Abstract
Previous studies have suggested that Helicobacter pylori (H. pylori) infection is associated with nonalcoholic fatty liver disease (NAFLD). The purpose of the present study was to investigate the effect of H. pylori eradication treatment on NAFLD patients. Two hundred NAFLD patients who tested positive for H. pylori infection were randomized into the H. pylori eradication treatment group or the control group. Metabolic and inflammatory parameters and FibroScan were measured in all subjects at baseline and 1 year after treatment. At 1 year after treatment, the decrease in metabolic indicators, such as fasting blood glucose, glycosylated haemoglobin, homeostasis model assessment of insulin resistance (HOMA-IR), triglycerides, body mass index and controlled attenuation parameter values, were more obvious in the treatment group. Moreover, the inflammatory indicators white blood count and high-sensitivity C-reactive protein (hs-CRP) and the inflammatory factors interleukin 6 (IL-6) and tumour necrosis factor-α (TNF-α) were also significantly decreased. H. pylori eradication can further reduce the metabolic indices of NAFLD and the degree of liver steatosis. H. pylori infection may participate in the occurrence and development of NAFLD through its influence on inflammatory factors. Thus, checking for the presence of H. pylori infection in patients at risk of NAFLD may be beneficial.
Similar content being viewed by others
Introduction
Helicobacter pylori (H. pylori) causes significant morbidity and mortality in gastric cancer, peptic ulcers, and chronic gastritis, which infect more than half of the world’s population1. The World Health Organization (WHO) has classified H. pylori as a class I carcinogen2.
Moreover, H. pylori infection has been defined as an infectious disease and is considered to be associated with many extragastrointestinal diseases3,4. Studies have suggested that H. pylori infection increases systemic inflammation by producing inflammatory factors, which results in the development of insulin resistance (IR) and metabolic syndrome (MetS)5,6,7. Therefore, H. pylori may be a risk factor for cardiovascular disease, diabetes and nonalcoholic fatty liver disease (NAFLD)8,9,10,11,12,13,14,15.
NAFLD is characterized by excessive fat deposition in liver cells, excluding secondary causes such as viral hepatitis, alcohol or hereditary liver diseases. It is closely related to IR and genetic susceptibility to metabolic stress-induced liver damage and ranges from isolated steatosis, nonalcoholic steatohepatitis (NASH) and cirrhosis16,17. Current routinely used modalities, such as laboratory tests and ultrasonography, cannot adequately determine the levels of steatosis and fibrosis, and liver biopsy is not accepted by most people. Among the noninvasive tests, transient elastography (FibroScan) has demonstrated good accuracy in quantifying the levels of liver steatosis and fibrosis in patients with NAFLD18,19.
Based on the above research, we hypothesized that H. pylori eradication treatment could reduce the inflammatory state of the body, thereby improving the pathophysiology of NAFLD. To our knowledge, few studies have evaluated the effects of H. pylori eradication treatment in NAFLD patients. Thus, we conducted a randomized controlled trial to investigate the effects of eradicating H. pylori infection in NAFLD patients.
Results
Clinical and demographic characteristics
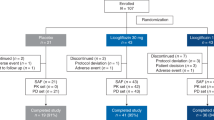
In this study, a total of 200 NAFLD patients with positive H. pylori infection were randomly divided into two groups. In the treatment group of 100 cases, 6 cases failed the first eradication. After adjusting the treatment plan according to the guidelines, a second eradication treatment was performed. After the second eradication, 2 cases of eradication failure were referred to the gastroenterologist. One year after successful eradication treatment, 2 patients with positive 13-C UBT were re-examined and thus withdrew from the study. In addition, 4 cases were lost to follow-up during the study (1 in the treatment group and 3 in the control group), and 1 case did not formally complete the eradication treatment due to a drug reaction (Fig. 1). Finally, a total of 94 patients were included in the treatment group. In this group, the average age was 50.20 ± 12.13 years old, and 65.2% were males. The H. pylori infection eradication percentage was 94%. Ninety-seven patients in the control group had an average age of 50.01 ± 10.11 years old, among whom 69.1% were males.
Comparison of the metabolic index, inflammatory parameters and FibroScan CAP values before and after treatment in the control group
In the control group, the diastolic blood pressure (80.85 ± 10.71 vs. 79.11 ± 8.61, p = 0.001), systolic blood pressure (133.7 ± 16.09 vs. 132.2 ± 13.44, p = 0.007), BMI (26.36 ± 2.09 vs. 26.16 ± 1.02, p < 0.001), ALT (32.15 ± 17.84 vs. 29.39 ± 13.26, p < 0.001), AST (26.81 ± 10.61 vs. 25.58 ± 9.88, p < 0.001), r-GT (36.53 ± 19.47 vs. 32.2 ± 15.30, p < 0.001), fasting blood glucose (5.55 ± 0.71 vs. 5.38 ± 0.63, p < 0.001), glycosylated haemoglobin (6.08 ± 0.76 vs. 5.80 ± 0.67, p < 0.001), HOMA-IR (3.11 ± 0.98vs 2.91 ± 0.97, p < 0.001), triglyceride (1.90 ± 0.94 vs. 1.67 ± 0.64, p < 0.001) and hepatic steatosis CAP values (245.53 ± 15.43 vs. 233.24 ± 10.29, p < 0.001) were significantly decreased. The inflammation indices WBC (6.83 ± 1.48 vs. 6.73 ± 1.26, p = 0.186) and hs-CRP (1.82 ± 1.99 vs. 1.66 ± 1.58, p = 0.088) and the inflammatory factors IL-6 6 (25.03 ± 4.09 vs. 24 ± 3.91, p = 0.063) and TNF-α (37.42 ± 6.71 vs. 35.68 ± 6.53, p = 0.088) were all decreased slightly, although significant differences were not observed (Table 1).
Comparison of the metabolic index, inflammatory parameters and FibroScan CAP values before and after treatment in the eradication group
In the H. pylori treatment group, the diastolic blood pressure (81.68 ± 11.27 vs. 78.34 ± 7.16, p < 0.001), systolic blood pressure (134.79 ± 16.67 vs. 131.28 ± 10.46, p < 0.001), BMI (25.99 ± 2.55 vs. 25.56 ± 2.19, p < 0.001), ALT (38.42 ± 22.32 vs. 33.89 ± 16.66, p < 0.001), AST (29.38 ± 12.18 vs. 25.64 ± 7.64, p < 0.001), r-GT (41.21 ± 21.38 vs. 34.05 ± 15.51, p < 0.001), fasting blood glucose (5.70 ± 0.91 vs. 5.32 ± 0.72, p < 0.001), glycosylated haemoglobin (5.87 ± 1.06 vs. 5.35 ± 0.81, p < 0.001), HOMA-IR (3.48 ± 1.40 vs. 3.07 ± 1.18, p < 0.001), triglyceride (2.07 ± 1.72 vs. 1.66 ± 0.67, p < 0.001) and hepatic steatosis CAP values (250.36 ± 17.15 vs. 226.34 ± 11.42, p < 0.001) were also significantly reduced after treatment. The inflammation indices WBC (7.06 ± 1.85 vs. 6.44 ± 1.45, p < 0.001) and hs-CRP (2.22 ± 3.33 vs. 1.46 ± 1.12, p < 0.001) and the inflammatory factors IL-6 (25.96 ± 5.91 vs. 21.10 ± 3.96, p < 0.001) and TNF-α (36.64 ± 7.23 vs. 28.55 ± 6.85, p < 0.001) were also significantly decreased (Table 2).
Comparison of the therapeutic effects between the treatment group and the control group
Before treatment, there was no statistically significant difference between the two groups in the metabolic indices at the basal value (p > 0.05). At 1 year after treatment, the treatment group presented a more obvious decrease in metabolic indicators, such as fasting blood glucose (0.38 ± 0.05 vs. 0.17 ± 0.02, p < 0.001), glycosylated haemoglobin (0.51 ± 0.06 vs. 0.28 ± 0.02, p = 0.001), HOMA-IR (0.41 ± 0.07 vs. 0.23 ± 0.03, p = 0.019), triglyceride (0.72 ± 0.14 vs. 0.20 ± 0.05, p = 0.001), body mass index (0.43 ± 0.07 vs. 0.20 ± 0.03, p = 0.003) and CAP values (24.02 ± 1.87 vs. 15.29 ± 0.84, p < 0.001), than the control group.
The inflammatory indicators WBC (24.02 ± 1.87 vs. 15.29 ± 0.84, p < 0.001) and hs-CRP (0.76 ± 0.28 vs. 0.16 ± 0.08, p = 0.045) and the inflammatory factors IL-6 (5.86 ± 0.33 vs. 1.03 ± 0.12), p < 0.001) and TNF-α (8.09 ± 0.44 vs. 1.74 ± 0.13, p < 0.001) were also significantly decreased in the treatment group.
There was no significant difference between the two groups in the degree of decline of the liver function indices ALT (4.53 ± 0.92 vs. 2.76 ± 0.68, p = 0.125), AST (2.74 ± 0.80 vs. 1.24 ± 0.29, p = 0.051), and r-GT (7.16 ± 1.27 vs. 4.33 ± 0.67, p = 0.054) (Table 3 and Figs. 2, 3, 4).
The treatment group presented a more robust decrease in metabolic indicators such as homeostasis model assessment of insulin resistance (a), glycosylated hemoglobin (b), fasting blood glucose (c), body mass index (d) and triglyceride (e) compared with the control group. * p < 0.05; HOMA-IR = homeostasis model assessment of insulin resistance, HbA1c glycosylated haemoglobin, FBG fasting blood glucose, BMI body mass index, TG triglyceride.
The treatment group presented a more robust decrease in inflammatory indicators white blood count (a) and high-sensitivity C-reactive protein (b) and the inflammatory factors Interleukin 6 (c) and Tumor necrosis facter-α (d) than the control group. * p < 0.05; WBC white blood cell, hs-CRP high-sensitivity C-reactive protein, IL-6 interleukin 6, TNF-α tumor necrosis factor-α.
Discussion
In our study, the influence of lifestyle intervention on NAFLD was considered in the design. In addition to the comparison before and after treatment in the treatment group, a control group with only lifestyle intervention was specifically added. In addition, the hepatic steatosis CAP value was used to evaluate the treatment effect, which made the results more objective. Our prospective study demonstrated the significant effect of eradicating H. pylori infection in NAFLD patients. Thus, H. pylori eradication therapy may improve the therapeutic effect of NAFLD by reducing inflammatory indicators in the body.
Although controversy remains, increasing evidence has revealed that there is an association between H. pylori infection and NAFLD. In particular, Doulberis et al. favoured an association between active H. pylori infection and NAFLD severity in morbidly obese patients subjected to bariatric surgery. The histological diagnostic “gold standard” for both main variables of interest (active H. pylori infection and NAFLD) was used. Specifically, the rates of NASH, as well as hepatic steatosis, inflammation, and fibrosis, were higher in H. pylori-positive patients than in H. pylori-negative patients20. In addition, the global incidence of NAFLD and H. pylori infection are high21,22, and both increase with age. The potential clinical significance of research into these diseases lies in the fact that if the two are related, then eradicating H. pylori infection may prevent NAFLD from further developing into advanced diseases, such as liver cirrhosis and even hepatic cancer.
Only a few studies have evaluated the therapeutic effect of eradicating H. pylori infection on NAFLD. Polyzos et al. conducted a 12-month prospective study11, in which NAFLD patients confirmed by liver biopsy were selected and divided into an H. pylori-positive group and an H. pylori-negative group. The H. pylori-positive group was treated with eradication therapy, and hepatic steatosis, NAFLD fibrosis score and HSENSI (homocysteine, serum glutamic oxaloacetic transaminase, erythrocyte sedimentation rate, nonalcoholic steatohepatitis index) were used to evaluate the efficacy of H. pylori eradication treatment on NAFLD. The eradication of H. pylori had no long-term effect on hepatic steatosis but improved the NAFLD fibrosis score and HSENSI index. However, another randomized open-label trial compared lifestyle modification alone and lifestyle modification combined with eradicating H. pylori eradication therapy in NAFLD patients23. After follow-up for 6 months, the liver function (AST, ALT and r-GT), liver fat content and HOMA-IR were improved in both groups. However, the values did not differ between the two groups, and only dyspeptic NAFLD patients were included in the study.
A recently published study further established the relationship between H. pylori infection and NAFLD and provided evidence for eradicating H. pylori in patients with NAFLD24. This is a multicentre cohort study of 369 adults without NAFLD at baseline who were followed up for 2 years. H. pylori infection was detected by stool antigen, and the patients were divided into positive and negative groups. During the follow-up period, the researchers observed a higher incidence of NAFLD in H. pylori-positive individuals and no NAFLD in the H. pylori-negative group. After 2 years of follow-up, the H. pylori-positive NAFLD patients were further treated with eradication therapy based on lifestyle modification. The eradication of H. pylori reduced HIS and NAFLD liver fat scores. During the 2-year follow-up, 23 new NAFLD patients were diagnosed, 18 (78.3%) NAFLD patients achieved remission after H. pylori eradication treatment, and only 5 remained unchanged (21.7%). More importantly, the HOMA-IR, adipokines, and inflammatory markers, which are considered to be key indicators of the occurrence and development of NAFLD, were also significantly improved.
Studies have revealed that due to low-grade inflammation in obese and type 2 diabetic patients, the tissue and serum IL-6 levels are abnormally increased, which promotes the occurrence and development of chronic inflammation25. IL-6 can inhibit insulin-mediated lipolysis in white adipose tissue, thereby increasing the delivery of FFAs to the liver and promoting the occurrence and development of NAFLD26. Studies have reported that the use of anti-IL-6 antibodies to treat obese mice can increase insulin sensitivity, indicating that IL-6 is involved in the pathogenesis of liver IR27. Similarly, elevated levels of TNF-α can disrupt insulin signalling through serine phosphorylation, thereby inducing insulin resistance in adipocytes and surrounding tissues and leading to the development of T2DM28.
WBC and hs-CRP are the most commonly used inflammatory indicators in clinical practice, and their detection is simple, easy and inexpensive. Many studies have reported that the WBC level is positively correlated with NAFLD29,30,31. In addition, other studies have revealed that hs-CRP is also a promising biomarker for screening cardiovascular metabolic diseases and NAFLD32,33. Moreover, a retrospective cohort study by Lee et al. found that even within the normal range33, a higher level of hs-CRP was still an independent risk factor for NAFLD.
The results of our study showed that after only one year of treatment with correct lifestyle changes, the control group presented decreases in various metabolic indices and hepatic steatosis CAP values, and the difference was significant. This indicates that simply relying on lifestyle intervention has a therapeutic effect on NAFLD. Similarly, our study indicated that 1 year after H. pylori eradication treatment with lifestyle intervention, the metabolic indices and hepatic CAP values were also significantly lower than those before treatment, and the difference was significant.
Then, we further analysed the difference in the final efficacy between the two groups. The results of our study indicated that compared with the control group, the treatment group had a more significant decrease in metabolic indices and hepatic steatosis CAP values. This demonstrated that on the basis of lifestyle intervention, the eradicating H. pylori treatment can significantly improve the therapeutic effect in NAFLD patients. Moreover, the inflammatory indicators WBC and hs-CRP and inflammatory factors IL-6 and TNF-α were all significantly decreased in the treatment group compared with the control group. Therefore, we believe that eradicating H. pylori infection helps to delay the occurrence and development of NAFLD by decreasing inflammatory factors. These results can be also reflected in the clinical routine inflammation indicators WBC and hs-CRP; therefore, the therapeutic effect of NAFLD can be followed-up by monitoring the changes in WBC and hs-CRP in the clinic.
This study has some limitations. First, we diagnosed NAFLD by ultrasonography, which was not as accurate as biopsy but was easily accepted by patients. We also used the hepatic steatosis parameter CAP value to evaluate the treatment effect. Second, patients with obvious abnormal liver function are not suitable for simultaneous H. pylori eradication therapy. The enrolled subjects were selected based on abnormal liver enzyme index values less than 2 times the UNL, and those with hypertension, diabetes, and hyperlipidaemia under treatment were excluded. Thus, selection bias may have occurred, which may explain the lack of significant differences in the changes in liver function indices between the two groups.
In conclusion, our study revealed that for patients subject to the same lifestyle management, treatment to eradicate H. pylori infection can further reduce the metabolic indices of NAFLD and the degree of liver steatosis. H. pylori infection may participate in the occurrence and development of NAFLD through its influence on inflammatory factors in the body. Clinically, the therapeutic effect of NAFLD can be followed-up by monitoring changes in WBCs and hs-CRP. We hope that additional well-designed large randomized controlled studies with longer follow-up durations will be performed to further explore the relationship between H. pylori infection and NAFLD patients.
Methods
Study design
The study design was a randomized controlled trial (Fig. 1). Briefly, the NAFLD subjects were defined as H. pylori positive or negative by 13C-UBT. The H. pylori-positive subjects were randomized into two different groups by a random number table: treatment or no treatment. Four weeks after treatment, the presence of H. pylori infection was assessed again in the treated subjects by 13C-UBT. Metabolic and inflammatory parameters and FibroScan were measured in all subjects at baseline and 1 year after the treatment.
Study participants
Participants who voluntarily underwent a general health screening from January to June 2017 were recruited from the International Health Care Centre of the Second Affiliated Hospital of Zhejiang University School of Medicine. The inclusion criteria were as follows: (1) ultrasonography for NAFLD; (2) alanine aminotransferase (ALT) levels less than 2 times the UNL; and (3) no symptoms of gastrointestinal tract disease and negative blood pepsinogen detection. The exclusion criteria were as follows: (1) the consumption of 3 or more alcoholic drink units per week; (2) any chronic liver disease; (3) a history of gastric surgery; (4) the use of bismuth, antibiotics, proton pump inhibitors or H2 blockers within the prior 4 weeks; (5) a significant mental or neurological disorder; (6) a history of cancer; and (7) patients on steatogenic medications, such as methotrexate and corticosteroids. All subjects underwent a detailed physical examination, including 13C-UBT detection of H. pylori infection, ultrasonography and FibroScan for NAFLD.
Ethics approval and consent to participate
All participants provided written informed consent before the examination. The present study was reviewed and approved by the Ethics Committee of the 2nd Affiliated Hospital, School of Medicine, Zhejiang University (2016–322). All methods were carried out in accordance with the principles expressed in the Declaration of Helsinki.
Registration
The trial was registered in the Chinese Clinical Trials Registry, with the name of the registry being “Helicobacter pylori Infection Eradication on Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial”. The trial registration number was ChiCTR2200061243 (retrospectively registered on 17/06/2022).
Questionnaires
The medical history of each participant was obtained using a questionnaire that included the history of the present illness, previous diagnoses of H. pylori infection, history of anti-H. pylori therapy, history of gastric surgery, history of significant mental or neurological disorders, history of cancer(s), use of bismuth, antibiotics, PPIs or H2 blockers within the previous 4 weeks, alcohol intake, and cigarette smoking.
Diagnosis of H. pylori infection
After fasting for at least 2 h, all participants underwent 13C-UBT at our centre. After a baseline breath sample was collected, the participants ingested a 13C-urea reagent that was dissolved in water. The second breath sample was collected 30 min later and analysed. A delta over baseline (DOB) value ≥ 4.0 indicated a positive result for H. pylori infection.
Definition of NAFLD
NAFLD was defined according to the guidelines published in 2012 by the American Association for the Study of Liver Diseases (AASLD), American College of Gastroenterology (ACG), and American Gastroenterological Association (AGA)34. In this study, the diagnosis of NAFLD required the following: (1) hepatic steatosis detected by ultrasonography; (2) no significant alcohol consumption (to strictly exclude the influence of alcohol, we chose individuals with alcohol consumption of less than 3 drink units per week); and (3) no coexisting causes of chronic liver disease, such as hepatitis C, medications, parenteral nutrition, Wilson’s disease or severe malnutrition.
Treatments
The enrolled NAFLD subjects were randomized to H. pylori eradication treatment or untreated groups based on standard instructions for diet and exercise according to the guidelines. The H. pylori eradication treatment consisted of 14 days of quadruple therapy (Esomeprazole 20 mg given twice daily + colloidal bismuth pectin 200 mg twice daily + amoxicillin 1000 mg twice daily + furazolidone 100 mg twice daily). If the patient was allergic to amoxicillin, it was substituted with clarithromycin (500 mg twice daily). Eradication was assessed by 13C-UBT 4 weeks after the end of treatment. Eradication was considered successful when the UBT was negative.
The untreated groups only accepted health education and lifestyle guidance.
Health education and lifestyle guidance
Two general practitioners (unknown about the research group) provided full-time guidance and monthly telephone or network follow-up supervision.
The specific content was based on the recommendations of the guidelines.
-
1)
Moderate-intensity calorie restriction and personalized daily calorie intake according to body mass index were recommended.
-
2)
Changes in the diet composition to a low-sugar and low-fat balanced diet. Reductions in sucrose beverages, saturated fats and trans fats and increases in fibre content were recommended.
-
3)
A moderate amount of aerobic exercise was recommended 5 times a week, and the cumulative exercise time should be at least 150 min.
Data collection
Blood pressure measurements were obtained after at least 10 min of rest. Body mass index (BMI) was defined as weight divided by height squared (kg/m2). The waist circumference (WC) was measured while standing with a measuring tape midway between the lowest rib and the iliac crest. The fasting plasma WBC, hs-CRP, fasting blood glucose, glycosylated haemoglobin (HbA1c), alanine aminotransferase (ALT), γ-glutamyltranspeptidase (γ-GT), aspartate aminotransferase (AST), total cholesterol (TC), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), and triglyceride (TG) levels were measured after an 8-h overnight fast (Beckman Coulter AU 5400). HOMA-IR was calculated according to the following formula: HOMA-IR = [FINS (µIU/mL) × FPG (mmol/L)]/22.5.
IL-6 and TNF-α
Venous blood (4 ml) was collected into a serum separation tube. After the blood clot formed, the sample was centrifuged, and the serum was collected. The total IL-6 and TNF-α contents in plasma were determined using ELISA (Abcam, USA).
FibroScan
Liver stiffness was evaluated using a FibroScan-502 touch (Echosense, Paris, France). The operation was carried out according to the user manual by two specially trained doctors who obtained the FibroScan operator certificate.
-
(1)
Fasting patients were in the supine position, and they held their head with their right hand to maximize the expansion of the intercostal space.
-
(2)
The probe was placed on the right lobe of the liver in the intercostal position.
-
(3)
The probe was kept perpendicular to the skin. When the pressure indicator was displayed in green, the M waveform on the display screen was consistent and evenly distributed, and the A waveform was linear, subsequently, the detection process started.
-
(4)
The unit of liver steatosis was dB/m, which was achieved in 10 replications with a success rate of 60% and an interquartile range of < 30%.
Statistical analysis
The statistical analysis was performed using SPSS 13.0 software. Measurement data are expressed as the mean ± standard deviation. A paired t test was used for comparisons before and after treatment within groups. A single factor analysis of variance was used for multigroup comparisons, and SNK was used for pairwise comparisons between groups. All p values were based on a two-sided test of statistical significance. Significance was accepted at the level of p < 0.05.
Data availability
The full trial protocol can be found at the Chinese Clinical Trials Registry (http://www.medresman.org.cn/uc/projectsh/projectedit.aspx?proj=4531). The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
McColl, K. E. Clinical practice: Helicobacter pylori infection. N. Engl. J. Med. 362, 1597–1604 (2010).
Lee, Y. C. et al. The benefit of mass eradication of Helicobacter pylori infection: A community-based study of gastric cancer prevention. Gut 62, 676–682 (2013).
Sugano, K. et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64, 1353–1367 (2015).
Malfertheiner, P. et al. Management of Helicobacter pylori infection-the maastricht V/florence consensus report. Gut 66, 6–30 (2017).
Longo-Mbenza, B., Nsenga, J. N. & Ngoma, D. V. Prevention of the metabolic syndrome insulin resistance and the atherosclerotic diseases in Africans infected by Helicobacter pylori infection and treated by antibiotics. Int. J. Cardiol. 121, 229–238 (2007).
Oshima, T. et al. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J. Am. Coll. Cardiol. 45, 1219–1222 (2005).
Pietroiusti, A. et al. Cytotoxin-associated gene-A–positive Helicobacter pylori strains are associated with atherosclerotic stroke. Circulation 106, 580–584 (2002).
Polyzos, S. A., Kountouras, J., Zavos, C. & Deretzi, G. The association between Helicobacter pylori infection and insulin resistance: A systematic review. Helicobacter 16, 79–88 (2011).
Chen, T. P. et al. Helicobacter pylori infection is positively associated with metabolic syndrome in Taiwanese adults: A cross-sectional study. Helicobacter 20, 184–191 (2015).
Polyzos, S. A. et al. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism 62, 121–126 (2013).
Polyzos, S. A. et al. Effect of Helicobacter pylori eradication on hepatic steatosis, NAFLD fibrosis score and HSENSI in patients with nonalcoholic steatohepatitis: A MR imaging-based pilot open-label study. Arq. Gastroenterol. 51, 261–268 (2014).
Takuma, Y. Helicobacter pylori infection and liver diseases. Gan To Kagaku Ryoho 38, 362–364 (2011).
Doğan, Z., Filik, L., Ergül, B., Sarikaya, M. & Akbal, E. Association between Helicobacter pylori and liver-to-spleen ratio: A randomized-controlled single-blind study. Eur. J. Gastroenterol. Hepatol. 25, 107–110 (2013).
Zhou, X., Liu, W., Gu, M., Zhou, H. & Zhang, G. Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J. Gastroenterol. 50, 1027–1040 (2015).
Sumida, Y. et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J. Gastroenterol. 50, 996–1004 (2015).
Samuel, V. T. et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 (2004).
Bhala, N., Younes, R. & Bugianesi, E. Epidemiology and natural history of patients with NAFLD. Curr. Pharm. Des. 19, 5169–5176 (2013).
Mikolasevic, I. et al. Transient elastography (FibroScan) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease: Where do we stand?. World J. Gastroenterol. 22, 7236–7251 (2016).
Sasso, M., Miette, V., Sandrin, L. & Beaugrand, M. The controlled attenuation parameter (CAP): A novel tool for the non-invasive evaluation of steatosis using fibroscan. Clin. Res. Hepatol. Gastroenterol. 36, 13–20 (2012).
Doulberis, M. et al. Active helicobacter pylori infection is independently associated with nonalcoholic steatohepatitis in morbidly obese patients. J Clin Med. 9, 933–944 (2020).
Zhu, J. Z. et al. Prevalence of fatty liver disease and the economy in China: A systematic review. World J. Gastroenterol. 21, 5695–5706 (2015).
Burucoa, C. & Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 22, e12403 (2017).
Jamali, R., Mofid, A., Vahedi, H., Farzaneh, R. & Dowlatshahi, S. The effect of Helicobacter pylori eradication on liver fat content in subjects with non-alcoholic fatty liver disease: A randomized open-label clinical trial. Hepat. Mon. 13, e14679 (2013).
Abdel-Razik, A. et al. Helicobacter pylori and non-alcoholic fatty liver disease: A new enigma?. Helicobacter 23, e12537 (2018).
Franckhauser, S. et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia 51, 1306–1316 (2008).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Klover, P. J., Clementi, A. H. & Mooney, R. A. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology 146, 3417–3427 (2005).
Aggarwal, B. B., Gupta, S. C. & Kim, J. H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 119, 651–665 (2012).
Alkhouri, N. et al. Neutrophil to lymphocyte ratio: A new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 32, 297–302 (2012).
Chao, T. T. et al. Use of white blood cell counts to predict metabolic syndrome in the elderly: A 4 year longitudinal study. Aging Male 17, 230–237 (2014).
Chung, G. E. et al. Associations between white blood cell count and the development of incidental nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2016, 7653689 (2016).
Yeniova, A. O. et al. High-sensitivity C-reactive protein is a strong predictor of non-alcoholic fatty liver disease. Hepatogastroenterology 61, 422–425 (2014).
Lee, J., Yoon, K., Ryu, S., Chang, Y. & Kim, H. R. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS ONE 12, e0172666 (2017).
Chalasani, N. et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 142, 1592–1609 (2012).
Funding
This work was supported in part by funding from the Zhejiang Provincial Natural Science Foundation of China (LGF19H030016) and the Medical and Health Technology Projects of Zhejiang Province (2018KY413, 2022KY815).
Author information
Authors and Affiliations
Contributions
Y.Y., L.W. and X.Y. designed the study, performed the experiment, supervised the findings of this work, and wrote the manuscript. Y.T. provided patients for the study, monitored the medical conditions of patients, helped in the design and interpretation of data, and critically reviewed the work. All the authors reviewed and approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Yy., Tong, Yl., Wu, Ly. et al. Helicobacter pylori infection eradication for nonalcoholic fatty liver disease: a randomized controlled trial. Sci Rep 12, 19530 (2022). https://doi.org/10.1038/s41598-022-23746-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23746-0
This article is cited by
-
Helicobacter pylori Eradication Treatment in Older Patients
Drugs & Aging (2024)
-
The possible role of Helicobacter pylori in liver diseases
Archives of Microbiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.