Abstract
The use of extended antibiotic (EA) prophylaxis (> 24 h) remains controversial in aseptic revision arthroplasty. We sought to determine whether EA prophylaxis reduces the risk of periprosthetic joint infection (PJI) in aseptic revision hip and knee arthroplasty. A total of 2800 patients undergoing aseptic revision hip and knee arthroplasty at five institutional databases from 2008 to 2017 were evaluated. One to two nearest-neighbor propensity score matching analysis was conducted between patients who did and did not receive extended antibiotic prophylaxis. The matching elements included age, sex, body mass index, Charlson comorbidity index, hospital distribution, year of surgery, joint (hip or knee), surgical time, CRP, preoperative hemoglobin, albumin, and length of stay. The primary outcome was the development of PJI, which was assessed at 30 days, 90 days, and 1 year following revision and analyzed separately. A total of 2467 (88%) patients received EA prophylaxis, and 333 (12%) patients received standard antibiotic (SA) prophylaxis (≤ 24 h). In the propensity-matched analysis, there was no difference between patients who received EA prophylaxis and those who did not in terms of 30-day PJI (0.3% vs. 0.3%, p = 1.00), 90-day PJI (1.7% vs. 2.1%, p = 0.62) and 1- year PJI (3.8% vs. 6.0%, p = 0.109). For revision hip, the incidence of PJI was 0.2% vs 0% at 30 days (p = 0.482), 1.6% vs 1.4% at 90 days (p = 0.837), and 3.4% vs 5.1% at 1 year (p = 0.305) in the EA and SA group. For revision knee, the incidence of PJI was 0.4% vs 0.9% at 30 days (p = 0.63), 1.8% vs 3.4% at 90 days (p = 0.331), and 4.4% vs 7.8% at 1 year (p = 0.203) in the EA and SA group. A post hoc power analysis revealed an adequate sample size with a beta value of 83%. In addition, the risks of Clostridium difficile and resistant organism infection were not increased. This multi-institutional study demonstrated no difference in the rate of PJIs between patients who received extended antibiotic prophylaxis and those who did not in aseptic revision arthroplasty. The risk of C. difficile and resistant organism infection was not increased with prolonged antibiotic use.
Similar content being viewed by others
Introduction
The use of perioperative antibiotics remains one of the important practices for reducing the rate of infection following total joint arthroplasty. In primary TJA, antibiotic prophylaxis is often administered for a maximum of 24 h, as this was the maximum time allowed by previous guidelines1,2. Some literature and recent guidelines even suggest one dose of preoperative antibiotic prophylaxis before incision without any additional antibiotic prophylaxis following primary TJA postoperatively3,4,5,6. However, in the revision setting, the risk of infection is substantially higher than during primary TJA, with rates reported as high as 7% in most literature7,8. This is often because of longer operative time, more complex nature, and the unexpected positive intraoperative cultures of the revision surgery compared to primary TJA9. Therefore, up to 60% of orthopedic surgeons continue antibiotic prophylaxis more than 24 h in their practice in a recent survey10.
The use of extended prophylactic antibiotics therapy in primary total hip (THA) and knee arthroplasty (TKA) has shown no decrease in the rate of periprosthetic joint infection (PJI) compared to those who received prophylaxis for 24 h11,12,13. However, in the revision TJA setting, the use of extended prophylactic antibiotics therapy has demonstrated conflicting results in the literature14,15,16,17,18. While some have found that prolonging antibiotic prophylaxis is a protective effect against PJI14,18, other studies found no association regarding a reduction in PJI15,16,17. Furthermore, the 2018 International Consensus Meeting on Musculoskeletal Infection stated that the evidence behind extended prophylactic antibiotics therapy for patients undergoing aseptic revision is limited2. Currently, we are aware of no strong evidence to suggest that extended antibiotic prophylaxis more than 24 h is beneficial for aseptic revision TJA. In addition to the conflicting literature regarding the benefits of extended antibiotics14,15,16,17, there is limited literature regarding the antibiotic stewardship implications and possible increase in antibiotic-related side effects such as the development of Clostridium difficile infection1 and resistant organisms19.
The aim of this study was to evaluate the rate of PJI and antibiotic-related complications with the administration of extended antibiotic prophylaxis (> 24 h) compared to standard antibiotic prophylaxis (≤ 24 h) following aseptic revision hip and knee arthroplasty.
Materials and methods
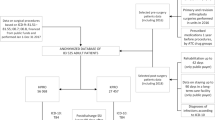
This study was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital, Taiwan (IRB No. 201901016B0C601) and carried out in accordance with the pertinent guidelines and regulations. Following IRB approval of the waiver for informed consent, we retrospectively reviewed the five institutional standardized electronic medical records from Chang Gung Research Database (CGRD)20,21 for a consecutive series of revision TJA (hip and knee) between 2008 and 2017 in Taiwan. Using the International Classification of Diseases, Ninth Revision, and 10th Revision (ICD-9 and ICD-10) procedure codes, electronic medical and operative notes were queried to identify aseptic revision TJA for a non-infective indication (loosening, polyethylene wear, tibia-femoral instability, stiffness, malrotation, dislocation, osteolysis, and periprosthetic fracture). Patients, who were younger than 18 years, underwent septic revision (ICD-9 code: 996.66 and 99667; ICD-10: T84.50XA, T84.60XA, and T84.7XXA), less than 1 -year follow up after index revision surgery, and those with one or more positive intraoperative culture during revision, and those without documented post-operative antibiotic duration, were excluded.
A total of 2800 aseptic revision cases were identified for analysis. Of those, 333 (12%) received standard antibiotic prophylaxis (≤ 24 h) and 2467 (88%) received extended antibiotic prophylaxis (> 24 h). Patient demographic factors (age, sex, body mass index [BMI]), comorbidities (diabetes mellitus, rheumatoid arthritis, renal failure and Charlson comorbidity index [CCI]), hospital distribution, joint (hip or knee), year of procedure, American Society of Anesthesiologists (ASA), the use of antimicrobial incise drape, component revision (total, partial, or linear exchange), surgeon volume (high or low), surgical time, blood transfusion, and length of stay (LOS) were queried. Preoperative lab data, including erythrocyte sedimentation (ESR), C-reactive protein (CRP), hemoglobin, platelet, creatine, and albumin were also queried within 2 weeks before revision surgery. Serological markers were obtained in all revisions followed by aspiration if ESR and/or CRP were high. The infection organisms were also recorded from cultures taken preoperatively and intraoperatively. Because the cutoffs for revision TJA volume are not defined in the literature, we considered low volume as less than 1 revision case per month and high volume as 1 or more cases per month.
Antibiotic regimen
All patients who underwent aseptic revision hip and knee arthroplasty received standard antibiotic prophylaxis protocol according to the respective policy of the institution15. Briefly, one gram of first-generation cephalosporin was prescribed within 1 h of skin incision. If patients exceeded 80 kg, 2 g of preoperative antibiotics were given. The antibiotics were re-dosed when the surgical time was longer than 4 h or if intraoperative blood loss exceeded 1500 ml. For patients who demonstrated an anaphylactic allergy to cephalosporin or penicillin, 1 g of clindamycin or vancomycin was administered. After aseptic revision surgery, patients received the standard antibiotic prophylaxis for an overall duration of 24 h. However, the extension of post-operative antibiotic prophylaxis for more than 24 h was based on the surgeon's discretion.
Outcome assessment
The primary outcome was the development of PJI at the following time points: within 30 days, 90 days, and 1 year after index aseptic revision surgery. PJI was defined using the definition from the 2013 ICM22. The operative reports were then manually reviewed to confirm that surgery had been performed for PJI. The secondary outcome was the occurrence of resistant organisms and C. difficile infection.
Statistical analysis
Statistical analysis was performed for continuous variables using Student t-tests and categorical variables were analyzed using the chi-square test or Fisher’s exact test. Missing data were filled by five imputations using multiple imputations by chained equations23. To provide the association of extended prophylactic antibiotic prophylaxis and PJI, covariates associated with PJI at p < 0.2 in the univariate analysis were added to a second multivariate logistic regression model. To mitigate the baseline differences between the two groups, a 1:2 ratio propensity score matching (PSM) technique was performed. We fitted a logistic regression model to estimate the propensity score using the following variables: age, sex, BMI, CCI, hospital distribution, year of surgery, joint (hip or knee), surgical time, CRP, and preoperative Hb, albumin and LOS. A nearest-neighbor matching procedure was applied, with the restriction of a caliper width of 0.25 units of each other. The difference of primary outcome between the matched group was calculated with conditional logistic regression. Post hoc power analysis using the difference between two dependent means (matched pairs) was performed to compare the PJI at 1 year in the matched group to determine the likelihood of a type 2 error (missing a significant difference between the standard and extended antibiotic groups when one in fact exists). Based on current information (beta = 0.83), the sample size was adequately powered at 83% to detect no difference between the treatment groups. A forest plot presented an adjusted odds ratio (OR) with 95% confidence interval (CI). The Kaplan–Meier survival curve was analyzed to calculate the cumulative incidence of PJI in two groups of the matched cohort. The group difference was assessed using the log-rank test. A p-value < 0.05 was considered statistically significant. All analyses were assessed using SPSS Statistics (version 22, IBM, Armonk, New York) and NCSS Statistical Software (version 10, NCSS, LLC, Kaysville, UT).
Result
The mean duration of antibiotic use was 3.9 ± 1.8 days (median 3.6 days; range 1.3–10.8) in the extended antibiotic (EA) group. Compared to patients in the standard antibiotic (SA) prophylaxis group, those with EA prophylaxis were hip predominant (71% vs. 65%, p = 0.040) and with a longer surgical time (3.3 \(\pm \) 1.2 h vs. 3.0 \(\pm \) 1.2 h, p < 0.001), and longer LOS (8.6 ± 6.2 days vs. 7.4 ± 4.7 days, p < 0.001). Meanwhile, patients in the EA prophylaxis presented a higher preoperative lab data, including CRP (9.7 ± 22.1 mg/L vs.8.4 ± 21.9 mg/L, p = 0.004), Hb (12.7 ± 1.9 g/dL vs. 12.4 ± 2.1, p = 0.007) and albumin (4.2 ± 0.5 g/dL vs. 4.1 ± 0.5 g/dL, p = 0.011). However, patients in the EA prophylaxis possessed a lower CCI (1.67 ± 2.19 vs. 1.83 ± 2.23, p = 0.035). After successful matching, CCI, joint, preoperative lab data (CRP, Hb, albumin), and LOS were similar between SA and EA cohorts (Table 1).
Overall, the incidence of PJI was 0.6% vs 0.3% at 30 days (p = 0.711), 1.7% vs 2.1% at 90 days (p = 0.657), and 3.7% vs 6.0% at 1 year (p = 0.051) in the EA and SA group (Fig. 1). In the matched cohorts, there continued to demonstrate no differences between the two groups with respect to PJI at 30 days (0.3% vs. 0.3%, p = 1.00), 90 days (1.7% vs. 2.1%, p = 0.62) and 1 year (3.8% vs. 6.0%, p = 0.109). The cumulative incidence of PJI was similar between the two groups (Fig. 2a). When stratified by hips and knees, the cumulative incidence of PJI remained with no statistical difference between the EA and SA groups (Figs. 2b,c). A sub-analysis demonstrated that the cause for aseptic revision was not associated with 1-year PJI rate between the EA and SA group (Table 2).
After controlling for potential confounders, there was no significant difference in 30-day PJI rate (adjusted OR 2.71, 95% CI 0.32–21.1), 90-day PJI (adjusted OR 1.02, 95% CI 0.42–2.48), and 1-year PJI (adjusted OR 0.69, 95% CI 0.40–1.17) between the EA and SA group. Results from a regression analysis of the PSM cohort showed no significant association between 30-day PJI and EA prophylaxis (adjusted OR 1.01, 95% CI 0.08–12.82). The 90-day PJI (adjusted OR 1.01, 95% CI 0.36–2.85) and 1-year PJI (adjusted OR 0.67, 95% CI 0.35–1.29) risk remained non-significant in patients with EA prophylaxis (Fig. 3).
Furthermore, when stratified by hips and knees in the unmatched cohort, there was no significant difference in any PJI rate between the EA and SA groups. For revision hip, the incidence of PJI was 0.6% vs 0% at 30 days (p = 0.62), 1.5% vs 1.4% at 90 days (p = 1.0), and 3.2% vs 5.1% at 1 year (p = 0.16) in the EA and SA group. For revision knee, the incidence of PJI was 0.7% vs 0.9% at 30 days (p = 0.59), 2.3% vs 3.4% at 90 days (p = 0.52), and 5.0% vs 7.8% at 1 year (p = 0.26) in the EA and SA group. When stratified by hips and knees in the matched cohort, the incidence of PJI remained with no statistical difference between the two groups (Fig. 4). Compared with the SA prophylaxis, the use of EA prophylaxis did not increase or decrease the incidence of any PJI rates in revision hip or revision knee at the unmatched and matched cohort after controlling for confounding variables (Figs. 5, 6).
Multivariable logistic regression results among the unmatched and propensity score–matched cohort for 30-day, 90-day and 1-year periprosthetic joint infection (PJI), stratified by hip. Adjusted OR and 95% CI for 30-day PJI could not be estimated because there was zero event with 30-day PJI in the ≤ 24 h group.
In patients who developed PJI within 1 year, there were no significant differences in the infecting organisms between the 2 groups (Table 3). The incidence of resistant organisms was 4% in the EA group and 5% in the SA group (p = 1.000). Six patients (0.24%) in EA group and 1 patient (0.30%) in the SA group developed C. difficile infection. The incidence of C. difficile infection was not significantly different between the two groups (p = 0.588).
Discussion
The results of the present study demonstrate no difference in 30-day, 90-day, and 1-year PJI between patients who received extended antibiotic prophylaxis and those who received routine antibiotic prophylaxis (maximum 24 h). This was present even after controlling for potential confounders with multivariate logistic analysis and PSM. Among those who received extended antibiotic prophylaxis, we found no increased rates of C. difficile infection and no differences in the infecting organisms, including resistant organisms. There is limited evidence to support extended antibiotic prophylaxis for patients undergoing aseptic revision hip and knee surgery in the published literature. Our multicenter study, with a larger sample size, statistical methodology, and adequate follow-up, improves the evidence on the previous literature.
The current literature is mixed regarding the utility of extended antibiotic prophylaxis for reducing PJI in the setting of aseptic revision TJA. Claret et al. reported prolonged post-operative antibiotic treatment up to 5 days was marginal significantly associated with a lower rate of 90-day PJI (2.2% versus 6.9%, p = 0.049) after aseptic revision knee arthroplasty14. Bukowski et al. demonstrated that extended antibiotic prophylaxis with a mean duration of 11 days was associated with a sevenfold decreased risk of any infection at 90 days compared to those without extended antibiotic prophylaxis after aseptic revision TKA (0.75% vs. 2.04%, p = 0.14)18. However, studies from Kuo et al. demonstrated that extended antibiotic prophylaxis have shown no added benefit on the risk reduction of 5- year PJI in aseptic revision knee (1.1% versus 3.9%, p = 0.14)15 and 1-year PJI in aseptic revision hip (4.8% versus 2.4%, p = 0.293)16. Recently, Villa et al. reported the incidences of PJI rate were not significantly different in the EA group (2.2%) versus in the SA group (3.5%) after aseptic total hip or knee arthroplasty revisions in a minimum follow-up of 1 year among 86% of their cases17. Our study evaluated three different time points (30 days, 90 days and 1 year) of PJI and revealed that EA prophylaxis did not positively affect any evaluation time point compared to those with SA prophylaxis. Although a longer follow-up is better to avoid bias in the results, prior studies have shown that 1-year follow-up is reliable because most subsequent infections occur within 1 year after revision arthroplasty8,24. Besides, the effect of reduction of PJI with extended antibiotics prophylaxis did not continue at 1 year and 5 years18. Based on these reasons, we did not follow up on these patients for more than 2 years after aseptic revision.
In the primary TJA literature, recent literature suggests that extended antibiotic prophylaxis provides some benefit. Inabathula et al. demonstrated that patients with 1 week of oral antibiotics were 5 times and 4 times less likely to develop 90-day PJI in high-risk patients than those who did not take antibiotics postoperatively in primary total knee arthroplasty and total hip arthroplasty, respectively25. Furthermore, after two-stage exchange arthroplasty for PJI, chronic suppression with extended antibiotic prophylaxis has demonstrated a clinically meaningful reduction in the infection and treatment failure rate26,27,28,29. However, we could not demonstrate the utility of extended antibiotic prophylaxis in aseptic revision TJA, unlike the studies in high-risk primary TJA or two-stage exchange arthroplasty. Studies have demonstrated an unexpected positive intraoperative culture (PIOC) is associated with the risk of subsequent PJI in the presumed aseptic knee and hip revisions30,31,32. The prevalence of unexpected PIOC in presumed aseptic revision ranges from 7.9 to 28%9,31,32,33, leading to the duration of extended antibiotics. In a large multicenter cohort evaluating the outcome of PIOC, the failure rate was 8.4% after an antimicrobial treatment for a median time of 56 days (IQR 42–90)34. The duration of antibiotic use for PJI treatment usually takes 6–12 weeks35. However, our study initially excluded patients with one or more positive intraoperative cultures during revision and the mean duration of antibiotic use was 3.9 ± 1.8 days (median 3.6 days; range 1.3–10.8) in the EA group. Therefore, we could not evaluate the extended antibiotics' role in patients with PIOC.
Long-term antibiotics utilization increases antibiotic resistance and Clostridium difficile infection36. The incidence of C. difficile infection is estimated to range from 0.4% after revision TKA37 to 1.7% after revision THA38. The incidence rates of C. difficile infection increase gradually from 1.6 to 2.1 times following 1–3 to 7–11 days of antibiotic exposure compared to those without prior antibiotic exposure39. The overall incidence of C. difficile infection in our study was 0.25%, which was similar to prior studies37,38. Although C. difficile infection is an uncommon complication following revision TJA, it can increase hospital stay, costs, and in-hospital mortality following revision surgery38. Surgeons should pay attention to high-risk patients and manage perioperative antibiotics judiciously.
Our study has several limitations that should be considered. First, the study was retrospective and non-randomized, which introduced the possibility of selection bias. For example, a higher CRP level was observed in the EA group, and surgeons may lead towards extended antibiotic use after revision surgery. Besides, CCI was calculated as a numerical variable based on the ICD diagnosis codes to represent comorbidity. Therefore, a lower CCI distribution was found in the EA group. Given that the duration of antibiotics administered was based on the surgeon's discretion, the comorbidities and complexity of the surgery may have influenced this decision. However, we utilized propensity-score matching analysis and multiple logistic regression to control for possible confounding factors. Besides, a post hoc power analysis revealed a beta value of 83%, indicating the sample size is adequate, and the likelihood of a type 2 error is low. Moreover, the reason for a surgeon selecting extended antibiotic prophylaxis was not recorded. Second, we could not control for factors that may have influenced the use of extended antibiotic prophylaxis, such as a positive nasal colonization screening for methicillin-resistant Staphylococcus aureus. Third, our database did not have socioeconomic status and education level, which has shown to be associated with PJI. Finally, we did not perform synovial fluid and histopathologic analysis during the revision in this study, and the diagnosis of aseptic revision was using ICD code rather than standard criteria (like ICM). This could under-diagnose septic revision in presumed aseptic revision and affect the antibiotic duration and the outcome.
Conclusion
Extended antibiotic prophylaxis may not reduce the PJI rate up to 1 year following aseptic revision TJA. Efforts are needed with evidence-based approaches regarding the duration of antibiotic prophylaxis. This supports current and previous guidelines that do not recommend extended antibiotic prophylaxis.
Data availability
All data generated or analysed during this study are included in this published article.
References
Anderson, D. J. et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect. Control Hosp. Epidemiol. 35, 605–627 (2014).
Aboltins, C. A. et al. Hip and knee section, prevention, antimicrobials (systemic): Proceedings of international consensus on orthopedic infections. J. Arthroplasty 34, S279–S288 (2019).
Allegranzi, B. et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 16, e288–e303 (2016).
Berríos-Torres, S. I. et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 152, 784–791 (2017).
Tan, T. L. et al. Perioperative antibiotic prophylaxis in total joint arthroplasty: A single dose is as effective as multiple doses. J. Bone Jt. Surg. Am. 101, 429–437 (2019).
Zastrow, R. K. et al. Characteristics of antibiotic prophylaxis and risk of surgical site infections in primary total hip and knee arthroplasty. J. Arthroplasty 35, 2581–2589 (2020).
Suarez, J. et al. Why do revision knee arthroplasties fail?. J. Arthroplasty 23, 99–103 (2008).
Badarudeen, S. et al. Complications after revision total hip arthroplasty in the medicare population. J. Arthroplasty 32, 1954–1958 (2017).
Neufeld, M. E. et al. The prevalence and outcomes of unexpected positive intraoperative cultures in presumed aseptic revision knee arthroplasty. J. Arthroplasty https://doi.org/10.1016/j.arth.2022.05.036 (2022).
de Beer, J. et al. Antibiotic prophylaxis for total joint replacement surgery: Results of a survey of Canadian orthopedic surgeons. Can. J. Surg. J. Can. Chir. 52, E229-234 (2009).
Nelson, C. L., Green, T. G., Porter, R. A. & Warren, R. D. One day versus seven days of preventive antibiotic therapy in orthopedic surgery. Clin. Orthop. 176, 258–263 (1983).
Williams, D. N. & Gustilo, R. B. The use of preventive antibiotics in orthopaedic surgery. Clin. Orthop. 190, 83–88 (1984).
Hansen, E. et al. Perioperative antibiotics. J. Arthroplasty 29, 29–48 (2014).
Claret, G. et al. A prolonged post-operative antibiotic regimen reduced the rate of prosthetic joint infection after aseptic revision knee arthroplasty. Surg. Infect. 16, 775–780 (2015).
Kuo, F.-C. et al. Extended postoperative prophylactic antibiotics with first-generation cephalosporin do not reduce the risk of periprosthetic joint infection following aseptic revision total knee arthroplasty. J. Knee Surg. https://doi.org/10.1055/s-0039-1683889 (2019).
Kuo, F.-C. et al. Extended antibiotic prophylaxis confers no benefit following aseptic revision total hip arthroplasty: A matched case-controlled study. J. Arthroplasty 34, 2724–2729 (2019).
Villa, J. M., Pannu, T. S., Braaksma, W., Higuera, C. A. & Riesgo, A. M. Extended oral antibiotic prophylaxis after aseptic total hip or knee arthroplasty revisions: A preliminary report. J. Arthroplasty https://doi.org/10.1016/j.arth.2022.08.003 (2022).
Bukowski, B. R. et al. Extended oral antibiotic prophylaxis after aseptic revision TKA: Does it decrease infection risk?. J. Arthroplasty 37, S997-S1003.e1 (2022).
Bryson, D. J. et al. Antibiotic prophylaxis in orthopaedic surgery: Difficult decisions in an era of evolving antibiotic resistance. Bone Jt. J. 98, 1014–1019 (2016).
Tsai, M.-S. et al. Chang gung research database: A multi-institutional database consisting of original medical records. Biomed. J. 40, 263–269 (2017).
Shao, S.-C. et al. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 28, 593–600 (2019).
Parvizi, J., Gehrke, T. & Chen, A. F. Proceedings of the international consensus on periprosthetic joint infection. Bone Jt. J. 95-B, 1450–1452 (2013).
Azur, M. J., Stuart, E. A., Frangakis, C. & Leaf, P. J. Multiple imputation by chained equations: What is it and how does it work?. Int. J. Methods Psychiatr. Res. 20, 40–49 (2011).
Xu, C., Tan, T. L., Li, W. T., Goswami, K. & Parvizi, J. Reporting outcomes of treatment for periprosthetic joint infection of the knee and hip together with a minimum 1-year follow-up is reliable. J. Arthroplasty 35, 1906-1911.e5 (2020).
Inabathula, A. et al. Extended oral antibiotic prophylaxis in high-risk patients substantially reduces primary total hip and knee arthroplasty 90-day infection rate. J. Bone Jt. Surg. Am. 100, 2103–2109 (2018).
Johnson, A. J. et al. Reduced re-infection rates with postoperative oral antibiotics after two-stage revision hip arthroplasty. BMC Musculoskelet. Disord. 14, 123 (2013).
Siqueira, M. B. P. et al. Chronic suppression of periprosthetic joint infections with oral antibiotics increases infection-free survivorship. J. Bone Jt. Surg. Am. 97, 1220–1232 (2015).
Frank, J. M. et al. The Mark Coventry, MD, Award: Oral antibiotics reduce reinfection after two-stage exchange: A multicenter, randomized controlled trial. Clin. Orthop. 475, 56–61 (2017).
Yang, J. et al. 2020 Mark Coventry Award: Microorganism-directed oral antibiotics reduce the rate of failure due to further infection after two-stage revision hip or knee arthroplasty for chronic infection: A multicentre randomized controlled trial at a minimum of two years. Bone Jt. J. 102, 3–9 (2020).
Parvizi, J., Suh, D.-H., Jafari, S. M., Mullan, A. & Purtill, J. J. Aseptic loosening of total hip arthroplasty: Infection always should be ruled out. Clin. Orthop. 469, 1401–1405 (2011).
Saleh, A. et al. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J. Arthroplasty 29, 2181–2186 (2014).
Jacobs, A. M. E., Bénard, M., Meis, J. F., van Hellemondt, G. & Goosen, J. H. M. The unsuspected prosthetic joint infection: Incidence and consequences of positive intra-operative cultures in presumed aseptic knee and hip revisions. Bone Jt. J. 99-B, 1482–1489 (2017).
Hipfl, C., Mooij, W., Perka, C., Hardt, S. & Wassilew, G. I. Unexpected low-grade infections in revision hip arthroplasty for aseptic loosening: A single-institution experience of 274 hips. Bone Jt. J. 103-B, 1070–1077 (2021).
Mancheño-Losa, M. et al. Prognosis of unexpected positive intraoperative cultures in arthroplasty revision: A large multicenter cohort. J. Infect. 83, 542–549 (2021).
Di Matteo, B. & Marcacci, M. Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N. Engl. J. Med. 386, 1001–1002 (2022).
Hong, C. S., Black, C. S., Ryan, S. P. & Seyler, T. M. Extended oral antibiotics and infection prophylaxis after a primary or revision total knee arthroplasty. J. Knee Surg. 33, 111–118 (2020).
Curtis, G. L. et al. Clostridium difficile colitis following revision total knee arthroplasty: Incidence and risk factors. J. Arthroplasty 34, 2785–2788 (2019).
Delanois, R. E. et al. Risk factors and costs associated with clostridium difficile colitis in patients with prosthetic joint infection undergoing revision total hip arthroplasty. J. Arthroplasty 33, 1534–1538 (2018).
Brown, K. A., Fisman, D. N., Moineddin, R. & Daneman, N. The magnitude and duration of Clostridium difficile infection risk associated with antibiotic therapy: A hospital cohort study. PLoS One 9, e105454 (2014).
Acknowledgements
We acknowledge and thank the Biostatistics Center at Chang Gung Memorial Hospital for their statistical work. This study is based in part on data from the Chang Gung Research Database provided by the Chang Gung Memorial Hospital (Grant number: CFRPG8J0161). The interpretation and conclusions contained herein do not represent the presentation of Chang Gung Memorial Hospital.
Author information
Authors and Affiliations
Contributions
F.-C.K. participated in the study with the primary responsibility in conception and design drafting, reference search, data analysis and writing of the manuscript. Y.-H.C. and T.-W.H. participated in the study with the primary responsibility in data collection. D.W.-C.C. and T.L.T. participated in the study with the primary responsibility in data statistical analysis. M.S.L. participated in the study with the primary responsibility in reference search, literature review, and final proof of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuo, FC., Chang, YH., Huang, TW. et al. Post-operative prophylactic antibiotics in aseptic revision hip and knee arthroplasty: a propensity score matching analysis. Sci Rep 12, 18319 (2022). https://doi.org/10.1038/s41598-022-23129-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23129-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.