Abstract
In 2019, in southern Italy (Campania) there was an outbreak of a sap beetle infesting stored walnut fruits. A monitoring activity started to assess the spread and impact of the pest in walnut orchards and in warehouses, and an integrative characterization led to identify the beetle as Carpophilus truncatus. This species has been in Europe for a long time, rare and harmless until recently. We show also that this species is the same recently recorded in other two continents, Latin America and Australia, where it is causing massive damage on walnut and almond fruits. The sharing of a mitochondrial haplotype among populations recorded on three continents suggests that a worldwide invasion might be ongoing. A Geographic Profiling approach has determined that the more virulent population was first introduced in Italy, and the climate conditions of areas where C. truncatus is currently widespread and harmful indicate that the entire walnuts world production is in jeopardy as this species could adapt to any of the main walnut and almond production areas.
Similar content being viewed by others
Introduction
Invasive insects and pathogens represent an increasing threat to agriculture and forestry worldwide1,2. Despite the implemented preventative measures, the transport of goods (stored products, living plants and fruits), and people facilitate the introduction of invasive species in new areas3,4. And climate change promotes modification in the distribution range of pests and can cause the weakening of host plants and a higher level of damage by pests5,6. In some cases, even a newly introduced population of an already present species may represent a threat, due to peculiar biological traits or eventual specialization or different adaptation7,8. Genetic studies of introduced populations can provide helpful information about the colonization processes, enabling the reconstruction of the route of invasion and possibly highlighting differences with the native ones9. Usually, introduced populations have poor genetic diversity due to bottleneck and founder effects10. However, despite the low genetic variability, the populations/species may still be able to efficiently colonize new areas, a phenomenon known as the ‘genetic paradox’11. A rapid identification of species potentially invasive is the first and essential step to implement adequate containment measures. Morphological identification is often time-consuming and requires highly specialized knowledge12. Furthermore, phenotypic plasticity can result in high intraspecific variability, making the identification hardly unambiguous12,13,14,15. An integrative approach, including DNA barcoding and morphological methodology, can be the best technique to identify non-native species16,17.
The walnut ecosystem (Juglans spp.) in Italy has been recently impacted by the arrival and establishment of several invasive pests such as Rhagoletis completa Cresson (Diptera: Tephritidae), Coptodisca lucifluella (Clemens) (Lepidoptera: Heliozelidae), and Pityophthorus juglandis Blackman (Coleoptera: Curculionidae)6,7,8,9,10,11,12,13,14,15,16,17,18,19,20. In 2019, inspective activities in a walnut warehouse led to the discovery of many sap beetles (larvae and adults) on stored walnuts, identified morphologically by us as belonging to the genus Carpophilus Stephens (Coleoptera: Nitidulidae)21. This genus includes more than 280 species, many of which now with a worldwide distribution, but native mainly to tropical and subtropical regions22,23,24, with some species being serious pests25. About fifteen Carpophilus species are present in Europe and in the Mediterranean basin, including Italy, mostly introduced through the trade of foodstuffs and subsequently acclimatized over the last 2–3 centuries23,26. Most species are phyto-saprophagous (they feed on decaying vegetable substances) and develop on fruits and other organic substrates as larvae. Carpophilus beetles mostly attack ripe and ripening fruit, but also stone fruits, cereals and dried fruit, sometimes causing serious damage to crops also by transmitting yeasts and bacterial pathogens23,27. Recently, there have been two reports of unprecedented massive damage by Carpophilus on stored walnuts in Argentina28 and almonds in Australia29,30,31.
Our first attempt to identify the Carpophilus species most frequently recorded during our samplings was not fully resolutive21, due to the uncertain taxonomy of the genus32,33. Some species of the C. dimidiatus complex (within the Myothorax subgenus) are, in fact, very difficult to distinguish by morphology alone24. For this reason, it is indispensable to integrate the morphological observation with a molecular approach. Unfortunately, only a few species of Carpophilus are already genetically characterized, with a rather small number of sequences available in the Genbank and BOLD databases. In some cases, very different sequences are associated with the same taxon, creating confusion and possible misidentification. Myothorax species are particularly challenging in this respect and clearly need a revision30.
This study aimed at: a) providing a correct identification thorough characterization of the recorded species with an integrative approach (morphological and molecular); b) assessing population-level diversity to identify the most likely point of introduction; c) carrying out a risk assessment based on the type and intensity of the damage and potential diffusion based on the current diffusion. We have also clarified some aspects of the biology of this pest.
Materials and methods
Monitoring activities
Sampling activity was carried out in 2019 and 2020 from October to December in the Campania region (southern Italy). Monitoring was conducted in dried fruit warehouses, specialized and non-specialized walnut orchards (Juglans regia L.), and farms with walnut orchards and storage rooms. In each warehouse, twenty walnut fruits were randomly collected, and categorized as follows: “sorted” included walnut fruits stored in warehouses that have undergone mechanical and/or manual sorting process; “unsorted” included walnut fruits stored in a warehouse waiting to be sorted; “discarded” included walnut fruits discarded during the sorting process. In each walnut field, 20 fruits were randomly collected, picking up four fruits from the ground under five trees. Each walnut tree was located at least 50 m from each other. Twenty and thirty fruits were collected from each farm, from the ground and from each category of stored walnuts in 2019 and 2020, respectively. Sampled fruits were contained in double plastic bags to prevent insects from escaping, labeled, brought to the laboratory of the Italian National Research Council (CNR)—Institute for Sustainable Plant Protection (IPSP) in Portici (Naples, Italy) and stored at 4 °C until analysis. Carpophilus specimens were collected from areas that had been invaded by this species in Italy. The fruits of Juglans regia used in this study were collected under the permission of owners and following Italian and international rules and regulations. Experimental research and field studies on plants were carried out in accordance with relevant institutional, national, and international guidelines and legislation.
Damage
Every sampled fruit was first inspected externally, to check for the presence of holes. After external observations, the shells were broken and insect presence, kernel damage and rotting symptoms were recorded. Adults and larvae of sap beetles (and moths, if present) were collected, counted, and placed in labeled Eppendorf tubes in 100% ethanol and stored at − 20 °C.
Morphological characterization
Sap beetle adults and larvae were examined under a Leica M165C auto montage microscope (Leica Microsystems, Mannheim, Germany) equipped with a Leica DFC450 digital photo camera to obtain multifocal images, which were then assembled with the Leica Application Suite software version 3.8.0 34. Specimens were tentatively identified using the available taxonomic keys23,32,35,36,37,38,39 and original descriptions30,40,41,42,43,44,45,46,47. Keys and descriptions are often based only on male characters (e.g., parameres and metatibia); this makes the identification of isolated females rather difficult for species belonging to the C. dimidiatus complex12,22,23,30. Fifteen adults of each sex, randomly selected, were measured. The characters examined and how they were measured are summarized in Figs. 1 and 2. For comparison, we measured also specimens of the private collection of author PA (Supplementary Table S1–S2). The following characters were also recorded: mandibles symmetry, antennomere coloration, relative length of the third and second antennomers, antennal club shape, pronotum setation length in the discal area, pronotum shape in the posterior part, tibiae’s shape, female pygidium lateral margin shape, apical margin shape, apical flexion, setation density and length.
Dorsal view of Carpophilus truncatus. a-b: Coloration and apical shape of elytra, posterior shape of the pronotum. Morphometric measures: c-n: Body length; d-f: Pronotum length; j-m: Elytral length; h–l: Body width; e-i: Pronotum width; g-k: Elytral width; o-p: Intraocular distance at narrowest point; q-r: Eye width at widest point in dorsal view; s: Antennomers (I-XI) length and width; t-x: Metatibia total length; u-w: Metatibia partial length; w-x: Metatibia distal width; u-v: Metatibia proximal width; y: Number of spurs; z: Number of spines.
The male genitalia of specimens characterized morphologically and genetically were extracted and observed through a temporary slide with glycerin under a Zeiss Axiophot 2 microscope (Carl Zeiss, Oberkochen, Germany); they were photographed with an Axiocam HRC digital camera attached to the microscope and the combineZP® software was used to obtain multifocal images (Fig. 2d). Morphology of study specimens was then compared with that of Carpophilus truncatus (Murray)28,35,44,46 sub “C. dimidiatus”47, Carpophilus jarijari Powell and Hamilton30, Carpophilus pilosellus Motschulsky23,40,45, Carpophilus floridanus Fall (synonym of C. pilosellus sensu Gillogly)36,41, and Carpophilus halli Dobson (synonym of C. pilosellus sensu Connell)22,32,37,43.
Ventral view of Carpophilus truncatus. Qualitative observation. a: absence of median longitudinal ridge on the mesosternum; b: shape of posterior rim of mesocoxal cavities and axillary space; c: setation on 5th ventrite and the supplementary segment of male and absence of depressions on 5th ventrite; d: Male genitalia, parameres (specimen AA046).
Molecular characterization
A Chelex and proteinase K protocol48 was applied to extract genomic DNA from a grinded (metathoracic) leg or from the larval head of each sample. The list of specimens analyzed is summarized in Supplementary Table S1. Molecular analysis was also performed on specimens obtained from the Audisio’s collection. Two sets of primers were used to obtain a ~ 1450 bp portion of the cytochrome c oxidase subunit I (COI): LCO-1490/HCO-219849 (the barcoding region), and C1-J-2183/TL2-N-301450. The choice of a long COI fragment derived from preliminary results showing marginal differences in Italian specimens and only in the COI barcode portion. When needed, to obtain longer and ambiguity-free sequences, primers CO1 lco hym and CO1 hco outout51 were used to amplify a COI fragment overlapping with the previously obtained sequences. To overcome amplification difficulties or low-quality COI sequences from museal specimens belonging to the Audisio’s collection, specific primers were designed based on COI sequences retrieved from other sequenced samples to obtain a ~ 300 bp fragment (hereafter COI-Aud). The primers CrP-F 5′-CCCCGGCTCACTAATCGGTA-3′; CrP-R 5′- ATGAACCTCCATGGGCGATA-3′ were designed using the Primer-Blast tool52. The ribosomal gene ITS2 along with the expansion segments D1-D2 of the 28S ribosomal subunit (28S-D1-2) (for an amplicon size of ~ 1200 bp) were amplified with primers ITS2F and D2R53. PCR amplifications were performed on an Eppendorf Nexus GX2 thermocycler in a 10-µl reaction volume: 5 μl DNA-free H2O, 2 μl 5X colorless GoTaq® reaction buffer (Promega), 0.8 μl of 0.25 µM dNTPs, 0.5 μl of each 10 µM primer, 0.2 μl of GoTaq® DNA Polymerase (Promega), and 1 μl of template DNA. Thermocycler conditions for COI fragments were set as reported in48 with the modification of the annealing temperatures to 50 °C and 57 °C for the pairs C1-J-2183/TL2-N-3014 and CrP-F/CrP-R, respectively. The PCR cycling program to amplify ITS2 and 28S-D2 was 3 min at 94 °C; 35 cycles of 45 s at 94 °C, 1 min at 52 °C, and 1 min at 72 °C; with a final extension at 72 °C for 5 min. PCR products were checked on a 1.2% agarose gel stained with Xpert Green DNA Stain (20,000X) and directly sequenced. Generated sequences were deposited in GenBank under the accession numbers reported in Supplementary Table S1 .
COI sequences were aligned using the ClustalW algorithm included in BioEdit54, ITS2-28S sequences (hereafter nuclear regions) were aligned as a whole using the G-INS-I algorithm in MAFFT 755 and this alignment was used for concatenated analysis. Ribosomal regions were also used separately in single-gene phylogenetic analyses. To perform phylogenetic reconstructions, all the homologous sequences of Carpophilus available in GenBank and BOLD were included in the analyses (www.ncbi.nlm.nih.gov/genbank/; www.boldsystems.org; last accessed on 21 November 2021) setting a threshold of 70% similarity with the sequences obtained.
Not all markers were available on GenBank (or BOLD for COI) databases for each species, hence different genes or their portions were analyzed both separately and combined to get a clearer picture of phylogenetic relationships. Phylogenetic reconstructions were performed by conducting a maximum likelihood (ML) analysis in RAxML version 8.2.1056 using the RAXMLGUI v. 2.0.557 for every marker (COI, ribosomal regions and combined molecular markers, and Bayesian inference (BI) in MrBayes 3.258 for the COI barcode portion and combined molecular markers. For the ML and BI analyses, the GRT + G + I evolutionary model selected by jModeltest59 was used for each genetic portion except ITS2, for which GRT + G model fit better. The phylogenetic reconstruction based on a concatenated dataset (COI + ribosomal regions) included all the Carpophilus species for which we obtained sequences of all markers for at least one specimen, and those available on public databases that were related to our samples based on single-gene phylogenetic analyses. Partitioned evolutionary models, according to the best partitioning scheme found by Partition Finder60, were used for the BI analysis on concatenated dataset. Trees were rooted by setting Aethina tumida (Coleoptera: Nitidulidae, Nitidulinae) as outgroup for COI and 28S or using the midpoint-rooted tree option for markers where outgroups were not available (acc. num.: KP134137 and KP134071). Phylogenetic trees were drawn by using FigTree software v1.4.261. COI genetic distances within and between lineages were calculated using the p-distance method in MEGA 6, only for the COI barcoding groups our samples clustered in62.
Intraspecific haplotype diversity assessment
Two methods were used to estimate the necessary sample size to evaluate the intraspecific genetic diversity of Carpophilus. Coalescent theory63 was used to calculate the probability (p) of sampling all haplotypes of the species with sample size (n) using the Eq. (1)64:
The Eq. (1) developed by Grewe et al. 65 was used to calculate the minimum sample size to exclude the presence of a given haplotype in a locality:
where p is the frequency of a given haplotype, and β is the desired confidence level.
Reconstructing the most likely area of introduction through the Geographic Profiling (GP) approach
To identify the possible areas of origin for the spreading of the population of sap beetle in Campania, a Geographic Profiling (GP) approach was used66,67,68. Although several GP algorithms include the presence of a buffer area, we used the Dragnet algorithm that excludes a buffer zone, because the dispersal capabilities of small coleopterans are rather limited67,69,70 and the spreading of small coleopteran pests is often more dependent on human activities than dispersal capabilities of the species71, but see exceptions72,73. To gain a more robust prediction, we used a bootstrap approach74 by repeating the Dragnet algorithm on 30 subsamples of the whole dataset, with each subsample including a random amount of the original data ranging between 25 and 75%. Finally, the mean of the scores was computed from the different models, as this approach has been shown to render the areas of highest priority (specificity), along with the surrounding area that can be considered analogous to confidence limits74. Because in Campania no individuals of the sap beetle under study were found in walnut fruits above 600 m a.s.l. (Supplementary Table S1), the reconstruction for the final mean model to all the local areas (to estimate the most likely area of first introduction) was restricted from 0 to 600 m a.s.l.
Statistic analysis
The ANOVA test was used to analyze data when the conditions of normality and homoscedasticity were respected. In the case where the conditions of normality and homoscedasticity weren’t satisfied, the non-parametric test Kruskall–Wallis was used, while a Box and Whiskers plot analysis was performed to separate medians graphically75.
Results
Monitoring activities
In 2019, 10 sites out of 18 (55.5%) were infested by Carpophilus sp. and 82 adults and 199 larvae were collected. In 2020, 28 out of 63 monitored sites (45.3%) resulted infested and 106 adults and 1123 larvae were collected (Fig. 3, 4). The northernmost plantation site, where Carpophilus was recorded, was located in Pignataro Maggiore (Caserta province), while the southernmost site was located in Massa Lubrense (Naples province). The plantation situated in Vico Equense (Naples province) was the one with the highest altitude (549 m a.s.l.). The walnut fruits stored in warehouses came from surrounding plantations, except for the walnut fruits collected in Sarno (Salerno province; L circle area in Fig. 4 on the right) that were moved to a warehouse in Giugliano in Campania (Naples province; L red little circle in Fig. 4 on the right). Locations A, B, D, E, and F (areas with a diameter of 3 km2 where Carpophilus was found) (Fig. 4) are usually used for cultivating of hazelnuts76. Locations C, I, L, M, and N were mainly situated in a flat area; some sites were near urban area, others were in an agricultural context (vegetable crops and fruit orchards). Location H and one site of the location L were located in more forested and hilly areas. Location G concerns the sites located on the Sorrento peninsula, where citrus are cultivated almost exclusively76.
Survey area of monitoring activities in 2019–2020. On the left: position and types of sites where walnut fruits were sampled; infested sites (red) and uninfested sites (green). On the right: distribution and frequency of haplotypes; each circle represents an area of 3 km2 which includes multiple infested sites.
Damage
The main evidence of Carpophilus in walnut fruits was the presence of different life stages or of light frass up to 0.1 mm diameter consisting of residues of walnut and excrements (Supplementary Fig. S1). Larvae and adults of Carpophilus dug tunnels flattened in the cross section on the kernel, eating the kernel’s center and leaving the darker skin on the outside intact. Sometimes, both sap beetles and moths infested the same walnut (about 3% of the walnuts examined). If no live insects or parts of them were found, the damage was attributed to moths if silk threads or frass larger than 0.5 mm diameter were found. The damage caused by Carpophilus sp. on walnut fruits is summarized in Table 1. No significant differences were found in the average number of sap beetles present within the walnut fruits of the different sampling types in 2019 (ANOVA F3,81 = 2.32, p = 0.017) (Table 1). Conversely, in 2020, there was a significant difference in the mean number of sap beetles present within the sorted walnut fruits and the discarded ones (Kruskall–Wallis, χ2 = 8.94, df = 228, p = 0.030) (Table 1). About 80% of the fruits that resulted infested with Carpophilus sp. showed a crack in the sheath between the two shells of the endocarp.
Morphological characterization
Measured characters and qualitative observations are summarized in Supplementary Tables S2–S4. Results of the comparison with Carpophilus species that most closely resembled the species here studied are shown in Supplementary Fig. S3. The closest species were C. truncatus and C. jarijari. The main discriminating characters were very similar in these two species, namely, parameres shape (Fig. 2d) and metatibiae (Fig. 1) (abruptly restricted proximally in males): in males of C. jarjari abruptly dilated in their distal half/third, while in males of C. truncatus abruptly dilated in their distal two thirds or three fifths: Fig. 1 t-x). However, male metatibiae of all our samples resulted identical to the typical shape above recorded for C. truncatus.
The parameres of our samples resembled more those of C. jarijari30, even if remarkable similarities with parameres of C. truncatus description, draws, and PA’s collection samples22,23,30,41,44,47 were noticed. Furthermore, the shape of parameres was found to be slightly variable among samples, in fact at least one specimen out of 15 examined showed parameres smaller and not regularly restricted up to the apex. Moreover, the parameres shape is strongly influenced by the angling placement on the slides, so a slight pressure on the slide cover, modifying their inclination, has a strong influence on the photo. This character was not correlated with the haplotype. The specimens of the most frequent species found in Italy were all identified as belonging to C. truncatus (= pilosellus auct., nec Motschulsky).
Molecular characterization
Mitochondrial COI sequencing revealed two haplotypes (Supplementary Table S1). BLAST search revealed that the haplotype B (hB) has 100% similarity with Carpophilus “dimidiatus (F.)” (accession number MG679359) found in Argentina 28; the haplotype A (hA) was recovered for the first time and differed from hB by 22/1448 bp. All the Italian C. truncatus samples, collected from different localities and on different dates, shared the same ribosomal genes sequence. No amplification was obtained from museal samples, except for samples AA103 and AA104, from which the COI-Aud portion was sequenced. These short sequences were identical to each other and to hB.
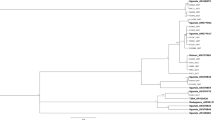
ML and BI phylogenetic reconstructions based on the barcoding region resulted in trees of identical topology but often with different statistical support (Fig. 5a). Our Carpophilus samples, from the field and from the Audisio’s collection, belonged to three different groups. Both mitochondrial haplotypes of C. truncatus clustered with C. “dimidiatus” sensu Reales et al.28 from Argentina, and this cluster was sister to the C. dimidiatus group (specimens from French Polynesia, China, and India) and C. pilosellus (represented by a single specimen), according to the recent reinterpretations of this taxon38,39. The last group showed C. davidsoni as the sister group of C. zeaphilus (CM45-CM44 from Malus sp.; AA049 from walnut) and C. nepos as the sister group of C. mutilatus. Phylogenetic reconstructions based on ribosomal genes (Supplementary Fig. S4) (GenBank accession numbers from ON555771 to ON555778) were partially congruent with that obtained with COI (for 28S-D2: CDN40 clusters with C. hemipterus while C. truncatus is sister group of the true C. dimidiatus). However, as shown more clearly by ITS2 phylogenetic reconstruction, the lack of the sequences of many species did not allow a complete analysis (data not shown). Phylogeny based on combined COI + ribosomal genes (Fig. 5b) showed that the phylogenetic relationships among the samples here studied were congruent with COI reconstruction (Fig. 5a).
BI majority-rule consensus tree for (a) COI barcoding portion and (b) concatenated COI + ITS2 + 28S-D2 genes of Carpophilus spp. Posterior probability values > 0.90 are shown above branches, along with bootstrap values > 70% for the topologically identical ML tree based on 10,000 rapid pseudoreplicates. Two parallel runs of four simultaneous Monte Carlo Markov chains were run for 5 and 1 million generations for COI barcoding and concatenated alignments respectively. ns = not significant; * indicate the placement of the samples here studied.
Uncorrected p-distances based on COI barcode within and between groups are reported in Table 2. The mean distance across the species was 14.8%. The highest interspecific distance was between C. dimidiatus and C. davidsoni (18.5%) while the lowest between C. davidsoni and C. zeaphilus (10.3%). The mean intraspecific distance was 1.39%, C. dimidiatus showed the highest value (3.52%) while the lowest value (0.62%) was found in C. truncatus and C. zeaphilus.
Intraspecific haplotype diversity assessment
Equation (1) showed that, although we examined 67 samples, 39 samples would have been sufficient to recover the genetic variations of the species (p = 95%). Only two mitochondrial haplotypes (A and B) were observed. Following an iterative approach, and starting from the preliminary frequency of the two recorded haplotypes, the minimum sample size resulted in 6.5. Both haplotypes were present in almost all localities (9) where at least two specimens were found. The frequency of the two haplotypes, calculated on the total number of sequenced specimens, was 53.8% for hA and 46.2% for hB (Supplementary Table S1; Fig. 4).
Reconstructing the most likely area of introduction through the Geographic profiling (GP) approach
The mean prediction after the bootstrapping procedure of the Dragnet GP algorithm is shown in Fig. 6. The model rendered three areas as the potential source for the spreading of the pests, two of them along the Sorrento peninsula (Fig. 6.1 and 6.2) and one between the Vesuvio and the mountains above Palma Campania (Fig. 6.3). In particular, the area with the highest score is located in the foothills of Monte Faito, one of the main peaks of the Lattari mountains, around the municipality of Vico Equense (Fig. 6.1). The latter area is the most likely area of origin for the spread of C. truncatus in Campania, while the other areas are secondary sources.
Rendering of the mean prediction for the Dragnet GP algorithm after bootstrapping. The areas are colored according to the score value of the prediction, which can be seen and increasing likelihood for the origin of the spreading for Carpophilus truncatus in Campania. The areas above 600 m a.s.l. have been discarded from the prediction.
Discussion
The correct identification and complete characterization of a harmful insect species is the first and indispensable step for any type of pest control. Sometimes, however, due to the scarce or absent knowledge of some emerging species and of the taxonomic groups to which they belong to, this first phase is an almost insuperable challenge16. Also in this case, the difficulties encountered in the identification of the species under study were considerable, due to the inadequacy of morphological and molecular knowledge of several Carpophilus species. However, our ultimately successful and comprehensive characterization of this species will allow for an easy identification in the future. Many species of Carpophilus have been described by observing a few or a single specimen, thus providing little if any data on the variability of each character. Having examined here 15 females and 15 males, we now also have a much better understanding of the intraspecific variability, including some characters considered diagnostic.
Our integrative approach has shown that the specimens found in Italy belong to C. truncatus. However, the identification process highlighted issues with the identification by other authors. Specimens collected in Italy share the same mitochondrial haplotype (hB) with specimens collected in Argentina on stored walnuts28 and with specimens collected in Australia on almonds29. Although Reales et al.28 adopted an integrated approach as well, it is evident that the species was misidentified. The authors identified the specimens collected as C. dimidiatus even though the morphology did not match the description, and the COI was not similar enough to other C. dimidiatus sequences deposited in GenBank. The identification of the Australian specimens is instead more controversial. Australian specimens were temporarily named Carpophilus near dimidiatus 29 and were successively described as the new species C. jarijari30, while other authors concluded that those individuals belong to C. truncatus31. Carpophilus jarijari is morphologically very close to C. truncatus and according to its description, it is distinguished by the particular shape of the parameres30. However, we have highlighted that the parameres exhibit a degree of variability that is not considered in any key, and the perception of their shape is strongly affected by the methodology with which this character is evaluated. This variability, together with the molecular correspondence between C. truncatus and Australian samples29,77, creates a need for the taxonomic position of C. jarijari to be re-evaluated. The unreliability of some morphological discriminating characters in this genus, if used alone, has been known for a long time32.
Molecular identification was hindered by the absence of sequences under the name of C. truncatus in the Genbank and BOLD databases. Furthermore, the presence of a sequence of C. pilosellus (in GenBank) renders the whole scenario even more complex. Carpophilus pilosellus Motschulsky (original description) is considered a synonym of C. mutilatus by78 and a synonym of C. dimidiatus44, but recent data38,39 consider the existence of the true C. pilosellus, as a distinct and valid species, at least in southern North America (Supplementary Table S5). Moreover, the descriptions of C. pilosellus after the original one refers instead to C. truncatus23 in Genbank (accessions NC046035-MN604383) entangled the identification process. However, the analysis of the interspecific p-distances between C. pilosellus and C. truncatus showed a genetic distance (13.4%) too much higher than the mean and the maximum intraspecific distance found in examined Carpophilus spp. (1.39% and 3.52% respectively) and this can have two possible explanations:
-
a)
C. pilosellus Motschulsky, 1858 (not pilosellus auct.) is truly a valid species (as informally introduced by Di Lorenzo et al.38,39) and the synonymy with C. dimidiatus or with C. mutilatus should be withdrawn;
-
b)
The sample to which the sequence belongs was misidentified and it belongs to an undescribed species or is to be referred to another taxon within the complex.
Phylogenetic reconstruction based on different genes highlighted several apparently polyphyletic “species” (Fig. 5a,b – Supplementary Fig. S4, S5), which confirms the existence of huge identification problems inside the genus Carpophilus. C. truncatus consistently resulted sister to C. dimidiatus, who in turn resulted polyphyletic based on 28S and the 3’ region of COI (Supplementary Fig. S4), where the samples identified by Cline in79 were included in one clade. Therefore, it is imperative to try using a different strategy. On the basis of both current results and results on other taxa80,81,82,83, the approach based on the combined molecular markers (COI + ITS2 + 28S-D2) is quite effective and should be used for future studies of this group.
The specimens under study also share the same mt haplotype (hB) and are morphologically identical to two specimens of C. truncatus from the Audisio’s collection. This result confirms that this haplotype was already present at least since 1996 in Italy (Supplementary Table S1), where until a few years ago it was sporadic and not harmful23. Some data on the species distribution and population density are unknown or not exhaustive, but the species is reported to be present but rare, in Italy and Europe since the 1800s and it never caused concern for agricultural production until 2019 (Supplementary Table S6). However, starting from 2013 in Australia29 and Argentina28 sub “C. dimidiatus”, and since 2019 in Italy21 sub “dimidiatus”, C. truncatus has begun to be harmful. The almost simultaneous appearance of a harmful population of C. truncatus in three different continents lets to consider it as an invasive species of stage V, widespread and dominant, sensu Colautti and MacIsaac84. What happened? A change has clearly occurred in the relationship between C. truncatus and its current hosts/habitat in recent years, and some hypotheses may be formulated to explain this process:
-
1)
The delay in the identification of an invasive species is, unfortunately, a recurring problem; often some species are not considered potentially invasive because they do not cause damage in the country of origin85. It is common for an invasion to be characterized by initial periods of inactivity that suddenly evolve into an explosion85. This period is called lag time and it is referred to the invader’s impact. However, if we consider that C. truncatus was already present on the Italian territory since the 1800s, that this species never impacted agricultural production until 201923,86, and that instead there was the almost simultaneous appearance of harmful populations in three different continents, this hypothesis becomes unlikely.
-
2)
The explosion of the species, which consists of an increase in population density and harmfulness, could have been caused by the recent invasion of other insect species such as R. completa or Apomyelois ceratoniae (Zeller) (Lepidoptera: Pyralidae), which might create more favorable conditions for C. truncatus damage20,87. These insects indeed damage the hull and uncover the fruits, favoring an early infestation by C. truncatus. A similar behavior was also observed in Australia, where C. truncatus infests almond fruits only when the nuts are uncovered29. Other Carpophilus species, such as C. hemipterus, have similar opportunistic habits, penetrating peaches through damage caused by other pests27,88.
-
3)
Some changes in the practices related to the management of walnut orchards and fruits (pest control, sorting process, harvest) may have influenced the explosion of this population. Until a few years ago, broad-spectrum insecticides were used in Italy and in other parts of the world to control many walnut pests89. Recently, many active ingredients, like dimethoate, were banned90. The pesticides currently used, not being systemic, may not be effective against C. truncatus.
-
4)
An alternative and more probable hypothesis is that a more virulent population has only recently developed that has also been introduced in Italy. This hypothesis is also corroborated by the evidence that the Italian population has adapted to a climate zone very different from that of origin and from the one where it has been harmful until now (see below). The genetic variability within the Italian population is very low, with only two haplotypes recorded thus far. This is a recurring pattern in invasive species and has been found multiple times6,13,91. To date, only one haplotype has been found in Argentina and Australia, but with a negligible number of specimens analyzed28,29. Recently, the sharp contraction of local production of walnuts, which are being replaced by the more profitable hazelnuts, has led to an increase import of walnuts from Argentina and Australia (bridgehead scenario) and from other unidentified areas (walnut growers, personal communication). This import may have introduced a more virulent population of C. truncatus than the one present so far. A similar phenomenon was recently recorded in Taiwan where the introduction of a non-native lineage resulted linked to a recent outbreak of the ant Dolichoderus thoracicus (Smith)92. The use of other markers, such as microsatellites or RAD-Seq, could confirm this hypothesis.
It is also likely that two or more scenarios listed above have contributed synergistically to favor the explosion of C. truncatus populations. The extent of the infestation and damage caused by the sap beetle in Italy is further evidence that the complex of walnut pests is constantly and rapidly changing, as recently reported20. Moreover, field sampling and observations have shown that C. truncatus adults lay their eggs already on the fruit on the trees. Therefore, the harvested fruits are already infested and this observation is congruent with the almond fruits observed in Australia29 and with the evidence that at least 20% of the infested ripe nuts did not present any alteration of the sheath that closes the two fruit valves. The damage caused by C. truncatus larvae and adults is relevant (up to average values of 22% on fruits collected on the ground) and is both direct and indirect. The direct damage is due to the feeding activity on the kernel, while the indirect one is due to the symbiotic fungi and bacteria carried by insects, and the difficulty in the detection of the infested fruits by external examination of the walnuts. There are no shell aesthetic deteriorations and, if the damage is not advanced, there are no significant weight variations of the fruits; therefore, the infested fruits can pass the sorting process, bringing inside the warehouses specimens that can thrive in such conditions. The sorting systems used to date are ineffective at discarding infested walnut fruits. The undetectability is particularly detrimental to walnut fruits, which are still widely commercialized with shells, unlike almonds. Carpophilus truncatus can overwinter inside the walnut fruits in the warehouse but also in the mummified fruits in the fields. Field and warehouse sanitation could therefore positively affect the state of infestation29. Although the type of C. truncatus was collected in Madagascar35, the current distribution of C. truncatus, suggests that this species could expand its diffusion where there are the world's largest producers of walnuts and almonds.
The intense monitoring performed in the Campania region allowed to assess that the distribution of sap beetles is patchy, with several areas still unaffected. Transporting of walnut fruits from the orchards to a distant warehouse seems to have played an important role in the dispersal of C. truncatus. An example of this phenomenon has been recorded in the case of walnut fruits collected in Sarno (SA) and transferred to Giugliano in Campania (NA) to be sorted (Fig. 4). However, there are two areas in the Sorrento Peninsula (Fig. 6.1, 6.2) and one in the area of Palma Campania (Fig. 6.3) where the presence of the sap beetle is more frequent. The orography of the territory, influencing the climatic conditions and the habitat quality, has certainly influenced the spread of the sap beetle, but the rather uneven distribution of walnut orchards in Campania also played an important role obviously. In Campania, few specialized walnut orchards are present, but walnut is a widespread tree and it is cultivated in many private gardens. However, the prediction made with the Dragnet GP algorithm indicates that the Sorrento peninsula (Fig. 6.1) is the most likely area where the pest has begun to spread. This result also seems to confirm that the recent demographic and harmful explosion of C. truncatus started from the recent import of walnuts, which has introduced an invasive population. It is important to remember that walnuts of the Sorrento cultivar are very renowned and that the contraction of local production has led to the importation of walnut fruits mainly from South America (pers. obs.). Some surveys were also carried out in other Italian regions (in Veneto, Lazio, and Calabria regions) but no specimens of C. truncatus were collected (Carmelo Bonsignore, Raffaele Sasso, Luca Mazzon, personal communications). This result is congruent with what is stated in previous studies that define C. truncatus as a Carpophilus species that probably encountered greater difficulties in acclimating in Euro-Mediterranean areas, where its presence is still relatively sporadic and mostly limited to urban and suburban areas or areas with anthropogenic influence23. At the moment the biological data about C. truncatus and in particular on this invasive population that infests walnuts, are scarce, but several other carpophiline species (such as Carpophilus hemipterus, C. nepos Murray, C. dimidiatus, Urophorus humeralis F.), due to their tropical origin, have a remarkable tolerance to high temperatures93,94,95. In Italy, C. dimidiatus, C. nepos, C. hemipterus, C. mutilatus, and C. quadrisignatus Erichson complete about 5–6 generations per year, with a peak of adults in summer and late summer88. Carpophilus truncatus seems instead to be univoltine in the field but it is able to complete more generations in the warehouse. The absence of C. truncatus in higher altitude areas (> than 250 m a.s.l.), except some findings in the Sorrentine peninsula (300–550 m a.s.l.), where the sea strongly mitigates the temperatures, seems to indicate that it has similar thermal needs of other species of the same genus (Figs. 4, 6). These results match with the observation that several Carpophilus species are more harmful at low altitudes96.
Conclusion
C. truncatus has been characterized by integrating multiple data, hence making its identification much easier from now on. The same population and, in particular, one of the two haplotypes found in Italy is behaving as an invasive species on three different continents. The multilocus phylogenetic reconstruction showed that several species are apparently polyphyletic, highlighting major taxonomic problems within the genus. The infestation occurs when the walnuts are still on trees, and on average 22% of the fruits are infested. The invasive process in Italy has started from the Sorrento peninsula. Lastly, C. truncatus appears capable of acclimatizing to the main walnut and almond production areas of the world. C. truncatus has all the features to be considered a quarantine pest.
Data availability
Data will be available upon the publication of the manuscript as supplementary material, furthermore all the voucher specimens are deposited in the Insect Collection of the Institute for Sustainable Plant Protection—National Research Council (IPSP-CNR), P.le E. Fermi, 1—80,055 Portici (NA) – Italy, and are available on request to Dr. Umberto Bernardo, umberto.bernardo@ipsp.cnr.it.
References
Paini, D. R. et al. Global threat to agriculture from invasive species. PNAS 113, 7575–7579. https://doi.org/10.1073/pnas.1602205113 (2016).
Molfini, M. et al. A preliminary prioritized list of Italian alien terrestrial invertebrate species. Biol. Invasions 22, 2385–2399. https://doi.org/10.1007/s10530-020-02274-w (2020).
Sweeney, J. et al. Special issue on invasive pests of forests and urban trees: pathways, early detection, and management. J. Pest Sci. 92, 1–2. https://doi.org/10.1007/s10340-018-01073-6 (2019).
Pace, R. et al. The bugs in the bags : The risk associated with the introduction of small quantities of fruit and plants by airline passengers. Insects 13, 617. https://doi.org/10.3390/insects13070617 (2022).
Nugnes, F., Russo, E., Viggiani, G. & Bernardo, U. First record of an invasive fruit fly belonging to Bactrocera dorsalis complex (Diptera: Tephritidae) in Europe. Insects 9, 182. https://doi.org/10.3390/insects9040182 (2018).
Bernardo, U. et al. Characterization, distribution, biology and impact on Italian walnut orchards of the invasive North-American leafminer Coptodisca lucifluella (Lepidoptera: Heliozelidae). Bull. Entomol. Res. 105, 210–224. https://doi.org/10.1017/S0007485314000947 (2015).
Saxena, R. C. & Barrion, A. A. Biotypes of insect pests of agricultural crops. Int. J. Trop. Insect Sci. 8, 453–458. https://doi.org/10.1017/s1742758400022475 (1987).
Bentur, J. S., Cheralu, C. & Rao, P. R. M. Monitoring virulence in Asian rice gall midge populations in India. Entomol. Exp. Appl. 129, 96–106. https://doi.org/10.1111/j.1570-7458.2008.00756.x (2008).
Lee, C. E. Evolutionary genetics of invasive species. Trends Ecol. Evol. 17, 386–391 (2002).
Prentis, P. J. et al. Adaptive evolution in invasive species. Trends Plant. Sci. 13, 288–294. https://doi.org/10.1016/j.tplants.2008.03.004 (2008).
Lack, J. B. et al. Comparative phylogeography of invasive Rattus rattus and Rattus norvegicus in the U.S. reveals distinct colonization histories and dispersal. Biol. Invasions 15, 1067–1087. https://doi.org/10.1007/s10530-012-0351-5 (2013).
Fišer Pečnikar, Ž. & Buzan EV. 20 years since the introduction of DNA barcoding: From theory to application. J. Appl. Genet. 55, 43–52, https://doi.org/10.1007/s13353-013-0180-y (2014).
Nugnes, F. et al. Genetic diversity of the invasive Gall Wasp Leptocybe invasa (Hymenoptera: Eulophidae) and of its Rickettsia endosymbiont, and associated sex-ratio differences. PLoS ONE 10, e0124660. https://doi.org/10.1371/journal.pone.0124660 (2015).
Nugnes, F., Bernardo, U. & Viggiani, G. An integrative approach to species discrimination in the Anagrus atomus group sensu stricto (Hymenoptera: Mymaridae), with a description of a new species. Syst. Biodivers. 15, 582–599. https://doi.org/10.1080/14772000.2017.1299811 (2017).
Packer, L., Gibbs, J., Sheffield, C. & Hanner, R. DNA barcoding and the mediocrity of morphology. Mol. Ecol. Resour. 9, 42–50. https://doi.org/10.1111/j.1755-0998.2009.02631.x (2009).
Dayrat, B. Towards integrative taxonomy. Biol. J. Linn. Soc. 85, 407–415 (2005).
Hebert, P. D. N. & Gregory, T. R. The promise of DNA barcoding for taxonomy. Syst. Biol. 54, 852–859. https://doi.org/10.1080/10635150500354886 (2005).
Faccoli, M., Simonato, M. & Rassati, D. Life history and geographical distribution of the walnut twig beetle, Pityophthorus juglandis (Coleoptera: Scolytinae), in southern Europe. J. Appl. Entomol. 140, 697–705. https://doi.org/10.1111/jen.12299 (2016).
Verheggen, F. et al. Walnut husk fly, Rhagoletis completa (Diptera: Tephritidae), invades Europe: Invasion potential and control strategies. Appl. Entomol. Zool. 52, 1–7. https://doi.org/10.1007/s13355-016-0459-7 (2017).
Gargiulo, S. et al. Insetti endemici e nuove invasioni: il complicato quadro dei fitofagi del noce. Entomata. 15, 73–83 (2021).
de Benedetta, F. et al. Carpophilus dimidiatus, nuova minaccia per la nocicoltura. Inf. Agr. 17, 57–59 (2020).
Dobson, R. M. The species of Carpophilus Stephens (Col. Nitidulidae) associated with stored products. Bull. Entomol. Res. 45, 389–402 (1954).
Audisio, P. Coleoptera: Nitidulidae – Kateretidae. Coleoptera Nitidulidaee Kateretidae Carpophilinae in Fauna d’Italia XXXII 226–269 (Calderini, 1993).
Powell, G. S., Cline, A. R., Duffy, A. G. & Zaspel, J. M. Phylogeny and reclassification of Carpophilinae (Coleoptera: Nitidulidae), with insights into the origins of anthophily. Zool. J. Linn. Soc. 189, 1359–1369. https://doi.org/10.1093/zoolinnean/zlaa001 (2020).
Bartelt, R. & Hossain, M. Chemical ecology of Carpophilus sap beetles (Coleoptera: Nitidulidae) and development of an environmentally friendly method of crop protection. Terr. Arthropod. Rev. 3, 29–61. https://doi.org/10.1163/187498310x489981 (2010).
Audisio, P. Fauna Europaea: Coleoptera, Carpophilinae, Carpophilus in Fauna Europaea version 2021.07 https://fauna-eu.org/ (2021).
Tremblay, E., Espinosa, B. & Baldini, C. Dannosità dei Carpofili (Coleoptera: Nitidulidae) alle pesche in Campania. Inf. Fitopatol. 34, 43–45 (1984).
Reales, N. et al. Morphological and molecular identification of Carpophilus dimidiatus (Coleoptera: Nitidulidae) associated with stored walnut in Northwestern Argentina. J. Stored Prod. Res. 76, 37–42. https://doi.org/10.1016/j.jspr.2017.12.002 (2018).
Hossain, M. Management of Carpophilus Beetle in Almonds. Hort Innovation - Final Report Project #A:1–93 (2018).
Powell, G. S. & Hamilton, M. L. Notes on the Carpophilus Stephens (Coleoptera: Nitidulidae) of Australia, with a new species from Victoria. Zootaxa 4701, 192–196. https://doi.org/10.1017/S0009840X0002730X (2019).
Boston, W., Leemon, D. & Cunningham, J. P. Virulence screen of Beauveria bassiana isolates for Australian Carpophilus (Coleoptera: Nitidulidae) beetle biocontrol. Agronomy 10, 1207. https://doi.org/10.3390/agronomy10081207 (2020).
Connell, W.A. A key to Carpophilus sap beetle associated with stored foods in the United States (Coleoptera: Nitidulidae). Department of Agriculture Cooperative Plant Pest Reports 23, 398–404 (1977).
Brown, S. D. J., Armstrong, K. F. & Cruickshank, R. H. Molecular phylogenetics of a South Pacific sap beetle species complex (Carpophilus spp., Coleoptera: Nitidulidae). Mol. Phylogenetics Evol. 64, 428–440. https://doi.org/10.1016/j.ympev.2012.04.018 (2012).
Leica Application Suite software version 3.8.0; Leica: Switzerland, (2011).
Murray, A. X. I. I. I. Monograph of the family of Nitidulariae. Trans. Linn. Soc. Lond. 24, 211–414. https://doi.org/10.1111/j.1096-3642.1863.tb00163.x (1864).
Gillogly, L. R. Insects of Micronesia Coleoptera: Nitidulidae*. Insects Micronesia 16, 133–188 (1962).
Connell, W.A. Sap Beetles (Nitidulidae, Coleoptera). in Insect and Mite pests in food, an illustrated key. 151–174 (1991).
DiLorenzo, C.L., Powell, G.S., Cline, A.R. & McHugh, J.V. Carpophiline-ID, a taxonomic web resource for the identification of Carpophilinae (Nitidulidae) of eastern North America. (2021a). https://site.caes.uga.edu/carpophiline-id/
DiLorenzo, C. L., Powell, G. S., Cline, A. R. & McHugh, J. V. Carpophiline-ID: An interactive matrix-based key to the Carpophiline sap beetles (Coleoptera, Nitidulidae) of Eastern North America. ZooKeys 1028, 85–93. https://doi.org/10.3897/zookeys.1024.59467 (2021).
Motschulsky, V. Insectes des Indes orientales. Etudes entomologiques 7, 20–122 (1858).
Fall, H. C. Miscellaneous notes and descriptions of North American Coleoptera. Am. Entomol. Soc. 36, 89–197 (1910).
Dobson, R. M. A new species of Carpophilus Stephens (Col. Nitidulidae) found on stored produce. Entomol’s Mon. Mag. 90, 299–300 (1954).
Connell, W. A. Carpophilus pilosellus Motschulsky, new synonymy and distribution (Coleoptera: Nitidulidae). Coleopt. Bull. 17, 89–90 (1963).
Kirejtshuk, A.G. Some results of study on the Nitidulidae from Namibia and adjacent territories. Part 1 Coleoptera, Cucujoidea, Nitidulidae. Mitteilungen aus dem Museum für Naturkunde in Berlin Zoologisches Museum und Institut für Spezielle Zoologie (Berlin) 72, 21–52, https://doi.org/10.1002/mmnz.19960720106 (1996).
Wang, D., Bai, X., Zhou, Y., Zhao, Y. Illustrated book of stored grain insects in China. 63–66 (China Press, 2008).
Brown, S.D.J. Molecular systematics and colour variation of Carpophilus species (Coleoptera: Nitidulidae) of the South Pacific. Dissertation, Lincoln University (2009).
Dasgupta, J., Pal, T. K. & Powell, G. S. Taxonomy of Carpophilinae (Coleoptera: Nitidulidae) from Tripura, India with a new species. Annal. Zool. 71, 627–649. https://doi.org/10.3161/00034541ANZ2021.71.3.003 (2021).
Gebiola, M. et al. Pnigalio agraules (Walker) and Pnigalio mediterraneus Ferrière and Delucchi (Hymenoptera: Eulophidae): Two closely related valid species. J. Nat. Hist. 43, 2465–2480. https://doi.org/10.1080/00222930903105088 (2009).
Folmer, R. H. A., Nilges, M., Folkers, P. J. M., Konings, R. N. H. & Hilbers, C. W. A model of the complex between single-stranded DNA and the single-stranded DNA binding protein encoded by gene V of filamentous bacteriophage M13. J. Mol. Biol. 240(4), 341–357 (1994).
Simon, C. et al. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87, 651–701. https://doi.org/10.1093/aesa/87.6.651 (1994).
Schulmeister, S., Wheeler, W. C. & Carpenter, J. M. Simultaneous analysis of the basal lineages of Hymenoptera (Insecta) using sensitivity analysis. Cladistics 18, 455–484. https://doi.org/10.1111/j.1096-0031.2002.tb00287.x (2002).
Ye, J. et al. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 13, 1–11. https://doi.org/10.1186/1471-2105-13-134 (2012).
Campbell, B. C., Steffen-Campbell, J. D. & Werren, J. H. Phylogeny of the Nasonia species complex (Hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28S rDNA sequences. Insect. Mol. Biol. 2, 225–237. https://doi.org/10.1111/j.1365-2583.1994.tb00142.x (1994).
Hall, T. A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033 (2014).
Edler, D., Klein, J., Antonelli, A. & Silvestro, D. RaxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 12, 373–377. https://doi.org/10.1111/2041-210X.13512 (2021).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61(3), 539–542 (2012).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 9, 772. https://doi.org/10.1038/nmeth.2109 (2012).
Lanfear, R., Calcott, B., Ho, S. Y. W. & Guindon, S. Partition Finder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 29, 1695–1701 (2012).
Rambaut, A., FigTree v1.4.2, A Graphical Viewer of Phylogenetic Trees. http://tree.bio.ed.ac.uk/software/figtree/ (2014).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. https://doi.org/10.1093/molbev/mst197 (2013).
Kingman, J. F. C. The coalescent. Stoch. Process. Appl. 13, 235–248. https://doi.org/10.1016/0304-4149(82)90011-4 (1982).
Austerlitz, F. et al. DNA barcode analysis: A comparison of phylogenetic and statistical classification methods. BMC Bioinform. 10, 1–13. https://doi.org/10.1186/1471-2105-10-S14-S10 (2009).
Grewe, P. M. et al. Mitochondrial DNA variation among lake trout (Salvelinus namaycush) strains stocked into Lake Ontario. Can. J. Fish. Aquat. Sci. 50, 2397–2403. https://doi.org/10.1139/f93-264 (1993).
Rossmo, D.K. Geographic profiling. CRC press, 1–378 (1999).
Le Comber, S. C. et al. Geographic profiling as a novel spatial tool for targeting infectious disease control. Int. J. Health Geogr. 10, 1–8. https://doi.org/10.1186/1476-072X-10-35 (2011).
Stevenson, M. D., Rossmo, D. K., Knell, R. J. & Le Comber, S. C. Geographic profiling as a novel spatial tool for targeting the control of invasive species. Ecography 35, 704–715. https://doi.org/10.1111/j.1600-0587.2011.07292.x (2012).
Gutiérrez, D. & Menéndez, R. Patterns in the distribution, abundance and body size of carabid beetles (Coleoptera: Caraboidea) in relation to dispersal ability. J. Biogeogr. 24, 903–914. https://doi.org/10.1046/j.1365-2699.1997.00144.x (1997).
Canter, D., Coffey, T., Huntley, M. & Missen, C. Predicting serial killers’ home base using a decision support system. J. Quant. Criminol. 16, 457–478. https://doi.org/10.1023/A:1007551316253 (2000).
Muirhead, J. R. et al. Modelling local and long-distance dispersal of invasive emerald ash borer Agrilus planipennis (Coleoptera) in North America. Divers. Distrib. 12, 71–79. https://doi.org/10.1111/j.1366-9516.2006.00218.x (2006).
Marchioro, M. & Faccoli, M. Dispersal and colonization risk of the walnut twig beetle, Pityophthorus juglandis, in southern Europe. J. Pest Sci. 95, 303–313. https://doi.org/10.1007/s10340-021-01372-5 (2022).
Meurisse, N. & Pawson, S. Quantifying dispersal of a non-aggressive saprophytic bark beetle. PLoS ONE 12, 1–24. https://doi.org/10.1371/journal.pone.0174111 (2017).
Papini, A. et al. The use of jackknifing for the evaluation of geographic profiling reliability. Ecol. Inform. 38, 76–81. https://doi.org/10.1016/j.ecoinf.2017.02.001 (2017).
Statgraphics Plus Version 3.0; Manugistics: Rockville, MD, USA, (1997).
Bagnaia, R. et al. Carta della Natura della Regione Campania: Carta degli habitat alla scala 1:25.000. ISPRA (2017).
Martoni, F., Piper, A. M., Rodoni, B. C. & Blacket, M. J. Disentangling bias for non-destructive insect metabarcoding. PeerJ 10, e12981. https://doi.org/10.7717/peerj.12981 (2022).
Jelinek, J. & Audisio, P. Elateroidea, Derodontoidea, Bostrichoidea, Lymexyloidea, Cleroidea and Cucujoidea in Catalogue of Palaearctic Coleoptera. 459–490 (Apollo Books, 2007)
Mbenoun, M., Garnas, J. R., Wingfield, M. J., Begoude Boyogueno, A. D. & Roux, J. Metacommunity analyses of Ceratocystidaceae fungi across heterogeneous African savanna landscapes. Fungal Ecol. 28, 76–85. https://doi.org/10.1016/j.funeco.2016.09.007 (2017).
Norris, L. C. & Norris, D. E. Phylogeny of anopheline (Diptera: Culicidae) species in southern Africa, based on nuclear and mitochondrial genes. J. Vector Ecol. 40, 16–27. https://doi.org/10.1111/jvec.12128 (2015).
Bernardo, U. et al. A new gall midge species of Asphondylia (Diptera: Cecidomyiidae) inducing flower galls on Clinopodium nepeta (Lamiaceae) from Europe, its phenology, and associated fungi. Environ. Entomol. 47, 609–622. https://doi.org/10.1093/ee/nvy028 (2018).
Bernardo, U. et al. An integrative study on Asphondylia spp. (Diptera: Cecidomyiidae), causing flower galls on Lamiaceae, with description, phenology, and associated fungi of two new species. Insetcs 12, 958. https://doi.org/10.3390/insects12110958 (2021).
Wacławik, B. et al. An integrative revision of the subgenus Liophloeodes (Coleoptera: Curculionidae: Entiminae: Polydrusini): taxonomic, systematic, biogeographic and evolutionary insights. Arthropod Syst. Phylogeny. 79, 419–441. https://doi.org/10.3897/asp.79.e64252 (2021).
Colautti, R. I. & MacIsaac, H. J. A neutral terminology to define ‘invasive’ species. Divers Distrib. 10, 135–141 (2004).
Crooks, J. A. Lag times and exotic species: The ecology and management of biological invasions in slow-motion. Ecoscience 12, 316–329. https://doi.org/10.2980/i1195-6860-12-3-316.1 (2005).
Jelínek, J. et al. Epuraea imperialis (Reitter, 1877). New invasive species of Nitidulidae (Coleoptera) in Europe, with a checklist of Sap Beetles introduced to Europe and Mediterranean areas. APP | Physical, Math. Nat. Sci. Accademia Peloritana dei Pericolanti, 94, 1–24, https://doi.org/10.1478/AAPP.942A4 (2016).
Benchi, D., Conelli, L. & Bernardo, U. L. mosca delle noci minaccia le produzioni campane. Inf Agr. 66, 74–76 (2010).
Pollini, A. Entomologia Applicata. (Edagricole, 2013).
Van Steenwyk, R.A. et al. Walnut husk fly control with reduced risk insecticides. Acta Hortic 861, 375–382, https://doi.org/10.17660/ActaHortic.2010.861.5 (2010).
EU (2019). Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019. Off. j. Eur. Union, Legis., L 319/1, 1–279. Retrieved from https://eur-lex.europa.eu/eli/reg_impl/2019/2072/oj
Russo, E. et al. Biological and molecular characterization of Aromia bungii (Faldermann, 1835) (Coleoptera: Cerambycidae), an emerging pest of stone fruits in Europe. Sci. Rep. 10, 1–10. https://doi.org/10.1038/s41598-020-63959-9 (2020).
Hsu, F. et al. Introduction of a non-native lineage is linked to the recent black cocoa ant, Dolichoderus thoracicus (Smith, 1860), outbreaks in Taiwan. Taiwania 67, 271–279. https://doi.org/10.6165/tai.2022.67.271 (2022).
Porter, J. Some studies on the life history and oviposition of Carpophilus dimidiatus (F.) (Coleoptera: Nitidulidae) at various temperatures and humidities. J. Stored Prod. Res. 22, 135–139. https://doi.org/10.1016/0022-474X(86)90006-8 (1986).
Potter, M. A. et al. A survey of sap beetles (Coleoptera: Nitidulidae) in strawberry fields in West Central Florida. Fla. Entomol. 96, 1188–1189. https://doi.org/10.1653/024.096.0363 (2013).
Burks, C.S., Yasin, M., El-Shafie, H.A.F. & Wakil, W. Pests of stored dates in Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges (eds Wakil, W., Romeno Faleiro J., Miller, T.A.) 237–286 (Springer, Zürich, Switzerland, 2015). https://doi.org/10.1007/978-3-319-24397-9
Akşit, T., Özsemerci, F. & Çakmak, İ. Studies on determination of harmful fauna in the fig orchards in Aydin province (Turkey). Türkiye Entomoloji Dergisi 27, 181–189 (2003).
Acknowledgements
The authors thank Marco Gebiola for critically reviewing the manuscript draft and Roberta Ascolese and Roberta Pace for their valuable help during the sampling activities; Carmela Carbone e Feliciana Pica for their support during reviewing activity and graphic designing. We thank the owners of the orchards and warehouses for allowing us to collect the walnuts, and to phytosanitary inspectors for their valuable assistance. Lastly, we thank two anonymous reviewers for their suggestions that improved our manuscript. A special thanks to Campania Region for funding this work with the project URCoFi (Unità Regionale di Coordinamento Fitosanitario—Strengthening of the supervision activities and control of pests).
Funding
This research was supported by the Campania Region-funded URCoFi project (Unità Regionale di Coordinamento Fitosanitario—Strengthening of the supervision activities and control of pests).
Author information
Authors and Affiliations
Contributions
Conceptualization: U.B.; methodology: F.de.B., S.G., and F.N.; formal analysis: F.de.B., F.N., P.A., M.I., and U.B.; investigation: F.de.B., F.M., S.G., L.F., and F.N.; resources: F.N. and U.B.; data curation: F.de.B., F.N., P.A., M.I., F.M., L.F., S.G., and U.B.; writing—original draft preparation: F.de.B., M.I., F.N., and U.B.; writing—review and editing: F.de.B., S.G., F.N., M.I., P.A., U.B.; supervision: U.B. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Benedetta, F., Gargiulo, S., Miele, F. et al. The spread of Carpophilus truncatus is on the razor's edge between an outbreak and a pest invasion. Sci Rep 12, 18841 (2022). https://doi.org/10.1038/s41598-022-23520-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23520-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.