Abstract
In non-anuric patients undergoing peritoneal dialysis (PD), residual kidney function (RKF) is a main contributor to fluid and solute removal and an independent predictor of survival. We investigated if urine volume could be used to estimate renal clearances and removal of urea, creatinine, and phosphorus in PD patients. The observational, cross-sectional study included 93 non-anuric prevalent PD patients undergoing continuous ambulatory PD (CAPD; n = 34) or automated PD (APD; n = 59). Concentrations of urea, creatinine and phosphorus in serum and in 24-h collections of urine volume were measured to calculate weekly residual renal clearance (L/week) and removed solute mass (g/week). Median [interquartile range], 24-h urine output was 560 [330–950] mL and measured GFR (the mean of creatinine and urea clearances) was 3.24 [1.47–5.67] mL/min. For urea, creatinine and phosphorus, residual renal clearance was 20.60 [11.49–35.79], 43.02 [19.13–75.48] and 17.50 [8.34–33.58] L/week, respectively, with no significant differences between CAPD and APD. Urine volume correlated positively with removed solute masses (rho = 0.82, 0.67 and 0.74) and with weekly residual renal clearances (rho = 0.77, 0.62 and 0.72 for urea, creatinine, and phosphorus, respectively, all p < 0.001). Residual renal clearances and urinary mass removal rates for urea, creatinine, and phosphorus correlate strongly with 24-h urine volume suggesting that urine volume could serve as an estimator of typical values of residual solute removal indices in PD patients.
Similar content being viewed by others
Introduction
In patients on peritoneal dialysis (PD), the presence of residual kidney function (RKF) is associated with increased fluid and solute removal, improved volume status, better nutritional status, reduced erythropoietin requirements and improved survival, and RKF should therefore, whenever appropriate, be considered in the evaluation of PD patients1,2. While increasing the dialysis dose has failed to have an impact on the mortality of dialysis patients, RKF has consistently been a potent predictor of improved survival for both hemodialysis and PD patients1,2. RKF is especially important to consider when prescribing incremental PD3,4,5 and for mathematical modeling of solute kinetics during dialysis6.
The estimated glomerular filtration rate (eGFR) calculated by formulas based on plasma creatinine are not reliable in patients undergoing dialysis. More exact methods to measure GFR, such as inulin based, or measuring creatinine clearance following administration of cimetidine to inhibit tubular secretion, are not feasible for routine clinical evaluation7,8. Routine assessment of RKF in PD patients should ideally be based on measurements of the mean of 24-h urinary creatinine and urea clearances to calculate measured glomerular filtration rate, mGFR9. However, measurements of urea and creatinine in 24 h urine collection are often not available in clinical practice and there is a need for simpler tools, such as urine volume, to estimate RKF and to estimate the contribution of RKF to the total solute removal. Moreover, impairment of urine excretion varies from substance to substance10,11,12.
Besides the classical markers of kidney function and dialysis adequacy, urea and creatinine, the impact of residual kidney function on phosphate metabolism is of recent interest in PD13,14,15,16,17 and hemodialysis18,19.
Information of a potential value of urine volume as an estimate of residual small solute removal in PD patients is lacking. We explored whether correlations of urine volume with different estimations of the residual renal function for urea, creatinine, and phosphorus, could be used to assess weekly residual renal clearances and renal mass removal for investigated solutes.
Methods
Patients and study design
This observational, cross-sectional study included prevalent PD patients at the dialysis facilities of RTS Versalles, Cali, Colombia, who were investigated as part of their routine clinical evaluation. We included 93 non-anuric (urine output ≥ 100 mL per 24 h) patients (53.8% men, median [interquartile range] age 59 [45–67] year, body mass index 25.7 [21.3–27.7] kg/m2 and measured glomerular filtration rate, mGFR, 3.24 [1.47–5.67] mL/min) undergoing continuous ambulatory (CAPD, n = 34) or automated (APD, n = 59) peritoneal dialysis (Tables 1, 2). Ninety percent of patients received furosemide and the dose was 40 mg in 5%, 80 mg in 69%, 120 mg in 10%, 200 mg in 1% and 240 mg daily in 5% of the patients. Urine volume (mL/day), weekly residual renal clearance (L/week), removed solute mass (g/week), solute concentration in urine and serum for urea, creatinine and phosphorus were estimated from 24 h collections of urine and determination of solute concentrations in urine and serum. All patients received instruction on how to perform the collection of urine which—as part of the measurement of KT/V—started 6 am when urine miction was discarded and lasted with collection of all urine until 6 am the following day. Urea and creatinine were assayed by routine methods and phosphorus concentration was determined using direct UV measurement of phosphomolybdate complex.
This study extends our analyses reported previously with detailed clarification of urine volume importance as an estimate of solute removal13,14.
Calculation of removed mass and residual renal clearance
Solute mass removed by the kidneys (Mrenal,solute) was evaluated from 24-h collection of urine as solute concentration in urine (Curine,solute) multiplied by urine volume (Vurine): \( {\text{M}}_{{{\text{renal,solute}}}} = {\text{C}}_{{{\text{urine,solute}}}} \cdot{\text{V}}_{{{\text{urine}}}} \), where solute is urea, creatinine or phosphorus. Weekly residual renal clearance was calculated as the mass removed by the kidneys Mrenal,solute over solute concentration in serum (Cserum,solute) normalized using body surface area (BSA) and recalculated to one week (7 days) interval:
Residual renal function was also assessed as the measured glomerular filtration rate, mGFR, calculated as the average of urea and creatinine residual renal clearances (in mL/min).
Normalized urine volume was calculated as:
Note that:
Statistical analysis
Data are expressed as median with interquartile range or as number and percentage. Chi-squared or exact Fisher test was used to compare categorical variables. Differences between continuous variable were investigated using Mann–Whitney and Kruskal–Wallis tests for two and more independent samples, respectively. Statistical dependence between variables was tested using Spearman’s correlation coefficient (rho). Observed effect was considered statistically significant at p value < 0.05; unless otherwise indicated. Statistical analyses were performed in Matlab R2021a (MathWorks, Natick, MA, USA).
Ethical approval
All procedures performed in the study involving patients were in accordance with the Declaration of Helsinki as part of the routine clinical evaluation. Approval was granted by the RTS Ethical and Investigation Committee (September 2016). The informed consent was not required for the time of study duration, but each patient gave informed consent to perform laboratory measurements and for data management.
Results
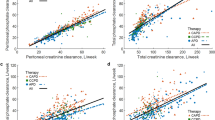
Median 24-h urine output was 560 [330–950] mL (Table 2). Renal mass removal for urea, creatinine and phosphorus was 10.12 [4.72–17.24], 3.53 [1.85–5.62] and 1.02 [0.49–1.72] g/week, respectively (Table 2). Serum creatinine correlated weakly and negatively with urine volume at rho = − 0.25, p < 0.05, but no such relationship was observed for urea and phosphorus (Table 3). Urine volume did not correlate with urine urea or urine phosphorus in contrast to urine creatinine that correlated (weakly) negatively at rho = − 0.28, p < 0.01 (Table 3). Urine volume correlated positively with urea, creatinine and phosphorus renal clearances at rho 0.77, 0.62 and 0.72, respectively (all p < 0.001, Table 3), and the mass of urea, creatinine and phosphorus removed by the kidney with rho = 0.82, 0.67 and 0.74, respectively (all p < 0.001, Table 3); Fig. 1A–C shows the linear regression of residual renal clearance of the solutes vs. normalized urine volume. The regression for mGFR was: mGFR [mL/min] = 0.0052 · normalized urine volume [mL/day] with rho 0.66 (p < 0.001, Table 4, Fig. 1D). The ratios of urine to serum concentration for phosphorus, creatinine and urea did not correlate with urine volume (Tables 3, 4, Fig. 2). Renal urea clearance (20.60 [11.49–35.79] L/week) correlated positively with creatinine renal clearance (43.02 [19.13–75.48] L/week), (rho = 0.92, p < 0.001), and with phosphorus renal clearance (17.50 [8.34–33.58] L/week), (rho = 0.88, p < 0.001), while renal creatinine clearance correlated positively with phosphorus renal clearance (rho = 0.85, p < 0.001), (Table 2; Fig. 3). Correlations of renal function parameters and normalized urine volume were similar as vs. urine volume, see Tables 3 and 4. No statistical difference between CAPD and APD groups were concluded unless mentioned (Tables 1, 2, 3, 4). No correlation was found between the dose of furosemide and urine volume, kidney residual clearances (Fig. 4), removed solute masses, and the urine to plasma concentration ratios for urea, creatinine, and phosphorus.
Residual renal urea clearance (panel A), renal creatinine clearance (panel B), renal phosphate clearance (panel C) and measured glomerular filtration rate (mGFR, panel D) as function of normalized urine volume. *** denote p value < 0.001. Solid line represents linear regression with equation displayed on the figure, dashed lines represent 0th and 100th percentile (minimum and maximum) of the data set.
Discussion
Residual kidney function (RKF) is an important contributor to fluid and solute removal and an independent predictor of survival in patients on peritoneal dialysis1,2 and hemodialysis20. While the amount of diuresis, by improving volume status, appears to have a stronger impact on survival than small solute removal in patients with kidney failure2, the control of plasma concentrations by urinary removal of small (sodium, potassium, phosphate) and large21 solutes accumulating in uremia is also of major importance for clinical outcomes. Regular measurements of urine volumes in kidney failure patients with urine production to assess kidney function and solute removal indices are of value to guide timing of dialysis initiation and adjusting dialysis prescriptions to optimize solute clearances especially in the case of incremental dialysis1,2,3,4,5,6.
Considering that calculations of the estimated glomerular filtration rate (eGFR) by formulas based on plasma creatinine are not reliable in patients undergoing dialysis and that measurements of solutes in 24 h urine collection are rarely available in clinical practice, we investigated if urine volume could be used to estimate residual renal clearances and urinary removal of solutes. The water and solute profiles are relatively stable in patients on PD, especially if compared to those on hemodialysis, and therefore we do not expect large variability of GFR and solute clearances in our data, as observed for interdialytic breaks between hemodialysis sessions6,8. In the present study, residual renal clearances and renal mass removal for urea, creatinine and phosphorus correlated strongly and positively with urine volume (Tables 3, 4, Fig. 1). This suggests that data on urine volume, if available, should be considered when investigating solute removal indices in PD patients. Among residual renal clearances for urea, creatinine, and phosphorus, two of them may be assessed based on measurements of the third one (compare Fig. 3).
The mean creatinine and urea clearance, mGFR, is a clinically useful estimator of residual GFR and as reported by Olden et al.7, mGFR correlated better with inulin clearance than creatinine clearance alone, and the average value of mGFR was closer to inulin clearance than creatinine clearance alone, with further improvement after addition of cimetidine. However, measurements of mGFR are rarely available in routine clinical care while measurements of urine volume are more common.
Tubular reabsorption is relatively more impaired than residual GFR in patients on dialysis with severely impaired kidney function, and, therefore, solute clearances and urine volume are more dependent on glomerular filtration in patients on hemodialysis than in the patients with normal renal function8. Our results confirm that urine volume is strongly associated with mGFR and solute clearances also in patients on peritoneal dialysis (Tables 3, 4, Fig. 1). More advanced methods such as urinary inulin clearance or administration of cimetidine to block tubular secretion of creatinine to obtain more exact determination of GFR provide better insight into residual kidney function but typically such studies are performed in small number of patients2,7,8. While urine volume can be increased by diuretics, urine volume, mGFR, residual clearances and the urine to plasma concentration ratios were not correlated to the dose of furosemide in the present study (Fig. 4). The apparent lack of any effect of furosemide on urine volume and adequacy indices may be due to the relatively low doses of furosemide used among our patients.
The relationship between residual clearance and normalized urine volume is via the ratio of solute concentrations in urine and plasma, Curine/Cserum, see Eq. (2). The Curine/Cserum ratio is an important marker of kidney concentration mechanisms22,23,24,25,26,27. The slopes of the regression lines in Fig. 1A–C are equivalent, after rescaling, to Curine/Cserum ratios of 4.97, 10.01, and 4.34 for urea, creatinine and phosphorus, respectively. These Curine/Cserum ratios obtained from the regression lines are close to the Curine/Cserum median values 4.81, 9.98, and 4.21 for urea, creatinine and phosphorus, respectively. Note that the CKD stage 5 patients—in spite of frequently very low urine volume—retain the classical relationship with Curine/Cserum for creatinine being about twice higher than Curine/Cserum ratios for urea and phosphorus22,23. The data points in Fig. 1 are scattered around the regression lines but lay inside characteristics cones created by the lines associated with minimal and maximal values of Curine/Cserum ratio for each solute respectively. All these associations are possible because Curine/Cserum in our data does not correlate with (normalized) residual urine volume (Fig. 2). Note however that the few patients with high urine output (normalized urine volume > 1500 mL/day) have rather low Curine/Cserum ratios; otherwise, they would not need dialysis (Fig. 2). Our finding that the urine to plasma concentration ratios for urea, creatinine and phosphate did not depend on urine volume in patients on peritoneal dialysis is unexpected and worth further investigation.
The regression lines in Fig. 1 do not describe the individual changes of residual clearance with declining urine volume—the individual history of this relationship may be considerably more sophisticated28. Furthermore, because high scattering of the Curine/Cserum values (solute concentration characteristics) for the same urine volume, see Fig. 2, one cannot use these regressions for the prediction of individual residual clearances with high accuracy. However, those regression lines allow for the estimation of the typical value of residual clearance given the urine volume, and the cone-like structure of distribution of data—for the assessment of likely values of the maximum and minimum residual clearances if the urine volume is known or assumed. The predicted typical values may be compared with real individual clearances or for the comparison of the mean values for different populations of patients. One can also derive from our data what is the expected range of residual clearances for a given normalized urine volume. Similarly, the typical values and range of mGFR can be predicted as a function of normalized urine volume (Fig. 1D). Our results are presented as normalized urine volume, mGFR, and residual clearances, i.e., scaled to the body surface area (BSA); this is because GFR is typically scaled to BSA29,30. However, the correlations between the non-scaled parameters were similar.
Some limitations of our study should be taken into account when interpreting the results. We analyzed a relatively small group of patients using two different modalities of peritoneal dialysis, CAPD and APD. However, we did not find significant differences between CAPD and APD in basic patient characteristics (except for age) and the normalized and non-normalized urine volume, mGFR, serum and urine concentrations, residual renal clearances and removed masses of urea, creatinine and phosphorus also did not differ (Tables 1, 2). Results from correlation analysis, performed separately for CAPD and APD, were generally consistent with data derived for the whole group (Tables 3, 4). An important factor in the assessment of residual urine output is the exact timing of 24 h. Strict control of this factor may be gained during hospitalization of the patients while in our patients who collected urine at home, the timing may vary to some extent. However, if the deviation from 24 h is random, as it might be expected, its impact on the correlation parameters should not significantly compromise the results. Our study was not designed to investigate the association of urine volume and solute clearance with survival. Further studies are needed to explore if there is a cut-off value of urine volume that assures a minimal solute clearance to support incremental prescriptions.
In summary, urine volume can be considered as a marker of residual urinary removal of urea, creatinine and phosphorus. The residual clearances of these three solutes correlate well to each other; two of them may be assessed based on measurements of the third one. The solute concentration mechanisms, i.e., interrelationship between serum and urine concentrations for urea, creatinine, and phosphorus in patients on peritoneal dialysis with residual function do not depend on urine volume.
Data availability
The data underlying this article were provided by RTS SAS under permission. Data will be shared on request to the corresponding author with permission of RTS SAS.
References
Perl, J. & Bargman, J. M. The importance of residual kidney function for patients on dialysis: A critical review. Am. J. Kidney Dis. 53, 1068–1081 (2009).
Krediet, R. T. Preservation of residual kidney function and urine volume in patients on dialysis. Clin. J. Am. Soc. Nephrol. 12, 377–379 (2017).
Blake, P. G., Dong, J. & Davies, S. J. Incremental peritoneal dialysis. Perit. Dial. Int. 40, 320–326 (2020).
Lee, Y. et al. Incremental peritoneal dialysis may be beneficial for preserving residual renal function compared to full-dose peritoneal dialysis. Sci. Rep. 9, 1–7 (2019).
Auguste, B. L. & Bargman, J. M. Incremental peritoneal dialysis: New ideas about an old approach. Semin. Dial. 31, 445–448 (2018).
Debowska, M., Waniewski, J. & Lindholm, B. Bimodal dialysis: Theoretical and computational investigations of adequacy indices for combined use of peritoneal dialysis and hemodialysis. ASAIO J. 53, 566–575 (2007).
Van Olden, R. W., Krediet, R. T., Struiji, D. G. & Arisz, L. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. JASN 7, 745–750 (1996).
Van Olden, R. W., Van Acker, B. A. C., Koomen, G. C. M., Krediet, R. T. & Arisz, L. Time course of inulin and creatinine clearance in the interval between two haemodialysis treatments. Nephrol. Dial. Transplant. 10, 2274–2280 (1995).
Grootendorst, D. C. et al. The MDRD formula does not reflect GFR in ESRD patients. Nephrol. Dial. Transplant. 26, 1932–1937 (2011).
Kashani, K., Rosner, M. H. & Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 72, 9–14 (2020).
Blaine, J., Chonchol, M. & Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 10, 1257–1272 (2015).
Hosten, A. O. BUN and creatinine. In Clinical methods: The history, physical, and laboratory examinations 874–878 (1990).
Debowska, M., Gomez, R., Pinto, J., Waniewski, J. & Lindholm, B. Phosphate clearance in peritoneal dialysis. Sci. Rep. 10, 17504 (2020).
Gomez, R., Waniewski, J., Zapata, A., Pietribiasi, M. & Lindholm, B. Phosphate equilibration rate and daily clearance in patients on CAPD, CCPD and APD. Int. J. Artif. Organs 39, 596–602 (2016).
Bernardo, A. P. et al. Peritoneal membrane phosphate transport status: A cornerstone in phosphate handling in peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 6, 591–597 (2011).
Sedlacek, M., Dimaano, F. & Uribarri, J. Relationship between phosphorus and creatinine clearance in peritoneal dialysis: Clinical implications. Am. J. Kidney Dis. 36, 1020–1024 (2000).
Courivaud, C. & Davenport, A. Phosphate removal by peritoneal dialysis: The effect of transporter status and peritoneal dialysis prescription. Perit. Dial. Int. 36, 85–93 (2016).
Penne, E. L. et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin. J. Am. Soc. Nephrol. 6, 281–289 (2011).
Kawasaki, T. et al. Urinary phosphorus excretion per creatinine clearance as a prognostic marker for progression of chronic kidney disease: A retrospective cohort study. BMC Nephrol. 16, 1–8 (2015).
Shafi, T. et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) study. Am. J. Kidney Dis. 56, 348–358 (2010).
Rosner, M. H. et al. Classification of uremic toxins and their role in kidney failure. Clin. J. Am. Soc. Nephrol. 16, 1918–1928 (2021).
Bankir, L., Bouby, N., Trinh-Trang-Tan, M. M., Ahloulay, M. & Promeneur, D. Direct and indirect cost of urea excretion. Kidney Int. 49, 1598–1607 (1996).
Underhill, S. W. F. The relative concentration ratios of some constituents of the urine. Br. J. Exp. Pathol. 4, 87–117 (1923).
Heida, J. E. et al. Use of the urine-to-plasma urea ratio to predict ADPKD progression. Clin. J. Am. Soc. Nephrol. 16, 204–212 (2021).
Carvounis, C. P., Nisar, S. & Guro-Razuman, S. Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 62, 2223–2229 (2002).
Pons, B. et al. Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: Multicenter cohort study. Crit. Care 17, 1–8 (2013).
Manoeuvrier, G., Bach-Ngohou, K., Batard, E., Masson, D. & Trewick, D. Diagnostic performance of serum blood urea nitrogen to creatinine ratio for distinguishing prerenal from intrinsic acute kidney injury in the emergency department. BMC Nephrol. 18, 1–7 (2017).
Johnson, D. W. et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit. Dial. Int. 23, 276–283 (2003).
Levey, A. S., Coresh, J., Tighiouart, H., Greene, T. & Inker, L. A. Measured and estimated glomerular filtration rate: Current status and future directions. Nat. Rev. Nephrol. 16, 51–64 (2020).
Hall, J. E. & Guyton, A. C. Textbook of Medical Physiology (Saunders Elsevier, 2011).
Author information
Authors and Affiliations
Contributions
The research idea and study design were carried out by J.W., M.D., R.G. and B.L. R.G. was responsible for data acquisition. J.P., M.D. and J.W. were responsible for the investigation. J.P. and M.D. were responsible for the statistical analysis. B.L. was responsible for supervision and mentorship. J.P., M.D. and J.W. were responsible for original draft preparation and editing. J.P., M.D., R.G., J.W. and B.L. were responsible for review of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Bengt Lindholm and Rafael Gomez are employed by Baxter Healthcare. Baxter Novum is supported by a grant from Baxter Healthcare to Karolinska Institutet. Joyce Pinto, Malgorzata Debowska and Jacek Waniewski declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto, J., Debowska, M., Gomez, R. et al. Urine volume as an estimator of residual renal clearance and urinary removal of solutes in patients undergoing peritoneal dialysis. Sci Rep 12, 18755 (2022). https://doi.org/10.1038/s41598-022-23093-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23093-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.