Abstract
IDH1-mutated cholangiocarcinomas (CCAs) are an interesting group of neoplasia with particular behavior and therapeutic implications. The aim of the present work is to highlight the differences characterizing IDH1m and IDH1wt CCAs in terms of genomic landscape. 284 patients with iCCA treated for resectable, locally advanced or metastatic disease were selected and studied with the FOUNDATION Cdx technology. A comparative genomic analysis and survival analyses for the most relevant altered genes were performed between IDH1m and IDH1wt patients. Overall, 125 patients were IDH1m and 122 IDH1wt. IDH1m patients showed higher mutation rates compared to IDH1wt in CDKN2B and lower mutation rates in several genes including TP53, FGFR2, BRCA2, ATM, MAP3K1, NOTCH2, ZNF703, CCND1, NBN, NF1, MAP3KI3, and RAD21. At the survival analysis, IDH1m and IDH1wt patients showed no statistically differences in terms of survival outcomes, but a trend in favor of IDH1wt patients was observed. Differences in prognostic values of the most common altered genes were reported. In surgical setting, in IDH1m group the presence of CDKN2A and CDKN2B mutations negatively impact DFS, whereas the presence of CDKN2A, CDKN2B, and PBRM1 mutations negatively impact OS. In advanced setting, in the IDH1m group, the presence of KRAS/NRAS and TP53 mutations negatively impact PFS, whereas the presence of TP53 and PIK3CA mutations negatively impact OS; in the IDH1wt group, only the presence of MTAP mutation negatively impact PFS, whereas the presence of TP53 mutation negatively impact OS. We highlighted several molecular differences with distinct prognostic implications between IDH1m and IDH1wt patients.
Similar content being viewed by others
Introduction
Cholangiocarcinoma (CCA) represents a heterogeneous group of malignancies that arises from biliary epithelium, and it is generally regarded as a rare tumor in Western countries1. Nevertheless, over the last 15 years its incidence has steadily increased worldwide, and now it represents the second most common type of primary malignancy in the liver (15–20% of cases) after hepatocellular carcinoma2,3. Nowadays, platinum-based chemotherapy constitutes the backbone treatment of advanced and metastatic setting, but prognosis remains dismal, with a five-year survival rate of about 2% for stage IV4. The unsatisfactory results obtained could be related to some intrinsic characteristics of CCA, and mainly to a general incomprehension of its underlying molecular pathways. Indeed, all the previous clinical trials considered CCA as a whole group of diseases without considering the molecular heterogeneity, thus hindering the development of the optimal therapy aimed at the specific type of biliary tract cancer. Starting from these premises, a better understanding of the biological pathways underlying the carcinogenesis in CCA and an individual characterization of these tumors at the genomic, epigenetic and molecular levels has turned to be an urgent need. Recent advances in technical innovations in high-throughput molecular analysis have led to the discover of new potential therapeutic targets, including tumor suppressor genes involved in DNA damage repair pathway, kinases such as FGFR1, FGFR2, FGFR3, PIK3CA, ALK, EGFR, ERBB2, BRAF and AKT3, and oncogenes such as CCND3, MDM2 and, notably, IDH1 and IDH25.
IDH1-mutated CCAs constitute a group of neoplasms of particular interest in the biliary tract cancer field, due to a particular behavior and therapeutic implications. IDH genes encode for three different IDH enzymes, which are known to play an important role in the Krebs cycle and in cell metabolism6,7. In physiological conditions, IDH1 and IDH2 enzymes are involved in a two-step reaction which converts the isocitrate (ICT) in α-ketoglutarate (α-KG) by reducing the NADP+ in NADPH8,9,10. Given the involvement of IDH1 and IDH2 in cell metabolism, gain-of-function mutations of these genes lead to the accumulation of the oncometabolite 2-hydroxyglutarate (2-HG), as consequence of the neomorphic ability to convert α-KG into 2-HG11. 2-HG does not participate in normal metabolic processes but instead interferes with theα-KG -dependent reactions, thus resulting in DNA and histone hypermethylation, genetic instability, hypoxia gene signature activation, oxidative stress and alteration of the mTOR pathway and the mitochondrial electron transport chain12. IDH1/2 mutated forms are mostly absent in perihilar CCA (pCCA) and distal CCA (dCCA)12, whereas represent the 25% of intrahepatic CCA (iCCA) cases, with some differences depending on the geographical location12.
The discovery of mutations in IDH genes (IDH1 and IDH2) has revolutionized the therapeutic approaches and opened a new research way focused on possible targeted therapies capable of inhibiting the aberrant activity of the mutated isoforms. Nowadays, a number of IDH1 inhibitors are under investigations13; among these, AG-120 (Ivosidenib) received the FDA approval in advanced and metastatic CCA patients with IDH1 mutations due to the promising results of the randomized phase III trial ClarIDHy14.
Recently, high-throughput genomic sequencing techniques permitted to highlight the heterogeneous genomic scenario of iCCA harboring IDH1 mutations12,15,16,17. Nevertheless, these data are still far to be conclusive, since a significant heterogeneity between the cohorts and differences in inclusion criteria have to be considered.
The aim of the present work is to highlight the molecular differences in IDH1 mutated versus IDH1wt CCAs, with a special focus on the most relevant genomic alterations and their prognostic value in both CCA patients receiving a surgical intervention and those treated with systemic therapy.
Material and methods
Patients’ enrollment and sample collection
For this study, we selected 284 patients with iCCA treated for resectable, locally advanced or metastatic disease in six Italian institutions and one Spanish institute from January 2013 to March 2021. The sample included two different cohorts of patients: the first one included patients diagnosed at local stage who received radical surgery, and the second one included patients who relapsed after surgery or who were diagnosed at locally advanced or metastatic stages and judged to be candidate to receive exclusively systemic treatment. All patients were reviewed to confirm the pathologic diagnosis of ICC and evaluated with a chest-abdomen computed tomography (CT) according to the 8th edition 2017 AJCC staging system. After exclusion of 37 patients for lack of clinic-pathological and genomic information (including 10 cases lost to follow-up, 15 cases who lack clinical information and 12 patients who lack genomic information), 247 patients were eventually used for comparison of clinical, molecular and genomic characteristics and survival analysis (Supplementary Figure). Formalin-fixed paraffin-embedded (FFPE) samples and hematoxylin–eosin staining slides of the 247 patients (surgical specimens for patients who underwent surgery and biopsy specimens for patients who did not undergo surgery during their clinical history) were collected from Pathology Department of each single institutions. A full histopathologic review was performed by an expert gastrointestinal pathologist. Genic analysis of the primary tumors was performed by the FOUNDATION Cdx technology.
Clinical data
Clinical data including the patients’ gender, age, Eastern Cooperative Oncology Group (ECOG) Performance Status, kind of treatment received (surgical versus systemic, and type of systemic therapy) and pathological data, including surgical records when available, primary tumor location, histological grading and TNM stage according to the 8th edition 2017 AJCC staging system were carefully collected at the baseline, and used for analysis. Response to systemic treatment was assessed using RECIST criteria. For patients receiving a radical surgery, the follow-up was planned after 4 weeks from the intervention, and then each three months by performing a chest-abdomen CT-scan, laboratory tests including the Ca 19.9 and CEA blood-levels and clinical examination, until the evidence of relapsed disease. For patients receiving systemic treatment, response was assessed every 8–12 weeks by performing a chest-abdomen CT-scan, according to each institution’s clinical protocol. Patients receiving systemic therapy were treated according to the physician choice. For patients treated surgically, disease free survival (DFS) and overall survival (OS) from surgery were calculated. DFS was measured from the date of surgery to the date of first recurrence or last follow-up, whereas OS from surgery was defined as the interval between the date of surgery and the date of death or last follow-up. For patients diagnosed for a locally advanced or metastatic disease, who were stained not eligible for surgery, progression free survival (PFS) and OS from the first line treatment were calculated. PFS was measured from the date of the start of the first line therapy to the date of first recurrence or last follow-up. OS from the first line treatment was defined as the interval between the date of the first-line start and the date of death or last follow-up.
Identification of genomic alterations
FFPE tumor tissues containing at least 20% of tumor cells were collected from patients for genomic analysis detection by the NGS-based FoundationOne (FoundationOneR, Foundation Medicine Inc., MA, USA) gene panel. Identified alterations included base substitutions, insertions/delections (1–40 bp), copy number alterations-amplifications (ploidy < 4, amplification with copy number ≥ 8), copy number alterations-delections (ploidy < 4, homozygous delections), rearrangements and microsatellite status (determined by assessing indel characteristics at 114 homopolymer repeat loci in or near the targeted gene regions of the FoundationOne test).. The Foundation Medicine assay used was designed to analyze all genes know to be somatically altered in human solid tumors that are validated targets for therapy, either approved or in clinical trials, and/or that are unambiguous drivers of oncogenesis based on current knowledge. The assay employed a single DNA extraction method from routine FFPE biopsy or surgical resection specimens; 50–1000 ng o DNA underwent whole-genome shotgun library construction and hybridization-based capture of all coding exons from 309 cancer-related genes, one promotor region, one non-coding (ncRNA), and selected intronic regions from 34 commonly rearranged genes, 21 of which also included the coding exons. In total, the assay detects alterations in a total of 324 genes. Using the Illumina® HiSeq 4000 platform, hybrid capture–selected libraries were sequenced to high uniform depth (targeting > 500× median coverage with > 99% of exons at coverage > 100×). Sequence data were then processed using a customized analysis pipeline designed to detect all classes of genomic alterations, including base substitutions, indels, copy number alterations (amplifications and homozygous gene deletions), and select genomic rearrangements (e.g., gene fusions)18.
A descriptive analysis of the molecular landscape in the entire sample and in the two groups of patients was performed.
Statistical analysis
Categorical variables were presented as totals and frequencies, then evaluated by Chi-squared test of Fisher exact test, as appropriate. Continuous variables were described as means with standard deviations or medians with ranges, and compared with T test. The genomic alterations present in ≥ 5% of the entire sample were considered for the analysis of distribution of genomic alterations in the two groups of patients (IDH1m versus IDH1wt). The distribution analysis was performed by Fisher exact test. For the IDH1m and IDH1wt patients, correlative analyses between genetic alterations and survival outcomes were performed. DFS and OS from surgery, as well as PFS and OS from first line therapy were calculated by Kaplan–Meier method, and assessed by log-rank test for univariate analysis. The results were recorded as hazard ratios (HR) and 95% confidence intervals (CIs). A two-tailed P values less than 0.05 was considered statistically significant. DFS and OS from surgery as well as PFS and OS from the start of the first line treatment were estimated by the Kaplan–Meier method and curves were compared by the log-rank test. A p value < 0.05 was considered statistically significant. A MedCalc package (MedCalc® version 16.8.4) was used for statistical analysis. For the comparative genomic analysis as well as for the survival analysis of the sample who underwent to surgery and the sample who received first line chemotherapy, we considered only the gene alterations which were present at least in 7% of the whole cohort of patients.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of San Raffaele Hospital with number of registry: 113/INT/2021. Under the condition of retrospective archival tissue collection and patients’ data anonymization, our study was exempted from the acquisition of informed consent from patients by the institutional review board.
Institutional review board statement
The Ethical Review Board of each Institutional Hospital approved the present study. This study was performed in line with the principles of the Declaration of Helsinki.
Informed consent statement
Written informed consent for treatment was obtained for all patients.
Results
Clinical characteristics in intrahepatic cholangiocarcinoma patients
Overall, 247 consecutive iCCA patients were retrospectively analyzed. 125 patients were IDH1m and 122 IDH1wt. 128/247 patients received surgical treatment. By considering the two populations, IDH1m and IDH1wt patients, no significative differences were found in terms of clinic-pathological characteristics, except for the gender (female 65.60% vs 40.98% in IDH1m group and IDH1wt group, respectively; p = 0.000126). The median age at diagnosis was 5928 in IDH1m group compared to 62 (33–83) in IDH1wt group. At the baseline, 7% and 2% of patients were diagnosed of CCA at stage I, whereas 76% and 43% were diagnosed at more advanced stages (II, III and IV), in the IDH1m and IDH1wt groups, respectively (p = 0.3304). At the start of the first-line therapy, 69/125 (55%) and 60/122 (49%) of patients presented an ECOG PS of 0 in IDH1m and IDH1wt groups of patients, respectively (p = 0.6587). In the IDH1m group, the 46% received surgical intervention with radical intention and 86% were treated with systemic therapy during their oncologic history; in the IDH1wt group of patients, 58% received surgical intervention with radical intention and 82% were treated with systemic therapy during their oncologic history. Finally, 90/125 (72%) of IDH1m patients and 75/122 (61.5%) of IDH1wt patients received the first line standard of care cisplatin plus gemcitabine, whereas 17/125 (13%) and 25/122 (20.5%) received other regimens in IDH1m and IDH1wt groups of patients, respectively (p = 0.120798). No patient received Ivosidenib or another IDH1 inhibitor as first line treatment in our sample (Table 1).
Genomic alterations in intrahepatic cholangiocarcinoma patients
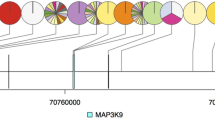
We first annotated alterations to specific genes. Overall, genomic sequencing performed by FoundatioOne assays identified a total of 1446 genomic alterations in the entire sample which involved 262 genes, with a mean of 4.65 alterations per gene (range 0–66). All the samples presented at least one genomic alteration, with a median of genomic alterations for patients of 7.21 (range 1–44). The most common genomic alterations were found in CDKN2A (27%), ARID1A (20%), CDKN2B (17%), PBRM1 (17%), KRAS/NRAS (16%), BAP1 (16%), TP53 (15%), FGFR2 (10%), BRCA2 (9%), PIK3CA (8.5%), ATM (7%), MTAP (7%) and MAP3K1 (7%) (Fig. 1).
Mutations of all remaining genes were detected less than 7% of the entire sample, and 15% of all the analyzed genes were mutated once in a single sample, including KIT, PIK3CG, NTRK2, GATA4, BCL2, FANCC, FGFR1, MAP2K1, MAPK1, RAD51 and RAD52.
Genomic alterations in IDH1m versus IDH1wt patients
A comparative analysis of the IDH1m group versus the IDH1wt group of patients highlighted several differences in terms of mutations distribution and molecular landscape. Overall, IDH1m CCA showed a lower incidence of detected mutations compared to IDH1wt CCA. If we focus on concrete gene alterations, IDH1m patients showed higher mutation rate compared to IDH1wt in CDKN2B (22% Vs 12%, p = 0.04) and lower mutation rate in TP53 (7% versus 23%, p = 0.0006), FGFR2 (4% versus 16%, p = 0.0013), BRCA2 (3% versus 15%, p = 0.0015), ATM (4% versus 10.5%, p = 0.047) MAP3K1 (2% versus 11%, p = 0.0053), NOTCH2 (1.5% versus 10%, p = 0.005), ZNF703 (1.5% versus 7%, p = 0.033), CCND1 (1% versus 7%, p = 0.01), NBN (0% versus 7%, p = 0.0015), NF1 (0% versus 6.5%, p = 0.003), MAP3KI3 (1% versus 6%, p = 0.034), RAD21 (0% versus 6.5%, p = 0.003), ESR1 (0% versus 6%, p = 0.007), GATA6 (0% versus 6%, p = 0.007), MYC (o% versus 5%, p = 0.014), AXIN1 (0% versus 5%, p = 0.014), TSC2 (0% versus 5%, p = 0.014), PARP2 (0% versus 5%, p = 0.014), WHSC1L1 (0% versus 5%, p = 0.014), ERBB3 (0% versus 5%, p = 0.014), ALK (0% versus 4%, p = 0.03), HSD3B1 (0% versus 4%, p = 0.03), PARK2 (0% versus 4%, p = 0.03), CTN1 (0% versus 4%, p = 0.03), MITF (0% versus 4%, p = 0.03), RICTOR (0% versus 4%, p = 0.03), BCOR (0% versus 4%, p = 0.03), NTRK1 (0% versus 4%, p = 0.03), EPHA3 (0% versus 4%, p = 0.03) and DOT1L (0% versus 4%, p = 0.03) (Table 2).
Targetable alterations
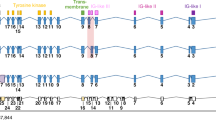
We identified 22 frequently (≥ 5%) mutated genes identified in the whole sample (Table 2), where 15 were highlighted to be actionable according to the TARGET database by the Broad Institute (http://archive.broadinstitute.org/cancer/cga/target), including: CDKN2A, CDKN2B, NRAS/KRAS, BAP1, TP53, FGFR2, BRCA2, PIK3CA, ATM, MDM2, PIK3C2B, NOTCH2, MCL1, MLL2. By comparing the two scenarios, those of the IDH1m patients and those of the IDH1wt patients, several differences in terms of incidence of targetable mutations has been highlighted (Fig. 2).
Survival analysis according to the genomic landscape
Resected patients
The survival analysis for DFS and OS from surgery was performed on the sample of resected patients (N = 128).
At the univariate analysis, IDH1m and IDH1wt patients showed no statistically differences in terms of DFS and OS from surgery (p = 0,6156, p = 0,2645; respectively). Nevertheless, a tendence toward a better OS was highlighted for IDH1wt patients compared IDH1m patients.
At the univariate analysis for DFS from surgery conducted for the most commonly altered genes in our sample, in the IDH1m group the presence of CDKN2A and CDKN2B mutations were highlighted to have a negative prognostic impact (CDKN2A HR 3.78, 95% CI 1.37–10.41, p = 0.0001; CDKN2B HR 3.46, 95% CI 1.68–10.28, p = 0.0004) (Fig. 3a,b). On the other hand, no one gene showed to affect prognosis in terms of DFS from surgery in the IDH1wt group of patients (Supplementary Table 1).
At the univariate analysis for OS from surgery conducted for the most commonly altered genes in our sample, in the IDH1m group of patients the presence of CDKN2A, CDKN2B, and PBRM1 mutations were highlighted to have a negative prognostic impact (CDKN2A HR 3.20, 95% CI 1.01–10.14, p = 0.0096; CDKN2B HR 3.20, 95% CI 1.01–10.14, p = 0.0096; PBRM1 HR 6.61, 95% CI 2.36–18.50, p = 0.04) (Fig. 3c–e). On the other hand, no one gene showed to affect prognosis in terms of OS from the surgical intervention in the IDH1wt group of patients (Supplementary Table 1). After adjustment for the clinical covariates known to be related to prognosis in this setting of patients (Stage disease and ECOG Performance Status), alterations in CDKN2A and MTAP were confirmed to be negative prognostic factor for DFS in this cohort of patients.
Patients treated with systemic therapy
The analysis for PFS and OS from the start of first line therapy was performed on the sample of patients receiving systemic treatments (N = 207).
At the univariate analysis, IDH1m and IDH1wt patients showed no statistically differences in terms of OS and PFS from the first-line treatment (p = 0.1179, p = 0.6203; respectively). Nevertheless, a tendence toward a better OS was highlighted for IDH1wt patients compared IDH1m patients.
At the univariate analysis for PFS from the first line therapy conducted for the most commonly altered genes in our sample, in the IDH1m group of patients, the presence of KRAS/NRAS and TP53 mutations were highlighted to negatively affect prognosis (KRAS/NRAS HR 2.06, 95% CI 0.94–4.51, p = 0.0136; TP53 HR 2.05, 95% CI 0.80–5.22, p = 0.0377) (Fig. 4a,b).
On the other hand, in the IDH1wt group of patients, only the presence of MTAP mutation was highlighted to have a negative prognostic impact on PFS from the first-line treatment (HR 3.40, 95% CI 1.11–10.46, p = 0.0327) (Fig. 4c) (Supplementary Table 2).
At the univariate analysis for OS from the first line therapy conducted for the most commonly altered genes in our sample, IDH1m and IDH1wt patients showed a different behavior. In particular, in the IDH1m group of patients, the presence of TP53 and PIK3CA mutations were highlighted to negatively impact the prognosis (TP53 HR 4.39, 95% CI 1.34–14.41, p = 0.0146; PIK3CA HR 2.67, 95% CI 1.09–6.57, p = 0.0320) (Fig. 4d,e).
On the other hand, in the IDH1wt group, only the presence of TP53 mutation was highlighted to have a negative prognostic impact in terms of OS from the first-line treatment (HR 1.97 95% CI 0.88–4.39, p = 0.0355) (Fig. 4f) (Supplementary Table 2). After adjustment for the clinical covariates known to be related to prognosis in this setting of patients (Stage disease and ECOG Performance Status), alterations in TP53 were confirmed to be negative prognostic factors for PFS and OS in this cohort of patients.
Discussion
The present work has the merit to be a comprehensive genomic analysis conducted on a large sample of iCCA patients, which include both IDH1m and IDH1wt cases, thus highlighting differences in terms of molecular profile between these two groups of patients. In the whole sample, our analysis reported a high incidence of genomic alterations in CDKN2A, ARID1A, CDKN2B, PBRM1, KRAS/NRAS, BAP1, TP53, FGFR2, which were highlighted in ≥ 10% of the entire sample of patients. Several studies recently analyzed molecular landscape of CCA by integrate genomic, transcriptomic and epigenomic data, some of them with a special focus on iCCA patients. Lowery et al. reported the genome profiling of CCA patients, including 152 iCCA and 43 eCCA, from Caucasian (89.2%, 174/195), Asian (7.1%, 14/195) and African American (3.6%, 7/195) patients and found that the most common mutations were IDH1, TP53, ARID1A, BAP1, KRAS, PBKM1, SMAD4 and ATM15. In the recent analysis from Jiang and collaborators, the most frequent mutated genes found in Chinese CCA patients were TP53 (41.27%, 26/63), KRAS (31.75%, 20/63), ARID1A and IDH1 (15.87%, 10/63, for both), SMAD4 (14.29%, 9/63), FGFR2 and BAP1 (12.70%, 8/63, for both) and CDKN2A (11.11%, 7/63)19. Recently, a bi-institutional study on 412 iCCA patients revealed as most common mutated genes IDH1 (20%), ARID1A (20%), TP53 (17%), CDKN2A (15%), BAP1 (15%), FGFR2 (15%), PBRM1 (12%) and KRAS (10%)19. Notably, these latter results were particularly similar to those highlighted in our analysis, with the exception of CDKN2A, which was more frequently mutated in our cohort of patients.
The differences reported between our analysis and the previous ones in terms of mutations frequencies could be explained by referring to the differences of the cohorts, as well as to the inclusion criteria. Indeed, our analysis included exclusively European iCCA patients from two different countries (Italy and Spain), whereas several previous experiences were conducted on mixed population which included Asiatic patients, or samples exclusively composed by Asiatic patients, which have been previously highlighted to carry different mutational profiles compared western populations19. Moreover, due to the rarity of the disease, the previous studies were not focused on IDH1m CCA patients, and the sample size of IDH1m patients in the cohorts were too small to characterize this subtype of iCCA.
By performing a comparative analysis, we highlighted two different molecular profiles for IDH1m and IDH1wt patients. More specifically, IDH1m samples showed a lower incidence of genomic alterations compared with IDH1wt samples, and were highlighted to be enriched in CDKN2A (29%), ARID1A (22%), CDKN2B (22%), PBRM1 (18%), NRAS/KRAS (13%), BAP1 (13%), PIK3CA (11%), TP53 (7%), MTAP (7%), MUTYH (5.5%), MDM2 (5%), MCL1 (5%) and DNMT3A (5%). If considering the most relevant genomic alterations in our sample, CDKN2B was highlighted to be more frequently mutated in IDH1m patients, whereas TP53, FGFR2, BRCA2, ATM, MAP3K1 and NOTCH2 resulted to be more frequently altered in IDH1wt patients. Consistently with these results, in a previous work Farshdifar and collaborators identified an IDHm-enriched subtype with distinct molecular features including low expression of chromatin modifiers (also cadherins), elevated expression of mitochondrial genes, and increased mitochondrial DNA copy number20,21.
Interestingly, several differences in terms of incidence of targetable mutations according the TARGET database by the Broad Institute (http://archive.broadinstitute.org/cancer/cga/target) have been highlighted between IDH1m and IDH1wt patients. Nowadays, the identification of targetable mutations is a hot topic in oncologic field, since patients carrying one or more actionable lesions could have broad opportunities for treatment. The identifications of differences in terms of targetable mutations’ incidence between IDH1m and IDH1wt patients could suggest novel therapeutic strategies which could be investigated in concomitating and/or in sequencing to the recently studied IDH inhibitors. In particular, our results could suggest that IDH1m patients may benefit from treatments which interfere with the cell cycle, such as CDK 4/6 inhibitors; on the other hand, IDH1wt patients may benefit from PARP inhibitors and FGFR2 inhibitors. Further studies are needed in order to verify our hypothesis, with the hope that new prospective trials investigating the efficacy of personalized target therapies could be designed in the next future for this setting of patients.
Concerning the survival analysis, several interesting considerations could be done. Firstly, in the cohort of patients receiving surgery, the analysis revealed CDKN2A/B alterations as negative prognostic factors in terms of DFS and OS from surgery in the subset of patients carrying IDH1 mutations, whereas no prognostic implication was highlighted in IDH1wt patients. Significantly, mutations in CDKN2A were confirmed to be negative prognostic factor in terms of DFS after adjustment for the clinical covariates known to impact prognosis in patients receiving surgery.
Cyclin-dependent kinase (CDK) inhibitor 2A (CDKN2A) and 2B (CDKN2B) are known to play an important role in cell-cycle regulation through inhibition of CDK4/6. CDKN2A/2B loss or mutation are associated with tumor progression, invasion and metastasis and have been reported in 7–18% of iCCAs22,23,24. Basing on these reports, CDK4/6 inhibitors have been recently tested in monotherapy in patients carrying CDKN2A alterations, without the wished results25. Previously, Lowery and collaborators showed that alterations in CDKN2A/B were associated with reduced survival and time to progression on chemotherapy in patients with locally advanced or metastatic disease15. Interestingly, the role of CDKN2A/B alterations in CCA is consistent with those found in other onco-hematologic settings, such as acute lymphoblastic leukemia, where CDKN2A/B mutations have been highlighted as independent poor prognostic markers and then included in the risk stratification26. Further genomic studies are necessary in order to define the prognostic and predictive role of CDKN2A/B mutations in CCA setting, and in order to explicate the different role revealed in IDH1m and IDH1wt patients in our analysis. Another important highlight in our analysis concerns the role of TP53 mutations in the cohort of patients treated with systemic therapy. In fact, alterations in TP53 were highlighted to negatively impact prognosis in both IDH1m and IDH1wt patients in this setting of patients. Curiously, alterations in KRAS and PIK3CA resulted to negatively affect prognosis in IDH1m patients, but not in IDH1wt patients. As reinforce to our result, mutations in TP53 were confirmed to be negative prognostic factors in terms of both PFS and OS in patients receiving a first line therapy after adjustment for the clinical covariates known to impact on prognosis in this subset of patients. The association between mutations in both TP53 and KRAS and poor prognosis is not novel for iCCA, since our data are consistent with previous reports16,22,27,28. Simbolo and collaborators performed a high-coverage target sequencing analysis on two groups of iCCA patients selected according to prognostic performance, and found that in the group of patients with poor prognosis (OS < 36 months) TP53 was the most mutated gene (p = 0.011) and exclusively present in these cases. At the multivariate analysis, mutations in TP53 have been confirmed to be independent predictors of poor prognosis17. The role of TP53 in CCA was further investigated by Tian and colleagues, who performed a comprehensive genomic analysis on 66 Chinese CCA patients, thus revealing TP53 as a suitable diagnostic and predictive biomarker in Chinese patients with CCA24. Association between KRAS mutation, perineural invasion, large bile duct type, and worse outcome after iCCA resection have also been reported22. In a further integrative genomic analysis, the authors found that for all patients TP53, KRAS and CDKN2A alterations predicted worse OS across all stages, even when controlling for known correlates of outcome (multifocal disease, lymph node involvement, bile duct type, periductal infiltration). In resected patients (n = 209), TP53 mutations and CDKN2A deletions independently predicted shorter OS; in unresectable iCCA, TP53, KRAS mutations and CDKN2A deletions similarly predicted worse outcome21.
All these data are partially consistent with our results, and our research could reinforce previous insights on the genomic landscape of CCA, mainly concerning the negative prognostic role of TP53. On the other hand, our analysis focused on the IDH1 mutations, thus providing, for the first time, a large sample of IDH1m patients.
Our research presents several limitations. Firstly, it was conducted as a retrospective investigation, thus several selection bias could be ascribed to the same nature of the study. Secondly, since several genes which resulted to significantly impact on OS at the univariate analysis belong to linked biological pathways, or to the same biological pathway, the eventual multivariate analysis would have been not informative, and for this reason has not been performed, thus reducing the powerful of the study. Moreover, in order to investigate the differences in terms of genomic landscape between IDH1m and IDH1wt CCA patients, we have recruited patients no consecutively, in order to define two large sample of patients to compare. Then, several clinic-pathological and familiar data have been excluded in our analysis, since our objective was to perform a pure genomic analysis with the definition of gene signatures able to stratify our patients. Finally, the results of our survival analysis have to be validated on an external cohort of patients. Nevertheless, it would be difficult to validate our results on an external cohort, since the most important cohort investigating CCA samples included a few proportion of IDH1m patients.
Conclusion
In conclusion, we performed a comparative genomic analysis on a large sample of iCCA patients, thus highlighted several molecular differences between IDH1m and IDH1wt patients, as well as interesting highlights on the prognostic role of genomic alterations. Once validated, our results could add new pieces to the puzzle of the heterogeneous scenario of CCAs, with the ultimate goal of opening the way to further researches focused on new therapeutic strategies depending on the genomic signatures.
Data availability
Data available on request from the authors (margherita.rimini@gmail.com).
References
Sia, D., Villanueva, A., Friedman, S. L. & Llovet, J. M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 152, 745–761 (2017).
Khan, S. A., Thomas, H. C., Davidson, B. R. & Taylor-Robinson, S. D. Cholangiocarcinoma. Lancet 366(9493), 1303–1314 (2005).
Petrick, J. et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEERmedicare. PLoS ONE 12, 10 (2017).
Howlader, N., Noone, A.M., Krapcho, M., et al. (Eds.) SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD, Based on November 2015 SEER Data Submission, Posted to the SEER Web Site; April 2016. http://seer.cancer.gov/csr/1975_2013/. Accessed 10 Dec 2016 (2016).
Rimini, M. et al. Cholangiocarcinoma: New perspectives for new horizons. Exp. Rev. Gastroenterol. Hepatol. 15(12), 1367–1383. https://doi.org/10.1080/17474124.2021.1991313 (2021) (epub 2021 Nov 9).
Waitkus, M. S., Diplas, B. H. & Yan, H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell 34(2), 186–195 (2018).
Dang, L., Yen, K. & Attar, E. C. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann. Oncol. 27(4), 599–608 (2016).
Fujii, T., Khawaja, M. R., DiNardo, C. D., Atkins, J. T. & Janku, F. Targeting isocitrate dehydrogenase (IDH) in cancer. Discov. Med. 21(117), 373–380 (2016).
Clark, O., Yen, K. & Mellinghoff, I. K. Molecular pathways: Isocitrate dehydrogenase mutations in cancer. Clin. Cancer Res. 22(8), 1837–1842 (2016).
Liu, X. & Ling, Z. Q. Role of isocitrate dehydrogenase 1/2 (IDH 1/2) gene mutations in human tumors. Histol. Histopathol. 30(10), 1155–1160 (2015).
Krell, D. et al. IDH mutations in tumorigenesis and their potential role as novel therapeutic targets. Future Oncol. 9(12), 1923–1935 (2013).
Boscoe, A. N., Rolland, C. & Kelley, R. K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest. Oncol. 10, 751–765 (2019).
Acher, A. W., Paro, A., Elfadaly, A., Tsilimigras, D. & Pawlik, T. M. Intrahepatic cholangiocarcinoma: A summative review of biomarkers and targeted therapies. Cancers (Basel). 13(20), 5169. https://doi.org/10.3390/cancers13205169.PMID:34680318;PMCID:PMC8533913 (2021).
Abou-Alfa, G. K. et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21(6), 796–807. https://doi.org/10.1016/S1470-2045(20)30157-1 (2020) (epub 2020 May 13. Erratum in: Lancet Oncol. 2020 Oct;21(10):e462).
Lowery, M. A. et al. Comprehensive molecular profiling of intra- hepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin. Cancer Res. 24, 4154–4161 (2018).
Nakamura, H. et al. Genomic spectra of biliary tract cancer. Nat Genet. 47(9), 1003–1010. https://doi.org/10.1038/ng.3375 (2015) (epub 2015 Aug 10).
Simbolo, M. et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 5, 2839–2852 (2014).
Current as of April 2022. https://www.foundationmedicine.com/f1cdx.
Jiang, G. et al. Characteristics of genomic alterations in Chinese cholangiocarcinoma patients. Jpn. J. Clin. Oncol. 50(10), 1117–1125 (2020).
Boerner, T. et al. Genetic determinants of outcome in intrahepatic cholangiocarcinoma. Hepatology 74(3), 1429–1444 (2021).
Farshidfar, F. et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep. 18, 2780–2794 (2017).
Churi, C. R. et al. Mutation profiling in cholangiocarcinoma: Prognostic and therapeutic implications. PLoS ONE 9, e115383 (2014).
Ross, J. S. et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 19, 235–242 (2014).
Tian, W. et al. Comprehensive genomic profile of cholangiocarcinomas in China. Oncol. Lett. 19(4), 3101–3110 (2020).
Al Baghdadi, T., Halabi, S., Garrett-Mayer, E. et al. Palbociclib in Patients with Pancreatic and Biliary Cancer with CDKN2A Alterations: Results from the Targeted Agent and Profiling Utilization Registry Study.
Zhang, W., Kuang, P. & Liu, T. Prognostic significance of CDKN2A/B deletions in acute lymphoblastic leukaemia: A meta-analysis. Ann. Med. 51(1), 28–40 (2019).
Javle, M. et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 122, 3838–3847 (2021).
Ruzzenente, A. et al. Cholangiocarcinoma heterogeneity revealed by multigene mutational profiling: Clinical and prognostic relevance in surgically resected patients. Ann. Surg. Oncol. 23, 1699–1707 (2021).
Author information
Authors and Affiliations
Contributions
Conception and design: A.C.-G., M.R. Acquisition of data (acquired and managed patients): All authors. Analysis and interpretation of data: A.C.-G., M.R. Writing, review, and/or revision of the manuscript: A.C.-G., M.R. Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
MN: Travel expenses from Celgene, speaker honorarium from Accademia della Medicina. Consultant honoraria from EMD Serono, Basilea Pharmaceutica, Incyte and MSD Italia. FdB: Honoraria from Roche, Pfizer, BMS, Merck, MSD, SERVIER, Sanofi, Amgen Astellas BioPharma, Incyte. Consulting or Advisory Role for Roche, Incyte, EMD Serono, BMS, Nerviano Medical Sciences, Sanofi, Novartis Italy, Menarini, research funding (institution): Novartis, Roche, Merck Serono, Pfizer, Servier, Philogen, Loxo, Tesaro, Nerviano Medical Sciences, Kymab. Research funding: BMS/Medarex, Merck KGaA, Ignyta, MedImmune, Exelis, Bayer health, Daiichi Sangyo Europe GmbH, Incyte, Basilea Pharmaceutical, jassen Oncology. TM: (SOBI) Swedish Orpahn Biovitrum AB, Ability Pharmaceuticals SL, Aptitude Health, AstraZeneca, Basilea Pharma, Baxter, BioLineRX Ltd, Celgene, Eisai, Ellipses, Genzyme, Got It Consulting SL, Hirslanden/GITZ, Imedex, Incyte, Ipsen Bioscience , Inc, Janssen, Lilly. Marketing Farmacéutico & Investigación Clínica, S.L, MDS, Medscape, Novocure, Paraxel, PPD Development, Polaris, QED Therapeutics, Roche Farma, Sanofi-Aventis, Servier, Scilink Comunicación Científica SC, Surface Oncology, and Zymeworks. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rimini, M., Fabregat-Franco, C., Burgio, V. et al. Molecular profile and its clinical impact of IDH1 mutated versus IDH1 wild type intrahepatic cholangiocarcinoma. Sci Rep 12, 18775 (2022). https://doi.org/10.1038/s41598-022-22543-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22543-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.