Abstract
Aiming to improve the postoperative outcome of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), the effect of physical prehabilitation (PP) was investigated in experimental model. Male Wistar rats (n = 106) divided to PP and sedentary (S) groups underwent ALPPS. Changes in liver weight, Ki67 index and liver volume by magnetic resonance imaging (MRI) were evaluated. Liver function was assessed by laboratory parameters and 99mTc-mebrofenin single-photon emission computed tomography (SPECT) hepatobiliary scintigraphy (HBS). Utilizing endotoxemia model mortality and septic parameters were investigated. Liver mass (p < 0.001), Ki67 index (p < 0.001) and MRI liver volume (p < 0.05) increased in the PP group compared to the S group. Both standard laboratory parameters (p < 0.001) and HBS (p < 0.05) showed enhanced liver function in the PP group compared to the S group. The vulnerability of animals improved in the PP group, as mortality decreased (p < 0.001), while septic laboratory parameters improved (p < 0.05) compared to the S group in the endotoxemia model. Our study demonstrated for the first time the beneficial role of PP on not only volumetric but also functional liver regeneration and postoperative vulnerability after ALLPS.
Similar content being viewed by others
Introduction
Associating liver partition and portal vein ligation for staged hepatectomy (ALLPS) is a two-stage hepatectomy offered to patients with inoperable tumors by standard procedures1. Its unique attribute is the capacity to induce robust and rapid regeneration of the future liver remnant (FLR), which could be in favor of advanced stage hepatic cancer patients, when extended resectability is needed2. Counterbalancing the remarkable advantages of the procedure, initially high morbidity and mortality rates were reported3. Although due to better patient selection and technical development these unfavorable aspects have improved since2, the patomechanism behind the high mortality and morbidity rates is still not completely elucidated.
Reciprocity between volumetric and functional regeneration of the FLR has been described following ALPPS, which results in a delayed functional recovery and undoubtedly contributes to the occurrence of postoperative complications4,5. In our previous studies we found that impaired mitochondrial function and biogenesis could be accounted for the delayed functional regeneration after ALPPS6. Seeking for methods to improve cell energy supply, we found that physical prehabilitation (PP) applied as preoperative exercise notably enhanced both mitochondrial biogenesis and function while also induced an even more potent regeneration following ALPPS7.
Therefore, we hypothesize that by stabilizing the cell-energy homeostasis PP could also improve liver function and the postoperative outcome following ALPPS. PP has been reported to enhance the function of various organs as enhanced respiratory and circulatory functions are associated with physical exercise8,9, which has been postulated to contribute to the overall recovery following liver surgery10. However, there is a paucity of data regarding the effects of exercise on liver function. To our knowledge, there is no literature data about the influence of PP on the postoperative outcome after ALPPS. Thus, in the current study we aimed to identify the effect of PP on liver regeneration, liver function and postoperative vulnerability following ALPPS.
Results
PP accelerates liver mass growth, cell proliferation and FLR volume (FLRV) increase after ALPPS
ALPPS induced liver growth of the FLR both in the sedentary (S) (238.85 ± 27.31 percent) and PP (299.16 ± 16.38 percent) groups, however the increase in liver mass was more expressed in the PP group, resulting in a significant difference between the groups from 48 h until 168 h (Fig. 1A). Validating our model, no difference could be observed between the two groups regarding neither the preoperative total liver weight, nor the weight of the FLR (Supplemental Figure SF1). Cell proliferation in the FLR also increased in both groups from 24 to 72 h, reached the peak at 48 h and decreased to the baseline by the end of the experiment. Supporting the increase observed in liver mass, Ki67 index was also higher in the PP group at 48 and 72 h compared to the S group (Fig. 1B,D). Magnetic resonance imaging (MRI) liver volumetry also showed increased volume of FLR from 48 to 120 h in the S and PP group, whereby FLRV in the PP group was higher both at 48 and 120 h than in the S group (Fig. 1C,E).
Liver regeneration of the future liver remnant (FLR). Increase in liver mass of the functional liver remnant (FLR) (A) and ki67 index (B, D) preoperatively (preop., 0 h), and at 24 h, 48 h, 72 h, and 168 h after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (N = 6 per time point per group). FLR volume (FLRV) (C) at 48 h and 120 h after ALPPS (N = 5 per time point per group). Representative magnetic resonance imaging (MRI)-volumetry picture (E) of volume increase of FLR (red mark) and volume decrease of the ligated lobes (green mark) in the S group 120 h after ALPPS. *p < 0.050, **p < 0.0010, *** p < 0.00010, **** p < 0.000010 physical prehabilitation (PP) versus sedentary (S).
PP enhances laboratory parameters of liver function after ALPPS
The level of alanine-aminotransferase (ALT) increased significantly at 24 h in the S group and abated towards baseline level by the end of the experiment, while in the PP group the increase was less explicit. Therefore, ALT level was significantly lower in the PP group at 24 h (Fig. 2A). Aspartate-aminotransferase (AST) level changed by the same dynamic, as it sharply peaked in the S group at 24 h and remained lower in the PP group during the experiment resulting in a disparity between the groups at 24 h (Fig. 2B). Similarly, total bilirubin (tBil) level increased in the S group at 24 h and decreased by the end of the experiment, while there was no change in the PP group. Consequently, tBil level was significantly lower in the PP group compared to the S group at 24 h (Fig. 2C).
Changes in liver function laboratory parameters. Level of alanine aminotransferase (A), aspartate aminotransferase (B) and total bilirubin (C) preoperatively (preop., 0 h), and at 24 h, 48 h, 72 h, and 168 h after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (N = 6 per time point per group). *p < 0.050, **p < 0.0010 physical prehabilitation (PP) versus sedentary (S).
PP ameliorates liver function measured by 99mTc-mebrofenin hepatobiliary scintigraphy (HBS) after ALPPS
Time of maximum (Tmax) in the FLR, which characterizes the organic anion uptake capacity of the liver, did not show difference between the S and PP group (Fig. 3A,E,F). By the same token, there was no significant disparity in 99mTc-mebrofenin uptake between the groups (Fig. 3B,E,F). However, tracer half-life (T1/2) in the FLR was significantly lower at 48 h in the PP group compared to the S group, indicating a more effective hepatic excretion after ALPPS in the PP group (Fig. 3C,E,F). Supporting this, 99mTc-mebrofenin washout was significantly higher in the PP group compared to the S group at 48 h (Fig. 3D,E,F), too.
Changes in liver function measured by 99mTc-mebrofenin hepatobiliary scintigraphy (HBS). Time of maximum (Tmax) 99mTc-mebrofenin concentration (A), total 99mTc-mebrofenin uptake (B), tracer half-life (T1/2) 99mTc-mebrofenin concentration and total 99mTc-mebrofenin washout (D) preoperatively (preop., 0 h), and at 24 h, 48 h, 72 h, and 168 h after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (N = 6 per time point per group). Representative curve of 99mTc-mebrofenin concentration in the change of time (E) with Tmax of the PP group (purple arrow), Tmax of the S group (blue arrow), T1/2 of the PP group (orange arrow) and T1/2 of the S group (yellow arrow). Representative figure of 99mTc-mebrofenin HBS 48 h after ALPPS in the PP group (F) showing a notable difference of 99mTc-mebrofenin uptake between the functional liver remnant (FLR) and the ligated lobes . *p < 0.050, **p < 0.0010 physical prehabilitation (PP) versus sedentary (S).
PP deteriorates vulnerability in the lipopolysaccharide (LPS) endotoxemia model after ALPPS
Vulnerability of the animals was assessed after the operation by inducing endotoxemia with LPS injection. A notable difference could be observed between the two groups, as the survival rate after LPS injection was 91.67 percent in the PP group, while only 41.67 percent in the S group, indicating an excessively increased stress-tolerance in the PP animals (Fig. 4A). Underpinning this, the Rat Grimace Scale (RGS) showed significantly lower values in the PP group compared to the S group, suggesting reduced pain in the PP animals after LPS injection (Fig. 4B). In line with the above, laboratory results also showed better stress-tolerance in the PP group, as the CRP level was higher (Fig. 4C), platelet count lower (Fig. 4D), neutrophil % higher (Fig. 4E) and lymphocyte % lower (Fig. 4F) in the S animals compared to the PP group, indicating markedly expressed inflammation, thrombocytopenia, neutrophilia and lymphocytopenia in the sedentary animals.
Changes in the vulnerability of the animals in lipopolysaccharide (LPS) endotoxemia model. Survival after LPS injection following associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) monitored for 168 h (A). Rat Grimace Scale (RGS) monitored at 4 h, 12 h and 24 h after LPS injection utilized following associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) (B). C-reactive protein (C), platelet count (D), neutrophil percent (%) (E) and lymphocyte % (F) monitored at 24 h after LPS injection utilized following associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). *p < 0.050, **p < 0.0010, ****p < 0.00001 physical prehabilitation (PP) versus sedentary (S) (N = 6 per time point per group).
PP improves body composition, which correlates with liver regeneration after ALPPS
Confirming that exercise was properly implemented, the body fat composition of the animals in the PP group improved, as the percentage of both visceral (Fig. 5A,F) and subcutaneous (Fig. 5B,F) fat was reduced in the PP group compared to the S group on the preoperative MRI. There was no significant difference between the two group regarding muscle volume (Fig. 5C,F).
Preoperative magnetic resonance imaging (MRI)-volumetry of body composition and correlation between subcutaneous fat and future liver remnant volume (FLRV). Changes in visceral fat (A), subcutaneous fat (B) and thigh muscle (C) preoperatively. Linear regression analysis of correlation between subcutaneous fat and FLRV at 48 h (D) and 120 h (E) after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Representative figure of preoperative MRI-volumetry (F) of the subcutaneous fat (blue), visceral fat (light green) and thigh muscle (dark green) in the PP group. *p < 0.050, **p < 0.0010 physical prehabilitation (PP) versus sedentary (S).
On univariable linear regression analysis, the subcutaneous fat percentage correlated with FLRV at 48 h, while only a tendentious correlation could be observed at 120 h (Fig. 5D-E). No correlations were found between either visceral fat percentage and FLRV, nor subcutaneous or visceral fat percentage and Tmax, T1/2, 99mTc-mebrofenin uptake and washout.
Discussion
ALPPS is a variation of two-stage hepatectomy that has been advocated for FLR augmentation to enable safe resection of hepatic tumors, which were initially considered unresectable1. Compared to conventional two-stage hepatectomies, ALPPS accelerates robust liver hypertrophy and reduces the time between the two steps of the surgery11. Along with its beneficial effects, adverse events also occurred postoperatively, as in the beginning morbidity and mortality rates (40% and 9% respectively) were undeniably high12. Considering these disadvantageous aspects, the potential perks of ALPPS over conventional liver volume manipulation techniques could only be in favor of patients if the postoperative outcome becomes at least comparable to these procedures. In the last few years, a new concept called prehabilitation has seen the light in oncological surgery, which aims to improve patients’ bearing capacity and decrease the perioperative stress13. One entity of prehabilitation is the improvement of the physical state, which is extensively investigated in colorectal surgery and its positive impact on postoperative outcome has been also supported by our previous study, which recommends preoperatively 4 weeks of walking-breathing exercise14. As with hepato-pancreato-biliary surgery, unfortunately literature data are scarcer and more controversial15,16,17,18, and the physiological effects of preoperative exercise are still less known. In our previous study PP was proven to stabilize mitochondrial function following ALPPS7. Based on these, we hypothesized that PP could also enhance the otherwise lagging functional recovery of the liver after ALPPS and hence, improve postoperative outcomes.
To our knowledge, this is the first study investigating the influence of PP on the postoperative outcomes of ALPPS. Remarkably, our main findings showed that PP enhanced not only [1] liver regeneration, [2] but also improved postoperative liver function and [3] reduced postoperative vulnerability.
Adding to the essentially robust liver regeneration induced by ALPPS, PP accelerated an even more pronounced liver growth, with an increase in liver mass of more than 60% compared to the sedentary animals, which is line with our previous investigation7. Serving as a proof of concept and model reliability, both cell division in the FLR and the FLRV measured by MRI were notably higher in the animals receiving PP.
Unfortunately, the rapid volumetric regeneration is not necessarily followed by the functional regeneration of the liver after ALPPS, resulting in functionally immature liver5,19. Our previous investigation on ALPPS showed the energetic disbalance of the regenerating liver caused by mitochondrial dysfunction, which could certainly contribute to the lagging functional recovery6. In our further study, PP has been proven to greatly enhance mitochondrial function after ALPPS7, hence, we postulated that it could also boost the functional regeneration of the liver. Confirming our hypothesis, liver transaminase and bilirubin levels did not raise in the PP group, indicating the functional intactness of the liver, while a sharp peak could be observed in the sedentary animals at 24 h, which is also supported by previous findings on ALPPS20. Unfortunately, standard laboratory parameters are only informative on whole liver function, greatly limiting their applicability. For this reason, we have also investigated liver function with the nuclear imaging method 99mTc-mebrofenin HBS, which could selectively measure the segmental function of the liver and allows the differentiation between hepatic uptake and excretion. As it was found to accurately measure FLR function after two-stage hepatectomy both in experimental and clinical reports, it is currently considered a highly relevant method for the assessment of hepatic function following ALPPS5,21. In accordance with laboratory parameters, 99mTc-mebrofenin HBS showed enhanced FLR function in the PP animals compared to the sedentary animals. Strikingly, the change was notable only in hepatic excretion, while no difference could be detected between the groups when uptake was assessed. As multidrug resistance-associated protein (MRP) 2 and MRP3 play a central role in the excretion of 99mTc-mebrofenin22, the altered function of these transporters could explain the improvement in the hepatic excretory function. Ventricular upregulation of MRP2 has been already revealed as an effect of exercise23. While further investigations about the influence of PP on liver transporters are needed for confirmation, this phenomenon is echoed in our results. Although literature data are available about the favorable aspects of prehabilitation after hepatectomy15,16,17,24, the present study is the first known demonstration of the effects of PP on both whole and regional liver function, moreover no previous study investigated these changes either after liver regenerative surgery, nor in healthy liver.
In the wake of experienced dramatic changes in hepatic functional regeneration during the first 24–48 h, which is the most vulnerable period following two-stage hepatectomies25, we embarked to further investigate whether PP has an impact on postoperative vulnerability. Utilizing an LPS-induced endotoxemia model after ALPPS, remarkable changes manifested in postoperative mortality. Survival was more than 90 percent in the PP group, whereas it dimidiated to close to 41 percent in the S group, demonstrating the improved stress-tolerance of the PP animals. Corroborating with this, sepsis-related laboratory parameters such as CRP, thrombocytopenia, neutrophilia and lymphocytopenia also showed improved values in the PP group.
Since our results are novum, there is a need to validate the physical prehabilitation model utilized in our experiments, therefore relevant data were benchmarked against reports in literature. Firstly, we observed notably decreased visceral and subcutaneous fat in the PP animals on preoperative MRI, which is utterly in accordance with previous findings26,27. Secondly, our model yielded the same results for the utilized exercise modality as described by others26,27,28. It has been already elucidated that while aerobic training improves body fat composition, it does not have an impact on muscle growth, as resistant training is the adequate modality to enhance muscle development28. Similarly, treadmill running used in our model as a classical form of aerobic training did not have an influence on muscles, corroborating with the evidence in literature.
Given the evidence of improved body fat composition in our model, we were eager to investigate whether a correlation occurs with volumetric and functional changes of the liver. Statistical analysis of our data shows an early supportive effect of decreased subcutaneous fat in liver regrowth. To our best knowledge, we have evidenced for the first time that the percentage of subcutaneous fat correlates with the volumetric growth of the liver in the early and most vulnerable phase of liver regeneration after ALPPS. Regarding functional regeneration no such correlation was found.
It must be acknowledged that animal models yield some limitation. The most important difference between the rat model and the clinical practice of ALPPS is set in the size and lobular structure of the liver. However, this being a standard model species in liver surgery, our rat model approach was carefully designed, as with the ligation of the right lateral, left part of the median, left lateral, and caudate lobes leading to the portal vein, 80 percent of the liver is deportalized and the transection is performed between the ischemic line of the right and left median lobe, which resembles well the clinical situation.
In conclusion, our study revealed the beneficial impact of physical prehabilitation on both volumetric and functional regeneration of the liver after ALPPS. Moreover, evidence was raised that preoperative exercise by enhancing stress-tolerance improves the postoperative outcomes following ALPPS. Based on these findings, the disadvantageous aspects of ALPPS could be mitigated with physical prehabilitation, therefore we propose the further clinical investigation of preoperative aerobic physical exercise protocols to improve patients’ safety after surgery.
Methods
All experiments were performed in accordance with the relevant guideline of the directive 2010/63/EU of the European Parlament, and all methods were reported in accordance with the ARRIVE guidelines28. Experiments were approved by the Scientific Ethical Committee on Animal Experimentation of the National Department of Food Chain Safety (approval number: PEI/001/1732–6/2015). Male Wistar rats (Toxicoop, Hungary) weighing between 220–250 g were housed in 12-h day-night cycle, with temperature (20–22 °C) and humidity (40–70%) controlled environment. Ad libitum access to water and standard chow (Toxicoop, Hungary) was provided. Before the inclusion to the experiment, animals were acclimated for 7 days.
Physical prehabilitation protocol and experimental design
PP protocol was established as previously described6,7. Animals in the PP group received PP one hour long 5 times/week for 5 weeks in form of treadmill running with 16 m/min. Animals in the sedentary group were housed in standard conditions for the same time interval (5 weeks) without receiving physical preconditioning. For detailed description of the protocol see Supplementary S1.
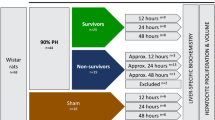
Experimental design consisted of three main groups (Supplemental Figure SF2). In experiment 1 (SF 1A) 60 animals were included (n = 30 S, n = 30 PP). Animals were terminated preoperatively and at 24, 48, 72, 168 h after surgery. Thereafter, liver weight, immunohistochemical and clinical chemistry analyzes were performed. In experiment 2 (SF 1B) 10 animals were included (n = 5 S, n = 5 PP). Liver volumetry analyzes MRI and liver function analyzes by 99mTc-mebrofenin hepatobiliary scintigraphy were carried out on the identical animals preoperatively and at 48 and 120 h after surgery. Animals were terminated at the end of the experiment. In experiment 3 (SF 1C) 36 animals were included (n = 18 S, n = 18 PP). Animals received LPS injection 24 h following surgery (see 2.6.1.) and the surviving rats were terminated 48 and 192 h after surgery. Thereafter, total blood count test and C-reactive protein measurement was performed. Overall survival and rat grimace scale (RGS) were also determined.
Surgical procedure
ALPPS operation was performed as previously described6,7. Briefly, following induction anesthesia (intraperitoneal injection of 75 mg/kg ketamine and 7.5 mg/kg xylazine in 1.5 mL saline solution) portal branches leading to the right lateral, left part of the median, left lateral, and caudate lobes were ligated. Alongside the transition line, which is situated between the left- and right part of the median lobe, transection was performed followed by careful electrocauterization of the liver wounds. Animals received antibiotic treatment (10 mg/kg body weight metronidazole intraperitoneally) and analgesia (1 mg/kg nalbuphine subcutaneously, repeated once 24 h post-operatively).
Sample extraction
Following intraperitoneal injection of 75 mg/kg ketamine and 7.5 mg/kg xylazine in 1.5 mL saline solution the animals were terminated by exsanguination via cardiopuncture. Blood was collected in tripotassium (K3) ethylenediamin tetra-acetic acid (EDTA), citrate and lithium heparin (Vacutainer) tubes. Following the in toto extraction of the liver, approximately 150 mg tissue of the right median lobe was fixed in 4% buffered formaldehyde for histology.
Assessment of morphologic alterations
Liver weight measurement
Wet weight of each lobe was determined by analytical scale (AG 245, Mettler-Toledo LLC, Columbus, OH; confidence: 0.01 mg/0.1 mg). Percentual increase of the FLR mass was defined by (lobe weight / body weight at the time of death) / (mean lobe weight at preoperative time point/ body weight at preoperative time point) × 100%.
Magnetic resonance imaging (MRI)-volumetry
In vivo liver lobe volumes were determined by 3 Tesla (3 T) MRI volumetry (nanoScan 3 T PET/MRI; Mediso Ltd., Budapest, Hungary) (for detailed description see S2). FLRV was expressed as a fraction of total liver volume and body weight.
Immunohistochemical analysis
Immunohistochemical analysis was performed as previously described7 (for detailed description see S3). The Ki67 index was calculated on the whole slide by the following formula: number of Ki67-positive cells / total number of cells.
Assessment of liver function
Clinical chemistry
Plasma AST, ALT and tBil level was measured by Vetlabor Veterinary Clinic and Laboratory (Budapest, Hungary).
99mTc-mebrofenin hepatobiliary scintigraphy (HBS)
Planar HBS (nanoScan SPECT/CT system, Mediso Ltd., Budapest, Hungary) was acquired after the injection of 99mTc-mebrofenin (combination of Bromo-Biliaron ready-to-use radiopharmaceutical kit (Medi-Radiopharma Ltd., Budapest, Hungary), and 99mTc isotope solution in physiological saline (Ultra-Technekow Technetium Generator, Mallinckrodt Medical, Petten, Netherlands)). For detailed methodology see S4. Characteristic parameters were computed from kinetic curves such as Tmax and T1/2. The uptake of the FLR can be characterized by the midpoint of the ascending section of the curve, which fits well with a linear function. The difference in isotope concentration in the bound lobe and in blood was determined at two time points. This value is then normalized by the applied activity. The washout was determined with same method at the descendent section of the kinetic curve after the inflexion point of the isotope activity curve (Fig. 3E,F).
Assessment of postoperative vulnerability
Postoperative vulnerability was assessed by creating a LPS induced endotoxemia model. LPS (O111:B4, L2630) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Rats received intraperitoneal LPS injection (25 mg/body weight kg) 24 h after surgery.
Determination of survival and evaluation of animal well-being
Animal survival was determined by consecutive monitoring after LPS injection. Administration of RGS, which features specific facial action units increasing in intensity in response to post-procedural pain29, was performed 4, 12 and 24 h after LPS injection.
Total blood count test and quantification of C-reactive protein
C-reactive protein level, platelet count, neutrophil percentage (%) and lymphocyte % was determined by Vetlabor Veterinary Clinic and Laboratory (Budapest, Hungary).
Assessment of body composition
In vivo fat and muscle volumes were determined by MRI volumetry (coronal T1-weighed gradient echo sequencing, 128 axial slices of 0.4 mm thickness) (nanoScan 3 T PET/MRI; Mediso Ltd., Budapest, Hungary) (Fig. 5F). In the first step the subcutaneous and visceral fat were manually roughly segmented using the appropriate size sphere. In the next step for clarification the subcutaneous and visceral fat layer were selected by choosing the density threshold between (10,000–18,000 voxel greyscale values in vivoQuant 1.22 software (inviCRO-Konica-Minolta Inc., Boston, US)). Total thigh volume was measured at the level of a single vertebral slice (lumbar L3). Measurements were performed in a semi-automated fashion with manual outlining of thigh muscle borders and setting the density.
Statistical analysis
Data is expressed as mean ± standard deviation. Normality and homoscedasticity of the data was analyzed with diagnostic tests. Statistical analysis was performed using two-way ANOVA with Tukey’s test for post-hoc analysis, Student's t-test, Mantel-Cox log-rank test and univariable linear regression. For analysis of non-parametric data Mann–Whitney-U test was performed. A P-value ≤ 0.05 was considered statistically significant. Calculations and visualisation were performed with GraphPad Prism 9 (GraphPad Software Inc., La Jolla).
Ethics declaration
All experiments are in compliance with the directive 2010/63/EU of the European Parlament, were reported in accordance with the ARRIVE criteria (11) and were approved by the Scientific Ethical Committee on Animal Experimentation of the National Department of Food Chain Safety (approval number: PEI/001/1732–6/2015).
Data availability
The data presented in this study are available upon request to the corresponding author.
References
Schnitzbauer, A. A. et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann. Surg. 255(3), 405–414 (2012).
Raptis, D. A. et al. Defining benchmark outcomes for ALPPS. Ann. Surg. 270(5), 835–841 (2019).
de Santibañes, M., Boccalatte, L. & de Santibañes, E. A literature review of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): so far, so good. Updates Surg. 69(1), 9–19 (2017).
Truant, S., Baillet, C., Deshorgue, A.C., Leteurtre, E., Hebbar, M., Ernst, O., et al. Drop of total liver function in the interstages of the new associating liver partition and portal vein ligation for staged hepatectomy technique: Analysis of the “Auxiliary Liver” by HIDA scintigraphy. Ann Surg. 2016;263(3). https://journals.lww.com/annalsofsurgery/Fulltext/2016/03000/Drop_of_Total_Liver_Function_in_the_Interstages_of.31.aspx
Olthof, P. B. et al. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: Liver volume overestimates liver function. Surgery. 162(4), 775–783 (2017).
Budai, A. et al. Mitochondrial function after associating liver partition and portal vein ligation for staged hepatectomy in an experimental model. Br. J. Surg. 106(1), 120–131 (2019).
Fard-Aghaie, M.H., Budai, A., Daradics, N., Horvath, G., Oldhafer, K.J., Szijarto, A., et al. The effects of physical prehabilitation: Improved liver regeneration and mitochondrial function after ALPPS operation in a rodent model. J. Hepatobiliary. Pancreat. Sci. 2021 Mar;
Jiang, H. K. et al. Aerobic interval training protects against myocardial infarction-induced oxidative injury by enhancing antioxidase system and mitochondrial biosynthesis. Clin. Exp. Pharmacol. Physiol. 41(3), 192–201 (2014).
Wang, H. et al. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell. Physiol. Biochem. 35(6), 2159–2168 (2015).
Wang, B. et al. Prehabilitation program improves outcomes of patients undergoing elective liver resection. J. Surg. Res. 251, 119–125 (2020).
de Santibañes, E. & Clavien, P. A. Playing play-doh to prevent postoperative liver failure: The “ALPPS” approach. Ann. Surg. 255, 415–7 (2012).
Schadde, E. et al. Early survival and safety of ALPPS: First report of the international ALPPS registry. Ann. Surg. 260(5), 829–838 (2014).
Silver, J.K., & Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013;92(8). https://journals.lww.com/ajpmr/Fulltext/2013/08000/Cancer_Prehabilitation__An_Opportunity_to_Decrease.9.aspx
Fulop, A., Lakatos, L., Susztak, N., Szijarto, A., Banky, B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: A randomised clinical trial. Anaesthesia. 2020 Aug;
Hao, S., Reis, H. L., Wercholuk, A. N., Snyder, R. A. & Parikh, A. A. Prehabilitation for older adults undergoing liver resection: Getting patients and surgeons up to speed. J. Am. Med. Dir. Assoc. https://doi.org/10.1016/j.jamda.2022.01.077 (2022).
Mylius, C. F. et al. Objectively measured preoperative physical activity is associated with time to functional recovery after hepato-pancreato-biliary cancer surgery: A pilot study. Perioper Med. (Lond) 10(1), 33 (2021).
Lin, F. P. et al. Prehabilitation-driven changes in frailty metrics predict mortality in patients with advanced liver disease. Off. J. Am. Coll. Gastroenterol. 116(10), 2105–2117 (2021).
Matsuo, K. et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery. 159(5), 1289–1298 (2016).
Uribe, M. et al. Insight on ALPPS – associating liver partition and portal vein ligation for staged hepatectomy – mechanisms: activation of mTOR pathway. HPB. 20(8), 729–38 (2018).
Tomassini, F. et al. Hepatobiliary scintigraphy and kinetic growth rate predict liver failure after ALPPS: a multi-institutional study. HPB. 22(10), 1420–8. https://doi.org/10.1016/j.hpb.2020.01.010 (2020).
Ghibellini, G., Leslie, E. M., Pollack, G. M. & Brouwer, K. L. R. Use of tc-99m mebrofenin as a clinical probe to assess altered hepatobiliary transport: integration of in vitro, pharmacokinetic modeling, and simulation studies. Pharm Res. 25(8), 1851–60 (2008).
Parry, T. L. & Hayward, R. Exercise training does not affect anthracycline antitumor efficacy while attenuating cardiac dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309(6), R675-83. https://doi.org/10.1152/ajpregu.00185.2015 (2015).
Dunne, D. F. J. et al. Randomized clinical trial of prehabilitation before planned liver resection. Br. J. Surg. 103(5), 504–512 (2016).
Kameoka, N. et al. Hepatic adenine nucleotides and DNA synthesis during the regenerative and atrophic process of the liver lobes after selective portal vein ligation. Eur. Surg. Res. 28(3), 212–21. https://doi.org/10.1159/000129459 (1996).
Mohammadi, H.R., Khoshnam, M.S., Khoshnam, E. Effects of different modes of exercise training on body composition and risk factors for cardiovascular disease in middle-aged men. Int. J. Prev. Med. 2018;9:9. https://pubmed.ncbi.nlm.nih.gov/29441186
Donges, C. E. & Duffield, R. Effects of resistance or aerobic exercise training on total and regional body composition in sedentary overweight middle-aged adults. Appl. Physiol. Nutrit. Metab. 37(3), 499–509. https://doi.org/10.1139/h2012-006 (2012).
Willis, L. H. et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 113(12), 1831–7 (1985).
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M. & Altman, D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8(6), e1000412 (2010).
Sotocina, S. G. et al. The rat grimace scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 7, 1744. https://doi.org/10.1186/1744-8069-7-55 (2011).
Acknowledgements
Mediso Ltd. is gratefully acknowledged for the joint collaboration in applications of nanoScan 3T PET/MRI. Erzsebet Kovacs is acknowledged for the contribution in the processing of the histological specimens.
The data that support the findings of this study are available from the corresponding author (ND) upon reasonable request.
Funding
Open access funding provided by Semmelweis University. The research leading to these results was supported by the European Union’s Horizon 2020 research and innovation program, grant agreement No 739593: HCEMM, supported by EU Program: H2020-EU.4.a. This work was also partly funded by grants from the Hungarian National Research, Development and Innovation Office (Thematic Excellence Program, TKP-BIOImaging, financed under the 2020–4.1.1-TKP2020 funding scheme to Semmelweis University), the Hungarian Government (grant VEKOP -2.3.3–15-2016–00005) and the Innovation Center of Semmelweis University (STIA-OTKA 1283978/IPK2020). ND is supported by the Semmelweis University 250 + PhD Excellence Grant (EFOP-3.6.3-VEKOP-16–2017 00009). AF is supported by Bolyai János Research Grant of the Hungarian Academy of Sciences. The founding source has no role in the design, practice or analysis of this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.F. and A.S.; methodology, A.F., M.D., K.S., N. K. and I.H.; validation, A.F. and N.D.; formal analysis, N.D.; investigation, K.L. and N.D.; resources, A.F. and A.S.; data curation, N.D.; writing—original draft preparation, N.D.; writing—review and editing, A.F., M.D. and A.S.; visualization, N.D.; supervision, A.F. and A.S.; project administration, A.S.; funding acquisition, A.F. and A.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Daradics, N., Levay, K., Horvath, I. et al. Physical prehabilitation improves the postoperative outcome of associating liver partition and portal vein ligation for staged hepatectomy in experimental model. Sci Rep 12, 19441 (2022). https://doi.org/10.1038/s41598-022-23744-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23744-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.