10.1007/s00464-019-07236-5 (2020).
Kim, T. K. & Yoon, J. R. Comparison of the neuroendocrine and inflammatory responses after laparoscopic and abdominal hysterectomy. Korean J. Anesthesiol. 59, 265–269. https://doi.org/10.4097/kjae.2010.59.4.265 (2010).
Watanabe, K. et al. High-dose remifentanil suppresses stress response associated with pneumoperitoneum during laparoscopic colectomy. J. Anesth. 28, 334–340. https://doi.org/10.1007/s00540-013-1738-x (2014).
Winterhalter, M. et al. Endocrine stress response and inflammatory activation during CABG surgery. A randomized trial comparing remifentanil infusion to intermittent fentanyl. Eur. J. Anaesthesiol. 25, 326–335. https://doi.org/10.1017/s0265021507003043 (2008).
Shinoda, T. et al. Effect of remifentanil infusion rate on stress response in orthopedic surgery using a tourniquet application. BMC Anesthesiol. 13, 14. https://doi.org/10.1186/1471-2253-13-14 (2013).
Koo, B. W. et al. Effects of deep neuromuscular blockade on the stress response during laparoscopic gastrectomy randomized controlled trials. Sci. Rep. 9, 12411. https://doi.org/10.1038/s41598-019-48919-2 (2019).
Savarese, J. J. & Lowenstein, E. The name of the game: No anesthesia by cookbook. Anesthesiology 62, 703–705. https://doi.org/10.1097/00000542-198506000-00001 (1985).
Klockgether-Radke, A. P., Haemmerle, A., Kettler, D. & Hellige, G. Do muscle relaxants influence vascular tone in isolated coronary artery segments?. Eur. J. Anaesthesiol. 17, 481–484. https://doi.org/10.1046/j.1365-2346.2000.00712.x (2000).
Gursoy, S. et al. Investigation of the relaxant effects of pancuronium, rocuronium, vecuronium and mivacurium on rat thoracic aorta. Eur. J. Anaesthesiol. 26, 155–159. https://doi.org/10.1097/EJA.0b013e32831a461f (2009).
Virmani, S. et al. Effect of muscle relaxants on heart rate, arterial pressure, intubation conditions and onset of neuromuscular block in patients undergoing valve surgery. Ann. Card. Anaesth. 9, 37–43 (2006).
Mathew, A. et al. Intraoperative hemodynamics with vecuronium bromide and rocuronium for maintenance under general anesthesia. Anesth. Essays Res. 10, 59–64. https://doi.org/10.4103/0259-1162.164740 (2016).
Huiku, M. et al. Assessment of surgical stress during general anaesthesia. Br. J. Anaesth. 98, 447–455. https://doi.org/10.1093/bja/aem004 (2007).
Rosenberg, J. et al. Deep neuromuscular blockade improves laparoscopic surgical conditions: A randomized, controlled study. Adv. Ther. 34, 925–936. https://doi.org/10.1007/s12325-017-0495-x (2017).
Choi, B. M. et al. Effects of depth of neuromuscular block on postoperative pain during laparoscopic gastrectomy: A randomised controlled trial. Eur. J. Anaesthesiol. 36, 863–870. https://doi.org/10.1097/eja.0000000000001082 (2019).
Kim, H. J., Lee, K. Y., Kim, M. H., Kim, H. I. & Bai, S. J. Effects of deep vs moderate neuromuscular block on the quality of recovery after robotic gastrectomy. Acta Anaesthesiol. Scand. 63, 306–313. https://doi.org/10.1111/aas.13271 (2019).
Zhao, M. & Joo, D. T. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology 109, 308–317. https://doi.org/10.1097/ALN.0b013e31817f4c5d (2008).
Yu, E. H., Tran, D. H., Lam, S. W. & Irwin, M. G. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain?. Anaesthesia 71, 1347–1362. https://doi.org/10.1111/anae.13602 (2016).
Carron, M., Zarantonello, F., Lazzarotto, N., Tellaroli, P. & Ori, C. Role of sugammadex in accelerating postoperative discharge: A meta-analysis. J Clin Anesth 39, 38–44. https://doi.org/10.1016/j.jclinane.2017.03.004 (2017).
Donati, F. & Brull, S. J. More muscle relaxation does not necessarily mean better surgeons or “the problem of muscle relaxation in surgery”. Anesth. Analg. 119, 1019–1021. https://doi.org/10.1213/ane.0000000000000429 (2014).
Armstrong, R. A., Mouton, R. & Hinchliffe, R. J. Routinely collected data and patient-centred research in anaesthesia and peri-operative care: A narrative review. Anaesthesia 76, 1122–1128. https://doi.org/10.1111/anae.15303 (2021).
Klein, A. A. et al. Recommendations for standards of monitoring during anaesthesia and recovery 2021: Guideline from the association of anaesthetists. Anaesthesia 76, 1212–1223. https://doi.org/10.1111/anae.15501 (2021).
Funding
This work was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.E.K.; methodology, I.K.Y. and J.E.K.; validation, H.H. and J.-S.K.; formal analysis, I.K.Y. and J.E.K.; investigation, I.K.Y., D.-G.H. and J.E.K.; data curation, I.K.Y. and J.E.K.; writing—original draft preparation, I.K.Y.; writing—review and editing, J.E.K.; visualization, I.K.Y.; supervision, J.E.K.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yi, I.K., Kim, JS., Hur, H. et al. Effects of deep neuromuscular block on surgical pleth index-guided remifentanil administration in laparoscopic herniorrhaphy: a prospective randomized trial. Sci Rep 12, 19176 (2022). https://doi.org/10.1038/s41598-022-23876-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23876-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.