Abstract
Apathy is frequently observed in idiopathic normal pressure hydrocephalus (iNPH) and worsens cognitive impairment and gait disturbance. In this study, we evaluated the regions associated with apathy in iNPH using statistical imaging analysis on the whole brain, both in terms of cerebral blood flow and gray matter volume. Twenty-seven patients with iNPH were assigned to two groups based on their scores on the neuropsychiatric inventory items related to apathy; 18 patients were assigned to the group with apathy (iNPH + APA) and 9 to the group without apathy (iNPH − APA). The magnetic resonance images and cerebral blood flow single-photon emission computed tomography data of the two groups were compared using statistical parametric mapping 12. The regional gray matter volume of the right precuneus was significantly larger in the iNPH + APA group than in the iNPH − APA group, but the regional cerebral blood flow in any region of the brain was not significantly different between the two groups. These results suggested that the larger gray matter volume, which is thought to reflect gray matter compression, in the precuneus might be involved in apathy in iNPH.
Similar content being viewed by others
Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is a slowly progressive syndrome caused by excessive accumulation of cerebrospinal fluid (CSF) and is typically characterized by a triad of symptoms, including gait, cognitive, and urinary disturbances, and ventricular dilation1,2. In addition to this triad of symptoms, apathy is frequently observed in iNPH3,4. Apathy has been generally known to worsen cognitive impairment and cause impaired activities of daily living (IADL) in patients with Alzheimer’s disease (AD)5,6 and Parkinson’s disease (PD)7. Similarly, gait disturbance and cognitive impairment in iNPH are more severe in patients with apathy than in those without apathy8,9. Therefore, apathy is an important symptom that can exacerbate cognitive and motor impairment, as well as IADL, regardless of the cause. However, the neurological basis of apathy that develops in iNPH is unclear.
The most distinctive morphologic changes to the brain in iNPH are enlargement of the ventricles and Sylvian fissures and tightness of the high-convexity and medial subarachnoid spaces10. These features were named disproportionately enlarged subarachnoid space hydrocephalus (DESH)11 and are also emphasized in the Japanese guidelines for the management of iNPH2. In addition to the DESH findings, a relatively high density of gray matter in the high-convexity areas, particularly the precuneus, was reported in patients with iNPH using voxel-based morphometry (VBM), a method of statistical imaging analysis12. Furthermore, this increase in gray matter density in the high-convexity areas on VBM was considered to share the same phenomenon with the tightness of sulci; therefore, an increase in gray matter density reflects compression of the brain in iNPH.
In a study on the relationship between the brain morphologic changes and clinical symptoms in iNPH, it was shown that a larger Evans index, which indicates the degree of enlargement of the lateral ventricles, measured on head computed tomography images was associated with severe urinary disturbance, sharper angle of the corpus callosum with cognitive impairment and motor disturbance, and ventricle width with the severity of all of the triad symptoms13. Moreover, it was found that severe thinning of the dorsal corpus callosum on magnetic resonance imaging (MRI) was associated with the severity of gait, cognitive, and urinary disturbance14. It was also reported that ventricular enlargement correlated with severe apathy in iNPH15. However, there had been no studies on the relationship between apathy and regional gray matter volume (rGMV) using VBM in a whole-brain analysis.
Cerebral blood flow (CBF) single-photon emission computed tomography (SPECT) studies have shown that anterior-dominant CBF reduction was the most common CBF alteration in iNPH, but mixed or diffuse and posterior-dominant CBF reduction have been shown to exist16. Although studies on the relationship between decreased CBF and the triad of symptoms in iNPH are abundant, their outcomes have not been consistent17,18,19,20,21,22,23. It was reported that apathy was associated with reduced CBF in the right caudate nucleus24. However, that study used the region of interest (ROI) method, and to the best of our knowledge, no studies have investigated the whole-brain to determine the relationship between CBF and apathy.
Apathy in iNPH may be associated with changes in brain morphology and CBF. Therefore, this study aimed to identify the brain regions associated with apathy in iNPH by analyzing both regional gray matter volume (rGMV) and regional CBF (rCBF) data with whole-brain analysis using statistical parametric mapping.
Methods
Flow of care for iNPH at Kochi University Medical Hospital
At Kochi University Medical Hospital, specialists in geriatric psychiatry, neurology, neuro-urology, and neuroradiology attend to the clinical and neuroimaging examinations of patients who meet the diagnostic criteria for possible iNPH, and neurosurgeons perform shunt surgery on patients deemed eligible. The diagnostic criteria and treatment procedures for patients with iNPH followed the Japanese clinical guidelines for iNPH second edition25.
Preshunt surgery examinations, including CSF tap test were primarily performed in the psychiatry department.
Cognition was assessed with the following tests: mini-mental state examination (MMSE)26, frontal assessment battery (FAB)27, digit symbol substitution test (DSST) of the Wechsler adult intelligence scale-III, a subtest of immediate and delayed recall of a short story of the Rivermead behavioral memory test (RBMT)28, and the Japanese version of the Addenbrooke’s cognitive examination 3 (ACE-3)29,30. The everyday memory checklist (EMC)31 was used to assess amnesia of the patient in daily life for the patient and caregivers.
Gait speed was assessed with the timed up and go test (TUG)32 and 10-m reciprocating walking test (WT). In addition, the quality of gait was assessed using the gait status scale-revised (GSSR)33, which examines 10 factors of gait disturbances: (1) postural stability (range, 0–4), (2) independence of walking (0–2), (3) wide base gait (0–1), (4) lateral sway (0–2), (5) petit pas gait (0–2), (6) festinating gait (0–2), (7) freezing of gait (0–2), (8) disturbed tandem walking (0–1), (9) shuffle (0–1), and (10) bow-leggedness (0–1). We used the total score of the 10 items of the GSSR to obtain scores that ranged from 0 to 18.
The urinary disturbance was assessed with the Overactive bladder symptom score (OABSS)34 and the international prostate symptom score (IPSS)35.
The triad of symptoms was evaluated with the iNPH grading scale (iNPHGS)33, a clinician-rated scale that separately evaluated the severity of each of the symptoms of the triad on a scale of 0 to 4. The Japanese version of the modified Rankin scale (mRS)36 assessed the level of independence in ADL, with possible scores of 0–6. The overall severity of dementia was rated on a 5-point scale (i.e., 0, 0.5, 1, 2, or 3) using the clinical dementia rating (CDR). The caregiver burden was assessed with the Zarit burden interview (ZBI)37. Higher scores indicate better performance in the DSST, RBMT story recall, MMSE, FAB, and ACE-3 and worse performance in the EMC, GSSR, OABSS, IPSS, iNPHGS, mRS, CDR, and ZBI.
Using the NPI38, psychiatric symptoms were assessed by asking the caregiver of the patient for the presence of 12 types of psychiatric symptoms within 30 days before the evaluation: delusions, hallucinations, agitation, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, nighttime behavioral disturbances, and appetite and eating abnormalities. Each symptom was graded in terms of its frequency on a scale of 1 to 4 and its severity on a scale of 1 to 3, and the total score (frequency × severity) was calculated. In addition, apathy was assessed using a self-rating Japanese version of the apathy scale (AS)39. Higher scores indicated worse symptoms in the NPI and AS.
A lumbar tap was performed for patients with possible iNPH. The CSF samples were transferred into a 15-mL polypropylene tube, centrifuged at 1500 rpm, and stored at − 80 °C. The phosphorylated tau level of the CSF sample was measured by enzyme-linked immunosorbent assay.
The neuroimaging tests performed on all patients were as follows: (1) head MRI (Phillips’ Ingenia 3.0 Tesla), including 3D volume T1-weighted, T2-weighted, fluid-attenuated inversion recovery, and susceptibility-weighted imaging sequences; (2) CBF SPECT, which was done 10 min after intravenous injection of 111-MBq N-isopropyl-p- [123I]-iodoamphetamine; and (3) [123I]-2beta-carbometoxy-3beta-(4-iodophenyl)-N-(3-fluoropropyl) nortropane (FP-CIT) (ioflupane) SPECT, which was done 3 h after intravenous injection of 167-MBq [123I] FP-CIT. The DaT View software (Nihon Medi-Physics, Tokyo, Japan) was used to calculate the Specific Binding Ratio (SBR)40.
Selection of subjects for this study
This was a retrospective study approved by the ethics committee of the Kochi University Medical Hospital (Kochi, Japan) and followed the Guidelines for Good Clinical Practice and the Declaration of Helsinki. As the study was a retrospective observational study, in which data were collected from medical records, an opt-out approach was used; that is, information regarding our study was provided on our homepage, and each participant’s consent to take part in our study was considered to be obtained unless they expressed their desire to be excluded. The exemption on informed consent and adoption of the opt-out approach were approved by the ethics committee of Kochi University Medical Hospital.
The inclusion criteria were as follows; (1) Patients who were hospitalized in the Department of Psychiatry between March 2018 and June 2021 and (2) those who met the Japanese clinical guidelines for iNPH second edition diagnostic criteria for probable iNPH25. The criteria for probable iNPH were as follows: (1) age > 60 years; (2) presence of at least one of the iNPH triad of symptoms; (3) presence of ventricular enlargement on MRI (Evans index > 0.3); (4) absence of other diseases, conditions, or radiologic findings that could be causing the clinical manifestations; (5) no history or evidence of underlying disease that can cause secondary NPH; (6) normal CSF pressure (i.e., ≤ 20 cm H2O) and contents; and (7) one of the following: gait disturbance and DESH or improvement of symptoms after removal of 30cc of CSF via lumbar tap. Improvement of symptoms after the lumbar tap was defined as an improvement of ≥ 3 points in the MMSE or improvement of 10% or more in the TUG test or WT.
Statistical analysis
This study classified the subjects into two groups, according to the presence (iNPH + APA group) or absence (iNPH − APA group) of apathy on NPI. The results of cognitive tests and clinical assessments of iNPH were compared between the two groups using Mann–Whitney U test or Fisher’s exact test. Statistical analyses were performed with SPSS ver.25 (IBM SPSS statistics 25), and the significance level was set at p < 0.05.
Intergroup comparison of voxel-wise rGMV
In this study, the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) algorithm implemented in the statistical parametric mapping 12 (SPM12; Wellcome Department of Cognitive Neurology, London, UK) was used to normalize the MR images. DARTEL is a suite of tools for achieving more accurate inter-subject registration of brain images. The MR images were segmented to gray matter, white matter, and CSF spaces, according to a tissue probability map. Segmentation results were visually confirmed for each patient by Y.C. and T.K. Using the segmented gray matter images of all the subjects, a DARTEL template was created. The original MR image of each subject was transformed into stereotactic anatomical space with deformation parameters and the template that was created in the DARTEL deformation process of the MR images. Next step, they were smoothed with an 8-mm Gaussian filter. Artifacts can be introduced when transformed into the stereotactic anatomical space, these effects are masked by the smoothing. After smoothing the images with an 8-mm Gaussian filter, SPM12 were used for voxel-wise comparisons between iNPH + APA and iNPH − APA groups using a two-sample t-test. A comparison of rGMV was performed using age; sex; the iNPHGS scores for cognitive impairment, gait disturbance, and urinary disturbance; the NPI score for dysphoria; the MMSE score and intracranial volume as covariates. The initial voxel threshold was set at p < 0.001 uncorrected. A cluster was considered significant if it was below a cluster-corrected p(FWE) < 0.05. The brain regions were identified using the atlas of neuromorphometrics implemented in SPM12.
Intergroup comparison of voxel-wise rCBF
Each SPECT image was first co-registered with the MR image of the subject, before being transformed into stereotactic anatomical space with deformation parameters and the template that was created in the DARTEL deformation process of the MR images. After smoothing the SPECT image with a 4-mm Gaussian filter, SPM12 was used for voxel-wise comparisons between iNPH + APA and iNPH − APA groups using a two-sample t-test. The covariates in the rCBF comparison were; age; sex; the iNPHGS scores for cognitive impairment, gait disturbance, and urinary disturbance; the NPI score for dysphoria; and the MMSE score. The initial voxel threshold was set at p < 0.001 uncorrected. The cluster was considered significant if it was below a cluster-corrected p(FWE) < 0.05. The brain regions were identified using the atlas of neuromorphometrics implemented in SPM12.
Ethical approval and consent to participate
This study was approved by the ethics review board of the Kochi University Medical Hospital, Japan (2021-85).
Results
Demographic data and results of clinical assessments of the iNPH patients
Of 28 consecutive patients diagnosed with probable iNPH, 27 who had available head MRI and CBF SPECT data were included. One case was excluded from the analysis due to incorrect MRI segmentation. The iNPH + APA group comprised 18 patients, whereas the iNPH – APA group had 9. The characteristics of each group are shown in Table 1. The NPI apathy score, the GSSR score, was significantly different between the two groups.
Comparison of rGMV between the two groups
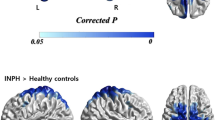
The rGMV of the right precuneus was significantly larger in the iNPH + APA group than in the iNPH – APA group [p(FWE) < 0.05]. There were no brain regions in which the rGMV was significantly smaller in the iNPH + APA group than in the iNPH-APA group (Table 2, Fig. 1).
Comparison of rCBF between the two groups
There were no brain regions with significant differences in rCBF between the iNPH + APA and iNPH – APA groups.
Discussion
To the best of our knowledge, this is the first study to investigate the neurological basis of apathy in patients diagnosed with probable iNPH using SPM12 to examine both rGMV and rCBF. The analysis showed that the rGMV of the right precuneus was larger in patients with iNPH with apathy than in those without apathy. In iNPH, a larger rGMV of high-convexity areas indicates greater grey matter compression12. However, there were no brain regions with significant differences in the rCBF between the two groups. These results suggested that the dysfunction in the precuneus might be involved in apathy in iNPH and that the grey matter compression might be more important than the abnormality of the rCBF for the apathy of iNPH. In addition, this is the first report to suggest the association between grey matter compression and clinical symptoms in iNPH; therefore, this study might facilitate the elucidation of the pathogenesis of the symptoms of iNPH. This study might also indicate that the VBM is a useful method of assessing grey matter compression in iNPH patients.
The iNPH patients with apathy had significantly worse GSSR scores and tended to have higher TUG, WT, and EMC scores and lower MMSE scores than those without apathy in this study, indicating presence of more severe gait and cognitive disturbances in those with apathy. The findings of this study are consistent with those of previous studies8,9. In addition, iNPHGS urinary and NPI dysphoria scores also tended to be worse in iNPH patients with apathy than in those without apathy in this study. To exclude these effects based on the above results, our SPM/VBM analysis included gait, cognition, and urination scores of iNPHGS, MMSE score, and NPI dysphoria score, in addition to age and sex, as covariates. In this analysis, which was adjusted for the effects of various factors, was possible because the specialists, including neuropsychiatrists, neurologists, urologists, radiologists, and neurosurgeons, cooperate well and the comprehensive evaluations for iNPH were performed at Kochi University Medical Hospital.
The results of this study showed no significant differences between the two groups in the number of patients with CSF phosphorylated tau levels > 50 and SBR values of FP-CIT SPECT. AD-related pathology is highly prevalent41,42 and striatal dopaminergic dysfunction was observed43 in patients with iNPH. Apathy in patients with neurocognitive disorders might be associated with AD-related pathology and dopaminergic dysfunction of the basal ganglia, in addition to age, sex, depression, and cognitive impairment44. However, AD comorbidity and dopaminergic dysfunction of the basal ganglia likely had little influence on the results of this study.
In this study, a relatively large rGMV in the right precuneus was shown to be associated with apathy. In NPH, vascular compliance is lower in a compressed superior sagittal sinus than in a straight sinus that is less compressed; the decreased vascular compliance of the compressed veins has believed to decrease the pulsation of the arteries45. Therefore, the right precuneus compression shown in this study may be presumed to have decreased the vascular compliance of the veins and arterial pulsation. Decreased arterial pulsation lowers the extravascular flow of CSF and lymphatic fluid and impairs the fundamental system of eliminating waste products from the interstitial space46. Furthermore, it has been suggested that the accumulation of waste products increases the osmotic pressure of spinal and extracellular fluids and that an elevated osmotic pressure may cause neuronal and axonal dehydration and contribute to neuronal and axonal damage and dysfunction in iNPH47. The right precuneus, which was shown to have a relatively large rGMV in this study, strong compression can impair the function of its neurons or axons; based on this, we surmised that right precuneus dysfunction was associated with apathy.
The results of this study suggested that the dysfunction in the right precuneus might be involved in apathy in iNPH. Among patients with PD, atrophy of the precuneus and decreased glucose metabolism were reported in those with apathy than in those without apathy, which revealed that precuneus volume and glucose metabolism were negatively correlated with the severity of apathy48,49. In addition, a previous study using functional MRI showed that the precuneus, along with the posterior cingulate cortex, is involved in both thinking about one's own intentions and consequent actions and bearing in mind an intention to make an action50. Specifically, in the right precuneus, a previous study using VBM on healthy participants reported that gray matter density positively correlated with the availability of manifold information, imagination, and problem solutions51. A functional MRI study of adolescents also reported that adolescents with higher-risk sexual behavior demonstrated greater activation in the right precuneus to social rewards52, suggesting that the right precuneus is involved in responses to social rewards. These findings of previous studies collectively led us to surmise that right precuneus dysfunction may impair the processes to generate behavior and presentation of apathy.
This study found no association between rCBF and apathy, although a previous study of 56 patients with iNPH demonstrated a relationship between apathy in iNPH and decreased CBF in the right caudate nucleus24. The methodological difference may have influenced the difference in the results; this study used whole-brain analysis, whereas the previous study used ROI analysis. A previous study on 97 patients with iNPH using whole-brain analysis showed an association between urinary disturbance and decreased rCBF in the right frontal lobe53. Therefore, no significant differences in rCBF between the two groups in this study might be due to the small sample size.
The small sample size of this study may bias the characteristics of the subjects and raise the concern that its results may not be those of a study conducted on common iNPH patients. First, in this study, we used strict diagnostic criteria for probable iNPH per the Japanese guidelines for the management of iNPH [Mori second version25], which resulted in a smaller sample size. Japanese guidelines for the management of iNPH emphasize DESH. DESH is a characteristic finding in iNPH that is useful differentiating it from other type of dementia11 and identifying iNPH patients who are more likely to benefit from shunt surgery54. In this study, the proportion of participants with DESH was high (85%), and we believe that we selected iNPH patients with a high degree of homogeneity. Second, we compared apathy between patients in the present study and those in two previous studies. A previous study evaluated psychiatric symptoms in 63 patients with iNPH3, and another study evaluated apathy in 56 patients with iNPH24. The prevalence of apathy was 66.7% in this study, 70.3% in Kito’s study, and 73.2% in Kanemoto’s study. The mean NPI-apathy score in iNPH patients with apathy was 4.8 in this study and 5.5 in Kanemoto’s study (no available data for Kito’s study). The prevalence and degree of apathy were a little less and milder in this study; however, apathy in iNPH patients in this study is similar to that in patients in previous studies. Third, we compared the triad of symptoms and characteristics between iNPH patients in this study and those in previous studies, including a multicenter prospective cohort study of 100 iNPH patients conducted in Japan11. In this study, the mean scores of iNPHGS gait, cognition, and urination were 2.0, 2.3, and 1.8, respectively. However, it is remarkable, that the mean of each of the three iNPHGS scores in Kito’s study (gait:2.0, cognition: 2.3, urination: 1.8) is the same. In SINPHONI, mean scores of iNPHGS gait, cognition, and urination were 2, 2, and 2, respectively (no available data for Kanemoto’s study). The mean MMSE scores were 21.8 in this study, 20.6 in Kito’s study, 22.2 in Kanemoto’s study, and 23.0 in SINPHONI. The mean age was 78.0 years in this study, 74.9 years in Kito’s study, 76.6 years in Kanemoto’s study, and 74.5 years in SINPHONI. The percentage of males was 59% in this study, 59% in Kito’s study, 55% in Kanemoto’s study, and 58% in SINPHONI. The mean Evans indices, which indicate the degree of enlargement of the lateral ventricles, were 0.34 in this study and 0.36 in SINPHONI. Therefore, the participants of this study can be considered common iNPH patients, although their mean age was a little higher than that of patients in previous studies.
This study had several limitations other than a small sample size. First, our data were not compared to those of normal controls; thus, we cannot conclude that the gray matter volume in the precuneus is larger than that of elderly normal control subjects. Second, apathy was examined in terms of gray matter volume and CBF, leaving open the possibility that other factors, such as white matter damage due to compression, may be related to apathy. Therefore, the results of this study should be reinforced in the future, and further studies using larger sample sizes and normal control groups are required to better understand the neural basis of apathy in iNPH. In addition, it is necessary to standardize research methods to allow for comparisons between different studies.
Conclusions
Patients with iNPH manifesting apathy were found to have large rGMV of the right precuneus, suggesting that the intense compression of the right precuneus is associated with apathy in patients with iNPH. In addition, this study shows that VBM is useful for indicating the degree of brain compression. Therefore, a longitudinal follow-up of patients who have DESH but do not have remarkable triad symptoms55 using VBM might reveal the pathogenesis of symptoms of iNPH.
Data availability
The materials and datasets are available from the corresponding author upon reasonable request.
Abbreviations
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- MRI:
-
Magnetic resonance imaging
- SPECT:
-
Single photon emission computed tomography
- VBM:
-
Voxel based morphometry
- ROI:
-
Region of interest
- DARTEL:
-
Diffeomorphic anatomical registration through exponentiated lie algebra
References
Adams, R. D., Fisher, C. M., Hakim, S., Ojemann, R. G. & Sweet, W. H. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure, A treatable syndrome. N. Engl. J. Med. 273, 117–126 (1965).
Nakajima, M. et al. Guidelines for management of idiopathic normal pressure hydrocephalus (Third Edition): endorsed by the Japanese society of normal pressure hydrocephalus. Neurol. Med. Chir. 61, 63–97. https://doi.org/10.2176/nmc.st.2020-0292 (2021).
Kito, Y. et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav. Neurol. 21, 165–174. https://doi.org/10.3233/BEN-2009-0233 (2009).
Mathew, R., Pavithran, S. & Byju, P. Neuropsychiatric manifestations of cognitively advanced idiopathic normal pressure hydrocephalus. Dement Geriatr. Cogn. Dis. Extra. 8, 467–475. https://doi.org/10.1159/000493914 (2018).
Starkstein, S. E. et al. Apathy predicts more severe parkinsonism in Alzheimer’s disease. Am. J. Geriatr. Psychiatry. 17, 291–298. https://doi.org/10.1097/JGP.0b013e31818a0e35 (2009).
Lechowski, L. et al. Persistent apathy in Alzheimer’s disease as an independent factor of rapid functional decline: the REAL longitudinal cohort study. Int. J. Geriatr. Psychiatry. 24, 341–346. https://doi.org/10.1002/gps.2125 (2009).
Dujardin, K., Sockeel, P., Delliaux, M., Destée, A. & Defebvre, L. Apathy may herald cognitive decline and dementia in Parkinson’s disease. Mov. Disord. 24, 2391–2397. https://doi.org/10.1002/mds.22843 (2009).
Allali, G. et al. Apathy and higher level of gait control in normal pressure hydrocephalus. Int. J. Psychophysiol. 119, 127–131. https://doi.org/10.1016/j.ijpsycho.2016.12.002 (2017).
Peterson, K. A. et al. Structural correlates of cognitive impairment in normal pressure hydrocephalus. Acta Neurol. Scand. 139, 305–312. https://doi.org/10.1111/ane.13052 (2019).
Kitagaki, H. et al. CSF spaces in idiopathic normal pressure hydrocephalus: Morphology and volumetry. AJNR Am. J. Neuroradiol. 19, 1277–1284 (1998).
Hashimoto, M., Ishikawa, M., Mori, E. & Kuwana, N. The study of INPH on neurological improvement (SINPHONI), diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cereb. Fluid Res. 7, 18. https://doi.org/10.1186/1743-8454-7-18 (2010).
Ishii, K. et al. Voxel-based analysis of gray matter and CSF space in idiopathic normal pressure hydrocephalus. Dement Geriatr. Cogn. Disord. 25, 329–335. https://doi.org/10.1159/000119521 (2008).
Lilja-Lund, O. et al. Wide temporal horns are associated with cognitive dysfunction, as well as impaired gait and incontinence. Sci. Rep. 10, 18203. https://doi.org/10.1038/s41598-020-75381-2 (2020).
Jinkins, J. R. Clinical manifestations of hydrocephalus caused by impingement of the corpus callosum on the falx: an MR study in 40 Patients. AJNR Am. J. Neuroradiol. 12, 331–340 (1991).
Peterson, K. A. et al. Apathy, ventriculomegaly and neurocognitive improvement following shunt surgery in normal pressure hydrocephalus. Br. J. Neurosurg. 30, 38–42. https://doi.org/10.3109/02688697.2015.1029429 (2016).
Ishii, K. et al. A multicenter brain perfusion SPECT study evaluating idiopathic normal-pressure hydrocephalus on neurological improvement. Dement Geriatr. Cogn. Disord. 32, 1–10. https://doi.org/10.1159/000328972 (2011).
Vorstrup, S. et al. Cerebral blood flow in patients with normal-pressure hydrocephalus before and after shunting. J. Neurosurg. 66, 379–387. https://doi.org/10.3171/jns.1987.66.3.0379 (1987).
Larsson, A. et al. Regional cerebral blood flow in normal pressure hydrocephalus: diagnostic and prognostic aspects. Eur. J. Nucl. Med. 21, 118–123 (1994).
Tanaka, A., Kimura, M., Nakayama, Y., Yoshinaga, S. & Tomonaga, M. Cerebral blood flow and autoregulation in normal pressure hydrocephalus. Neurosurgery 40, 1161–1165. https://doi.org/10.1097/00006123-199706000-00009 (1997).
Yoon, B. et al. Voxel-based analysis of Tc-99m ECD brain perfusion SPECT in patients with normal pressure hydrocephalus. Appl. Radiat. Isot. 67, 1377–1381. https://doi.org/10.1016/j.apradiso.2009.02.080 (2009).
Owler, B. K. et al. Normal pressure hydrocephalus and cerebral blood flow: a PET study of baseline values. J. Cereb. Blood Flow Metab. 24, 17–23. https://doi.org/10.1097/01.WCB.0000093326.88757.49 (2004).
Graff-Radford, N. R. et al. Regional cerebral blood flow in normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry. 50, 1589–1596 (1987).
Takeuchi, T. et al. Pathophysiology of cerebral circulatory disorders in idiopathic normal pressure hydrocephalus. Neurol. Med. Chir. (Tokyo). 47, 299–306. https://doi.org/10.2176/nmc.47.299 (2007).
Kanemoto, H. et al. Apathy and right caudate perfusion in idiopathic normal pressure hydrocephalus: a case-control study. Int. J. Geriatr. Psychiatry. 34, 453–462. https://doi.org/10.1002/gps.5038 (2019).
Mori, E. et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol. Med. Chir. (Tokyo) 52, 775–809 (2012).
Folstein, M., Folstein, S. & McHugh, P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–98. https://doi.org/10.1016/0022-3956(75)90026-6 (1975).
Dubois, B., Slachevsky, A., Litvan, I. & Pillon, B. The FAB: a frontal assessment battery at bedside. Neurology 55, 1621–1626. https://doi.org/10.1212/wnl.55.11.1621 (2000).
Wilson, B., Cockburn, J., Baddeley, A. & Hiorns, R. The development and validation of a test battery for detecting and monitoring everyday memory problems. J. Clin. Exp. Neuropsychol. 11, 855–870. https://doi.org/10.1080/01688638908400940 (1989).
Hsieh, S., Schubert, S., Hoon, C., Mioshi, E. & Hodges, J. R. Validation of the Addenbrooke’s cognitive examination III in frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 36, 242–250. https://doi.org/10.1159/000351671 (2013).
Takenoshita, S. et al. Validation of Addenbrooke’s cognitive examination III for detecting mild cognitive impairment and dementia in Japan. BMC Geriatr. 19, 123. https://doi.org/10.1186/s12877-019-1120-4 (2019).
Kazui, H., Watamori, T. S., Honda, R. & Mori, E. The validation of a Japanese version of the everyday memory checklist. No To Shinkei 55, 317–325 (2003) ((in Japanese)).
Podsiadlo, D. & Richardson, S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 39, 142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x (1991).
Kubo, Y. et al. Validation of grading scale for evaluating symptoms of idiopathic normal-pressure hydrocephalus. Dement Geriatr. Cogn. Disord. 25, 37–45. https://doi.org/10.1159/000111149 (2008).
Homma, Y. et al. Symptom assessment tool for overactive bladder syndrome- overactive bladder symptom score. Urology 68, 318–323. https://doi.org/10.1016/j.urology.2006.02.042 (2006).
Barry, M. J. et al. The American Urological Association symptom index for benign prostatic hyperplasia. J. Urol. 197, S189–S197. https://doi.org/10.1016/j.juro.2016.10.071 (1992).
Shinohara, Y., Minematsu, K., Amano, T., Ohashi, Y., mRS Reliability Study Group. Reliability of modified Rankin scale -Introduction of a guidance schema and a questionnaire written in Japanese. Jpn. J. Stroke 29, 6–13 (2007) ((in Japanese)).
Zarit, S. H., Reever, K. E. & Bach-Peterson, J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 20, 649–655. https://doi.org/10.1093/geront/20.6.649 (1980).
Cummings, J. L. et al. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. https://doi.org/10.1212/wnl.44.12.2308 (1994).
Okada, K., Kobayashi, S., Yamagata, S., Takahashi, K. & Yamaguchi, S. Poststroke apathy and regional cerebral blood flow. Stroke 28, 2437–2441. https://doi.org/10.1161/01.str.28.12.2437 (1997).
Tossici-Bolt, L., Hoffmann, S. M. A., Kemp, P. M., Mehta, R. L. & Fleming, J. S. Quantification of [123I] FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur. J. Nucl. Med. Mol. Imag. 33(12), 1491–9. https://doi.org/10.1007/s00259-006-0155-x (2006).
Hamilton, R. et al. Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann. Neurol. 68(4), 535–540. https://doi.org/10.1002/ana.22015 (2010).
Elobeid, A., Laurell, K., Cesarini, K. G. & Alafuzoff, I. Correlations between mini-mental state examination score, cerebrospinal fluid biomarkers, and pathology observed in brain biopsies of patients with normal-pressure hydrocephalus. J. Neuropathol. Exp. Neurol. 74(5), 470–9 (2015).
Todisco, M. et al. Clinical outcome and striatal dopaminergic function after shunt surgery in patients with idiopathic normal pressure hydrocephalus. Neurology 96(23), e2861–e2873. https://doi.org/10.1212/WNL.0000000000012064 (2021).
Lanctôt, K. L. et al. Apathy associated with neurocognitive disorders: Recent progress and future directions. Alzheimer’s Dementia. 13, 84–100. https://doi.org/10.1016/j.jalz.2016.05.008 (2017).
Bateman, G. A. Vascular compliance in normal pressure hydrocephalus. AJNR Am. J. Nwuroradiol. 21, 1574–1585 (2000).
Ringstad, G., Vatnehol, S. A. S. & Eide, P. K. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691–2705. https://doi.org/10.1093/brain/awx191 (2017).
Chrysikopoulos, H. Idiopathic normal pressure hydrocephalus: thoughts on etiology and pathophysiology. Med. Hypo. 73, 718–724. https://doi.org/10.1016/j.mehy.2009.04.044 (2009).
Reijnders, J. S. et al. Neuroanatomical correlates of apathy in Parkinson’s disease: a magnetic resonance imaging study using voxel-based morphometry. Mov. Disord. 25, 2318–2325. https://doi.org/10.1002/mds.23268 (2010).
Shin, J. H. et al. Precuneus degeneration and isolated apathy in patients with Parkinson’s disease. Neurosci. Lett. 653, 250–257. https://doi.org/10.1016/j.neulet.2017.05.061 (2017).
den Ouden, H. E. M., Frith, U., Frith, C. & Blakemore, S.-J. Thinking about intentions. Neuroimage 28, 787–96. https://doi.org/10.1016/j.neuroimage.2005.05.001 (2005).
Fink, A. et al. Gray matter density in relation to different facets of verbal creativity. Brain Struct. Funct. 219, 1263–1269. https://doi.org/10.1007/s00429-013-0564-0 (2014).
Eckstrand, K. L. et al. Heightened activity in social reward networks is associated with adolescents’ risky sexual behaviors. Dev. Cogn. Neurosci. 27, 1–9. https://doi.org/10.1016/j.dcn.2017.07.004 (2017).
Sakakibara, R. et al. Correlation of right frontal hypoperfusion and urinary dysfunction in iNPH: a SPECT study. Neurourol. Urodyn. 31, 50–55. https://doi.org/10.1002/nau.21222 (2012).
Virhammar, J., Laurell, K., Cesarini, K. G. & Larsson, E. M. Preoperative prognostic value of MRI findings in 108 patients with idiopathic normal pressure hydrocephalus. AJNR Am. J. Neuroradiol. 35, 2311–2318 (2014).
Iseki, C. et al. Asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on MRI (AVIM) in the elderly: a prospective study in a Japanese population. J. Neurol. Sci. 277, 54–57. https://doi.org/10.1016/j.jns.2008.10.004 (2009).
Funding
This work was supported by MHLW Research on Dementia Program Grant Number JP22GB1002 and JSPS KAKENHI Grant Number JP19H03585.
Author information
Authors and Affiliations
Contributions
Y.C., T.K., and H.K. designed the study. H.K., T.Y., H.F., T.U., M.S., K.I., N.K., M.A., R.F. and A.T. supervised the data collection. Y.C., T.K., R.T., and T.Y. assisted in the analysis of imaging data. Y.C. was responsible for the statistical design and analysis and wrote the first manuscript draft. All authors were involved in the interpretation and presentation of the data, reviewed and revised the initial draft and subsequent versions of the manuscript, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
H. Kazui has received speaker’s honoraria from Integra Japan K.K. and Nihon Medi-Physics Co., Ltd. T. Kashibayashi has received speaker’s honoraria from Nihon Medi-Physics Co., Ltd. R. Takahashi has received speaker’s honoraria, research funds from Nihon Medi-Physics Co., Ltd. T. Yamagami has received research funds from Nihon Medi-Physics Co., Ltd. and FUJIFILM Toyama Chemical Co., Ltd. Y Chadani, T Yamamoto, A Tsuda, R Fujito, M Akamatsu, N Kamimura, H Furuya, T Ueba, M Saito and K Inoue declare no potential conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chadani, Y., Kashibayashi, T., Yamamoto, T. et al. Association of right precuneus compression with apathy in idiopathic normal pressure hydrocephalus: a pilot study. Sci Rep 12, 20428 (2022). https://doi.org/10.1038/s41598-022-23800-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23800-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.