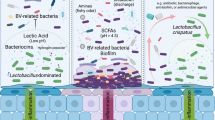
Abstract
Bacterial vaginosis (BV), the overgrowth of diverse anaerobic bacteria in the vagina, is the most common cause of vaginal symptoms worldwide. BV frequently recurs after antibiotic therapy, and the best probiotic treatments only result in transient changes from BV-associated states to “optimal” communities dominated by a single species of Lactobacillus. Therefore, additional treatment strategies are needed to durably alter vaginal microbiota composition for patients with BV. Vaginal microbiota transplantation (VMT), the transfer of vaginal fluid from a healthy person with an optimal vaginal microbiota to a recipient with BV, has been proposed as one such alternative. However, VMT carries potential risks, necessitating strict safety precautions. Here, we present an FDA-approved donor screening protocol and detailed methodology for donation collection, storage, screening, and analysis of VMT material. We find that Lactobacillus viability is maintained for over six months in donated material stored at − 80 °C without glycerol or other cryoprotectants. We further show that species-specific quantitative PCR for L. crispatus and L. iners can be used as a rapid initial screening strategy to identify potential donors with optimal vaginal microbiomes. Together, this work lays the foundation for designing safe, reproducible trials of VMT as a treatment for BV.
Similar content being viewed by others
Introduction
Bacterial vaginosis (BV)—a syndrome characterized by vaginal discharge, odor, and discomfort—affects 30% of women worldwide1. It is associated with the presence of high diversity, Lactobacillus-deficient anaerobic vaginal microbiota, and it carries increased risk for a number of adverse sexual and reproductive outcomes, such as preterm birth, miscarriage, cervical dysplasia, and sexually transmitted infections, including HIV2. Antibiotic treatment, most commonly with metronidazole, reduces the absolute quantity of BV-associated microbes and temporarily improves symptoms, but BV recurrence is as high as 30–60% one month after completion of therapy1,3,4.
The two most prevalent and abundant vaginal Lactobacillus species are Lactobacillus crispatus, which is generally associated with beneficial health outcomes, and Lactobacillus iners, which is linked to microbial community instability, adverse outcomes, and transition to diverse, BV-like communities5,6,7,8. Delivery of exogenous L. crispatus to the vagina as a live biotherapeutic has been studied for prevention of recurrent BV, but multiple studies have shown only modest benefit9,10,11. In a Phase 2b trial of a probiotic consisting of a single strain of L. crispatus (LACTIN-V), after 5 daily doses and twice-weekly dosing for 11 weeks, 30% of women in the treatment arm had BV recurrence immediately after cessation of treatment, with an increase to 39% after an additional 12 weeks without treatment12. Single strain probiotics likely demonstrate only partial efficacy for a variety of reasons: one hypothesis being that the diverse array of bacteria, phages, and small molecules comprising the entirety of a healthy vaginal ecosystem, along with the interactions between them, may be required to support lactobacilli and sustainably shift the community. Similar hypotheses have been proposed to explain the success of fecal microbiota transplants (FMT) for the treatment of recurrent Clostridium difficile colitis in the gut13,14,15,16.
Vaginal microbiota transplantation (VMT), the transfer of whole vaginal fluid from a healthy person with high Lactobacillus abundance, has been proposed as a solution to establish a healthy vaginal microbiome after antibiotic treatment for BV. In 2019, the first report of VMT in humans demonstrated remission of BV in 4 of 5 women treated multiple times over 2 years with antibiotics followed by a small amount of fresh vaginal fluid17. The authors reported no significant side effects and no serious adverse events. While the lack of adverse outcomes reported in this study is reassuring, the small study size and lack of a placebo arm make it difficult to interpret whether VMT provided an added benefit over antibiotics alone. A separate group published a more extensive proposal for characterization of donors to ensure safety in VMT and described properties of collected vaginal fluid18. However, for VMT to be a viable treatment for BV, strategies for efficient identification of suitable donors, as well as optimized methods for collection, screening, and storage of donated material are needed.
Here we describe the recruitment and enrollment of three vaginal fluid donors under the first Food and Drug Administration (FDA)-approved Investigational New Drug (IND) protocol for VMT. In addition to screening for general donor health and the absence of infectious diseases in the donors, we outline additional measures for safety screening of donors and donated material. Furthermore, we demonstrate comparability of donations and aliquots reserved for analysis and show stability of viable Lactobacillus in donations over time. This protocol, and the described donations meeting all quality metrics, will be used in a planned randomized trial of VMT to promote a Lactobacillus-dominant vaginal microbial community in patients diagnosed with recurrent BV.
Results
Donation collection optimization
We enrolled a pilot donor (Donor 0) to initially optimize sample collection and processing procedures, and to confirm the feasibility of the protocol. Vaginal fluid was collected using a disposable menstrual cup and donation material from Donor 0 was homogenized with sterile saline (see Methods). The donation material was then split into several “analysis aliquots,” which were stored at − 80 °C to test for Lactobacillus viability over time, and a remaining “donation aliquot” to be used for eventual transplantation. Donor 0 provided five donations over nine days, with a median of 400 μL of vaginal fluid per donation (range < 100–1000 μL). After 13 months stored at − 80 °C, Lactobacillus Colony Forming Unit (CFU) counts were similar in the two types of aliquots: median 3.7 × 107 CFU/mL in the donation aliquot compared to 5.3 × 107 CFU/mL in an analysis aliquot (Fig. 1a).
Microbial community profiling and absolute quantification of pilot donation fluid. (a) Lactobacillus colony forming unit (CFU) quantification for each donation and analysis aliquot. Total donation volume is listed below donation numbers. (b) Microbial species in donation (“D”) and analysis (“A”) aliquots of pilot donor (Donor 0) samples using bacterial 16S rRNA amplicon sequencing. D1 and D2 refer to two different donation aliquots from the same donation collection.
Microbial community composition of the donation and analysis aliquots by bacterial 16S rRNA amplicon sequencing revealed that the bacterial community profile of both aliquot types was nearly identical at each timepoint (Fig. 1b). The relative abundances of L. crispatus and L. iners varied over time across the five donations, but L. crispatus was consistently at a higher relative abundance (66.9–96.0%) than L. iners (0.35–24.9%). Non-Lactobacillus taxa represented < 0.8% of total relative abundance for each sample. Based on the lower CFU count in the single donation with the lowest volume, we set a conservative minimum threshold of 700 μL for a donation to be acceptable for use. However, the reduction in CFU counts for this sample was likely in part due to the higher relative abundance of L. iners, which typically does not grow on the MRS (deMan, Rogosa, and Sharpe) agar used for L. crispatus culturing.
Donor enrollment
After demonstrating the feasibility of donation collection, we received FDA approval for our Investigational New Drug protocol (#018173). Between March 2019 and November 2020, we screened 49 additional women by telephone and conducted 8 in-person screening visits to enroll three donors (Donors 1–3) (Fig. 2a). Women were considered for the study if they were premenopausal, had a Nugent score of 0–3, agreed to abstain from sexual activity during the entire donation period, and denied any history of BV. An extensive set of inclusion and exclusion criteria were used for final screening (see Methods). Four potential donors failed screening because of a Nugent score > 3, and one person could not have screening labs drawn due to a recent blood donation and did not return for a follow-up visit to complete screening.
Donor screening and donation schedule. (a) 50 total women, including the pilot donor (light green), were pre-screened over the phone. Eight potential donors completed an in-person screen and testing, three of whom went on to become donors (dark green), four of whom were ineligible due to high Nugent scores (red), and one of whom was ineligible due to a recent blood donation and no subsequent follow-up (pink). Twenty-three potential donors (grey) were uninterested in following up or were disqualified for logistical reasons (e.g., travel), 18 completed pre-screening and asked for later follow up (yellow). (b) Donation screening and schedule with detailed testing for donors and donations before, during, and after donating.
Donor screening
All three donors identified as white, cis-gender women and reported a preference for male sexual partners. Donors 1 and 3 were using oral contraceptives and Donor 2 was using a Mirena IUD for contraception. A full list of screening tests is outlined in Table 1, which include tests to screen for underlying medical comorbidities (acceptable ranges in Table 1 and Table S1). Donor demographics including age, race, and BMI are included in Table S2. Enrolled donors went through extensive testing for potentially transmissible infections at enrollment, at the final donation visit, and 30–45 days after the final donation (Fig. 2B). This ensured identification of an incident infection during the entire donation period. Participants agreed to remain sexually abstinent during the donation period. Each individual donation was tested for prostate specific antigen (PSA) at the time of processing to confirm the absence of semen, and analysis aliquots for each donation were tested for HPV and again for sperm using a Y-chromosome-specific PCR assay. Starting in June 2020, all donors underwent nasal swab PCR testing for SARS-CoV-2 prior to enrollment, were screened for symptoms before each donation, and each donation was tested for SARS-CoV-2 by RT-PCR.
Donation collection for potential VMT
Donor 1 provided eight donations over 12 days, with a median volume of 0.4 mL (range 0.1–0.8 mL) and a median weight of 1.42 g (range 1.0–1.8 g) (Fig. S1). Donor 2 provided 20 donations over 37 days, with a median volume of 0.75 mL (range 0.3–1.1 mL) and a median weight of 1.3 g (range 1.1–2.0 g). Donor 3 provided 14 donations over 40 days, with a median volume of 0.55 mL (range 0.4–0.9 mL) and a median weight of 1.1 g (range 0.5–1.5 g). All donations had a pH < 4.8 and all donors had a Nugent score < 4 at the time of donation.
Donation characteristics for potential VMT
For Donor 1, the Nugent scores for all donations ranged from 0–1 (Fig. 3a). For most gram stain slides, there were no white blood cells (WBCs) present or < 1 WBC/epithelial cell, but > 1 WBC/epithelial cell was seen on 2 donation samples (1 and 6) (Fig. 3a). High numbers of white blood cells often indicate a vaginal infection, but this donor had negative testing for known infections. For Donor 2, the Nugent score ranged from 0 to 3. Similar to Donor 1, there were no WBCs present or < 1 WBC/epithelial cell except for 1 day (sample 20). Donor 3 had Nugent scores of 0–1, and no samples had > 1 WBC/epithelial cell.
Microbial community profiles of VMT donation samples. (a) Microbial community and metadata. Relative abundance of the bacterial community in the donation material was determined using 16S rRNA amplicon sequencing for each donation. Nugent score and white blood cell (WBC)/epithelial cell ratio are presented below each donation. (b) Absolute quantification of L. crispatus and L. iners in donation samples using species-specific qPCR. Detection limit of the assay is depicted by dashed lines. (c) Donation stability measured as Lactobacillus CFUs streaked and counted on MRS agar at long-term intervals post freezing.
To determine the bacterial community composition of the VMT donors, we used 16S rRNA amplicon sequencing along with species-specific qPCR assays for L. crispatus and L. iners. We found Donor 1 and Donor 3 were L. crispatus dominant for the entire donation period (Fig. 3a). Donor 2, however, had varying levels of L. jensenii and L. iners, which together made up nearly the entire community for all donation samples.
Using species-specific qPCR assays, L. crispatus and/or L. iners were found to be almost mutually exclusive in these donors. In Donor 2, qPCR results showing high quantities of L. iners and no detectable L. crispatus demonstrated that despite normal Nugent scores, the primary Lactobacillus species was not L. crispatus, but rather L. jensenii. Additionally, despite L. jensenii having a higher relative abundance in the 16S rRNA sequencing, the absolute quantity of L. iners in the donations from Donor 2 was high (Fig. 3b).
Lactobacillus viability after untreated storage at − 80 °C
Although a previous study used fresh donations for VMT, for long term use and scalability of the donated material, optimal storage protocols are needed. We aimed to maximize viability of the bacteria in the samples over time and to minimize any modifications to the material. We thus froze analysis aliquots of each donation without the addition of glycerol or other cryoprotectants at − 80 °C and used the aliquots to quantify the stability of Lactobacillus. To measure viable Lactobacillus CFUs after storage at − 80 °C, aliquots from each donation were serially diluted and plated on Lactobacillus-selective MRS agar plates (Fig. 3c). For Donor 1, Lactobacillus viability (CFU/mL of sample) was stable between 30 days (median 2.8 × 108, range 4.5 × 107–1.0 × 109), 3 months (median 5.2 × 108, range 5.3 × 107–9 × 108), 6 months (median 3.7 × 108 range 1.2 × 108–1.5 × 109), and 11 months (median 1.7 × 108 range 5.5 × 107–1.1 × 109) after collection. For Donor 2, Lactobacillus viability (CFU/mL) was initially stable between 30 days (median 2.0 × 108, range 4.7 × 107–5.6 × 108) and 3 months (median 1.1 × 108, range 1.9 × 107–2.9 × 108) but declined at 9 months (median 5.2 × 106, range 8 × 105–1.1 × 107). This decline may be attributed to the difference in makeup of the community and associated differences between L. crispatus compared to the mixture of L. jensenii and L. iners, the latter of which does not typically grow on MRS. Donor 3 was similar to Donor 1, with stable CFU counts when comparing data at collection (median 1.8 × 108 CFU/mL, range 1.5 × 107–5.8 × 108 CFU/mL), 3 months (median 1.6 × 108 CFU/mL, range 3.6 × 107–2.8 × 108 CFU/mL) and 6 months (median 1.2 × 108 CFU/mL, range 3.4 × 107 to 3.8 × 108 CFU/mL). This suggests that donations with L. crispatus dominant communities can be stored without need for cryoprotectants.
Discussion
While VMT has been proposed as a potential strategy to prevent recurrent BV, detailed safety, feasibility, and efficacy testing is needed before this can be considered as an option for clinical care. Here we describe a protocol for extensive safety screening and collection of vaginal fluid donations for VMT. In our screening strategy, we test participants before, during, and after the donation period to ensure that they do not have or did not recently become infected with common sexually transmitted pathogens. We also confirm absence of semen or sperm in donation materials. We show that this collection protocol is feasible, with our enrollment of 3 donors, who together provided a total of 42 doses of at least 700 μL each. Additionally, our processing protocol resulted in donation aliquots with viable lactobacilli over several months of storage at – 80 °C, and our analysis aliquots reflected the characteristics of the donation aliquots, which will be important in characterizing successful donations.
While all three donors showed stable Lactobacillus viability at − 80 °C storage up to 3 months, there was a 1–2 order of magnitude decline in viability of the samples from Donor 2 at 9 months. For this donor, the 6-month time point was not performed due to the COVID-19 pandemic. Molecular analysis demonstrated that this donor had a majority of L. jensenii and high absolute quantities of L. iners, in contrast to the other two donors who had high relative and absolute abundance of L. crispatus. It is possible that the different Lactobacillus species survive freezing differently, or that another property of the vaginal fluid that differed between the donors (e.g., glycogen content) contributed to this discrepancy in long-term viability. For our first randomized trial of vaginal fluid transplant, our goals are to 1) prioritize recipient safety, and 2) to limit our manipulation of the vaginal fluid so that we are best able to identify features associated with benefit (or lack thereof). For this reason, we did not include cryoprotective solutions when processing the donations—we were concerned that these might increase the risk for vulvovaginal candidiasis in recipients and that they might obscure our ability to characterize donation characteristics. Based on our results, we feel that use of cryoprotectants is not necessary if a minimum concentration of 1 × 106 CFU/mL of L. crispatus is present. Additionally, because of the differences in viability we decided only to include donations that are L. crispatus dominant.
Our initial intent was to do as little characterization of the microbiota as possible prior to selection of donation material, to remain agnostic to what might be the beneficial component or optimal composition of vaginal fluid. However, the difference in viability of the donation material highlights a challenge in using Nugent score to screen donors. Although the Nugent method is efficient and quick, it led to the enrollment of a donor with a L. jensenii dominant community with a high absolute quantity of L. iners. This donation material was less stable when stored at − 80 °C than L. crispatus-dominant donations from the two other donors. Using species-specific qPCR assays for L. crispatus and L. iners to test the donation material clearly distinguished the two optimal donors from the donor with a higher proportion and quantity of L. iners. Based on these results, we propose screening future donations with qPCR and excluding donors or donations with competing absolute abundances of L. iners compared to L. crispatus. We have chosen to focus on these two species as they are the most commonly identified dominant lactobacilli in the vagina, and because of the differences in viability seen when levels of L. iners are high and L. crispatus is absent. We suggest the concentrations of L. crispatus to be at least three orders of magnitude higher than that of L. iners. The proposed qPCR strategy is faster and cheaper than completing 16S rRNA amplicon sequencing for each donation and will allow rapid assessment of the suitability of donations while awaiting the more comprehensive characterization of the microbiota that will occur in the analysis phase of the study.
Our safety screening of donors and donations was extensive. Of note, no individual donation tested positive for HPV, suggesting no intermittent shedding of HPV in the donors, all of whom had a negative HPV test with the screening Pap smear. Additionally, no individual donations tested positive for PSA or Y-chromosome, suggesting donors also abstained from penile-vaginal intercourse during the donation period. The assay can detect semen to a 1:512 dilution and has been shown to detect semen for up to 5 days after a sexual encounter19. This extensive testing for sperm/semen minimizes risk of inducing pregnancy in the recipient and minimizes the possibility of acquisition of sexually transmitted infections. Furthermore, viable sperm are unlikely to survive the freeze/thaw process, adding an additional level of safety.
VMT is a promising strategy to promote a Lactobacillus-dominant vaginal microbiota in people with recurrent BV. Studies of VMT will also provide a novel opportunity to identify host and microbial determinants of Lactobacillus colonization and persistence. This report describes a feasible, reproducible protocol for the collection of donated vaginal fluid with safeguards to ensure recipient safety. Our protocol differs from the published report of VMT in our method of collection, storage of frozen donations until multiple safety tests have been completed, and creation of analysis aliquots directly from the donated material (rather than swabs collected at the same time). The minimal processing in our protocol maintains viability of lactobacilli and will allow us to assess the impact of whole vaginal fluid on recipient microbiota and mucosal immunity, without being confounded by the addition of cryoprotectant or stabilizing compounds. With the proposed minimal processing of donated material, we consistently count between 108 and 109 Lactobacillus CFU/mL, which means a dose of 700 μL will deliver between 107 and 109 CFU. This concentration is comparable to the dose of Lactin-V, the L. crispatus live biotherapeutic used in a Phase2b clinical trial in 201912. Finally, the retention of analysis aliquots will facilitate identification of features linked to successful outcomes (e.g., specific strains associated with success).
Despite the high prevalence of BV, and lack of durable treatment response with current therapies, there has not been a fundamentally new therapy for BV in decades. VMT is a strategy to gain insights into determinants of vaginal colonization with beneficial lactobacilli and contribute to the development of novel strategies for BV treatment and prevention. Successful collection, testing, and storage of donated vaginal fluid is a first step toward that goal.
Methods
Donor recruitment
Potential donors were recruited by website postings, flyers, recruitment from previous studies, and advertisements. Posters were placed in sexual health and gynecology clinics as well as around the campuses of local universities.
We defined eligible donors as healthy, pre-menopausal (age 18–40), non-pregnant individuals who had never had a diagnosis of BV, were sexually experienced (thus have had the opportunity to acquire BV), and willing to abstain from any sexual activity for the duration of the screening and donation period. At initial screening, a donor needed to have a vaginal fluid pH < 4.8 and a Nugent score between 0 and 3. Potential donors underwent screening based on the American Association of Blood Banks (AABB) donor questionnaire for exposure to infectious agents. As part of the AAB questionnaire, potential donors are also asked about their medications and excluded if taking medications not deemed safe for blood donors. After June 2020, the protocol was amended to exclude participants that declared having symptoms of COVID-19 during the phone screen. Exclusion criteria also include: history of non-BV cervicovaginal infections in the past year; abnormal Pap smear or positive test for high risk HPV within the past year; use of probiotics within prior 30 days; oral or vaginal antibiotics within prior 3 months; history of genital herpes; abnormal findings on physical exam; personal travel in prior 6 months to a country or territory with active Zika outbreak (based on CDC guidelines) or unprotected sex in the preceding month with a partner who traveled to an area with active Zika in the prior 6 months.
Donor screening
Donors were tested for viral, bacterial, and fungal infections (HIV, Hepatitis A, B, C, Herpes Simplex Virus (HSV), Cytomegalovirus (CMV), Syphilis, Human T-lymphotrophic Virus (HTLV 1 and 2), Epstein-Barr virus (EBV), Gonorrhea, Chlamydia, Trichomonas, Candida, and Mycoplasma genitalium) prior to, at the end of, and 30 days after the donation period (Table 1, Fig. 2b). In addition, at enrollment, donors had a complete blood count, basic metabolic panel, hemoglobin A1C, and liver function tests checked to screen for underlying medical comorbidities. After June 2020, all donors also underwent nasal swab PCR testing for SARS CoV-2 prior to their enrollment appointment. Urine toxicology screen for illicit drugs, pregnancy test, and Pap smear with HPV testing was also performed at the time of enrollment. All testing was performed in the CLIA-certified hospital lab, according to routine clinical testing procedures. Any positive test (with the exception of HSV1 IgG or CMV IgG) resulted in exclusion. Donor 1 had positive tests for HSV1 IgG and CMV IgG. Our protocol specifies that in those cases each donation will be tested for HSV or CMV with PCR and excluded if positive. However, due to the COVID-19 pandemic these donations expired before confirmatory testing was performed. Donors consented to abstain from sexual contact (including oral, vaginal, anal giving or receiving) during the period of donation.
Donation collection and testing
The donation period started 3–10 days after the last day of a participant’s menstrual period. Per protocol, donors could provide up to 20 donations in a 45-day window. To donate vaginal fluid, participants inserted a disposable menstrual cup (Softdisc) 6–12 h prior to arriving at the clinical site, and removed the cup on site, where study staff processed the donation immediately.
Vaginal secretions were collected from the Softdisc by centrifugation in a sterile 50 mL conical tube at 810×g at 4 °C for 10 min, diluted with 500 μL of sterile saline and sheared with a blunt 16-gauge needle to homogenize the solution. A swab of material left on the Softdisc was used to make a slide for Nugent scoring and to test for semen using a prostate-specific antigen (PSA) card19. Each donation was aliquoted into treatment doses (at least 700 μL), and several 50 μL analysis aliquots to perform longitudinal Lactobacillus viability testing and to characterize the components of a successful transplant dose.
Analysis aliquots were used to test for the presence of semen using a Y-chromosome PCR20, and for high risk HPV using BD Oncoclarity test in the hospital clinical lab21. Lactobacillus CFU counts were quantified by thawing a separate analysis aliquot, performing serial dilutions, and plating 10 µl of diluted samples (1:103–1:106) in triplicate on MRS agar plates (Hardy Diagnostics) under anaerobic conditions (5% H2/5%CO2/90%N2 anaerobic mixed gas). Plates were incubated at 37 °C in a Bactron anaerobic chamber for two days prior to counting. The number of colonies was counted manually for each dilution with less than 100 colonies per 10 µl replicate.
DNA was extracted from an additional analysis aliquot using a manual phenol chloroform extraction and used for sequencing of the V4 region of the 16S rRNA gene as previously described22. Single-end sequencing was performed on an Illumina MiSeq using a v2 300-cycle kit. Data was demultiplexed using a custom python script and denoised using DADA223. Taxonomy of amplicon sequence variants was assigned using RDP24 in addition to manually curated assignments. Finally, data was analyzed in R using phyloseq25,26.
Quantification of L. crispatus and L. iners was performed using TaqMan qPCR assays as previously described27,28. Briefly, 5uL of DNA was used in a total 20uL reaction with species-specific primers and probe over 45 amplification cycles. A plasmid standard curve was used to calculate quantity of 16S rRNA gene copies per reaction and data were normalized to the volume of donation sample. Specific conditions are provided in Table S3.
Ethical parameters
All participants signed informed consent, and all procedures were performed per the human subjects approved protocol (Partners Human Subjects Committee, 2018P000057).
Data availability
Sequencing data is available from the NCBI Sequence Read Archive (BioProject ID: PRJNA812510). Code for data processing of 16S amplicon data available from Zenodo (https://doi.org/10.5281/zenodo.6374205).
References
Muzny, C. A. & Kardas, P. A narrative review of current challenges in the diagnosis and management of bacterial vaginosis. Sex. Transm. Dis. 47, 441–446 (2020).
Peebles, K., Velloza, J., Balkus, J. E., McClelland, R. S. & Barnabas, R. V. High global burden and costs of bacterial vaginosis: A systematic review and meta-analysis. Sex. Transm. Dis. 46, 304–311 (2019).
Fredricks, D. N., Fiedler, T. L., Thomas, K. K., Mitchell, C. M. & Marrazzo, J. M. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J. Clin. Microbiol. 47, 721–726 (2009).
Mitchell, C., Balkus, J., Agnew, K., Lawler, R. & Hitti, J. Changes in the vaginal microenvironment with metronidazole treatment for bacterial vaginosis in early pregnancy. J. Womens Health 18, 1817–1824 (2009).
Gosmann, C. et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46, 29–37 (2017).
Petrova, M. I., Reid, G., Vaneechoutte, M. & Lebeer, S. Lactobacillus iners: Friend or foe?. Trends Microbiol. 25, 182–191 (2017).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. 108, 4680–4687 (2011).
Munoz, A. et al. Modeling the temporal dynamics of cervicovaginal microbiota identifies targets that may promote reproductive health. Microbiome 9, 163 (2021).
Lambert, J. A., John, S., Sobel, J. D. & Akins, R. A. Longitudinal analysis of vaginal microbiome dynamics in women with recurrent bacterial vaginosis: Recognition of the conversion process. PLoS ONE 8, e82599 (2013).
van de Wijgert, J. & Verwijs, M. C. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: A systematic review and recommendations for future trial designs. BJOG Int. J. Obstet. Gynaecol. 127, 287–299 (2020).
Verwijs, M. C., Agaba, S. K., Darby, A. C. & van de Wijgert, J. H. H. M. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am. J. Obstet. Gynecol. 222(157), e1-157.e13 (2020).
Cohen, C. R. et al. Randomized trial of Lactin-V to prevent recurrence of bacterial vaginosis. N. Engl. J. Med. 382, 1906–1915 (2020).
Gerardin, Y., Timberlake, S., Allegretti, J. R., Smith, M. B. & Kassam, Z. Beyond fecal microbiota transplantation: Developing drugs from the microbiome. J. Infect. Dis. 223, S276–S282 (2021).
Draper, L. A. et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome 6, 220 (2018).
Gianotti, R. J. & Moss, A. C. Fecal microbiota transplantation: From Clostridium difficile to inflammatory bowel disease. Gastroenterol. Hepatol. 13, 209–213 (2017).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Lev-Sagie, A. et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 1–5 (2019). https://doi.org/10.1038/s41591-019-0600-6.
DeLong, K. et al. Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Front. Cell. Infect. Microbiol. 9, (2019).
Boward, E. S. & Wilson, S. L. A comparison of ABAcard® p30 and RSID™-Semen test kits for forensic semen identification. J. Forensic Leg. Med. 20, 1126–1130 (2013).
Chomont, N. et al. Detection of Y chromosome DNA as evidence of semen in cervicovaginal secretions of sexually active women. Clin. Diagn. Lab. Immunol. 8, 955–958 (2001).
Castle, P. E. et al. Evaluation of a new DNA test for detection of carcinogenic human papillomavirus. J. Clin. Microbiol. 49, 3029–3032 (2011).
Bloom, S. M. et al. Cysteine dependence in Lactobacillus iners constitutes a novel therapeutic target to modify the vaginal microbiota. bioRxiv (2021). https://doi.org/10.1101/2021.06.12.448098.
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Cole, J. R. et al. Ribosomal database project: Data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42, D633–D642 (2014).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
R Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2019).
Srinivasan, S. et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS ONE 5, e10197 (2010).
McClelland, R. S. et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect. Dis. 18, 554–564 (2018).
Acknowledgements
The authors would like to thank the study participants and clinical staff.
Funding
This article was funded by Ragon Institute of MGH, MIT and Harvard, National Institutes of Health (1R01AI158836-01A1).
Author information
Authors and Affiliations
Contributions
Conceptualization: C.M., L.Y., D.K. Methodology: C.M., L.Y., F.A.H., A.R., A.B., D.W., D.K. Investigation: L.Y., F.A.H., A.B., with assistance from J.X., I.G., S.B., and N.M. Visualization: F.A.H., L.Y., C.M. Funding acquisition: C.M., D.K. Project administration: C.M., A.R., D.W. Supervision: C.M., D.K. Writing: F.A.H., L.Y., C.M., with contributions from D.K., S.B., and J.X. Editing and review: F.A.H., L.Y., C.M., D.W., J.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yockey, L.J., Hussain, F.A., Bergerat, A. et al. Screening and characterization of vaginal fluid donations for vaginal microbiota transplantation. Sci Rep 12, 17948 (2022). https://doi.org/10.1038/s41598-022-22873-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22873-y
This article is cited by
-
The Endometrial Microbiota’s Role in Fertility: Updates and Future Directions
Current Obstetrics and Gynecology Reports (2024)
-
The evolving facets of vaginal microbiota transplantation: reinvigorating the unexplored frontier amid complex challenges
Archives of Microbiology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.