Abstract
Over the past few years, evidence of a positive relationship between inflammation and depression has grown steadily. The aim of the current study was to investigate whether such depression-related inflammation could also be associated with altered microstructural changes in the white matter. FA and serum cytokines (IL-1β, IL-6, TNF-α, and IFN-γ) were measured in 25 patients with depression (DE) and 24 healthy controls (HC). Diffusion tensor imaging was performed. Fractional anisotropy (FA) was calculated using the FSL pipeline for Tract-Based Spatial Statistics (TBSS). Both voxelwise and mean whole-brain FA were analyzed using general linear models (GLM). Higher concentrations of IL-1β were associated with lower whole-brain fractional anisotropy, particularly in people with depression (ρ = − 0.67; p < 0.001). TNF-α shared some variance with IL-1β and also showed a negative relationship between TNF-α concentrations and FA in depression (F1,46 = 11.13, p = 0.002, η2p = 0.21). In detail, the voxelwise analysis showed that the regression slopes of IL-1β on FA were more negative in the DE group than in the HC group, mainly in the corpus callosum (cluster statistics: genu corpus callosum, p = 0.022; splenium of corpus callosum, p = 0.047). Similar effects were not found for the other remaining cytokines. This study clearly demonstrated an association between peripherally measured IL-1β and white matter integrity in depression as assessed by DTI. The results suggest that microstructural changes in the corpus callosum are associated with increased peripheral IL-1β concentrations in depression.
Similar content being viewed by others
Introduction
Fractional anisotropy (FA) is a standard measurement in diffusion tensor imaging (DTI), which describes the movement of water molecules, from isotropic (FA = 0) to anisotropic movement (FA = 1). Examples of isotropic states are the cerebrospinal fluid (CSF) and the restriction of the movement of water molecules through membranes in fiber bundles for anisotropic states1,2,3.
A decrease in FA is associated with changes in white matter (WM), such as decreased axonal density and demyelination. In most cases, this correlates with a disease or a neuroinflammatory process4,5,6. Reduced FA levels have also been reported in different medical conditions, particularly mental disorders7,8,9, including depression10. In participants with depression, for instance, reduced FA values were observed for various WM fiber tissues11,12,13,14,15,16,17,18. Most of these studies included participants with first-episode depression19,20,21, acute exacerbation of depression11,13, drug-naïve patients17,18,19, and participants in inpatient or outpatient treatment15,16,22,23,24,25. Although an increasing body of literature supports reduced FA levels in depression, the pathophysiology of WM structural changes in depression is not clearly understood.
Likewise, alterations of the WM structure are observed when peripheral inflammation is present. Post-mortem and animal studies have demonstrated that peripheral inflammation is associated with WM injury26,27. Moreover, other experimental studies have also shown that psychological stress could cause the downregulation of the claudin-5, promoting the entrance of peripheral pro-inflammatory cytokines into the central nervous system and damaging the white matter integrity28,29,30. In addition, many studies have highlighted in the past decade the in vivo relationship between depression and peripheral inflammation31,32,33,34,35. Two meta-analyses by Köhler et al. and Dowlati et al. pointed out, for example, that patients with major depressive disorder (MDD) had increased peripheral pro-inflammatory cytokines such as IL-6 and TNF-α compared to healthy controls31,36. Another meta-analysis showed that IL-6 was increased in depressed subjects compared to healthy subjects32. A meta-analysis by Howren et al. showed positive correlations between depression and the cytokines IL-1 and IL-6 in patients with depression37. In addition, recent studies have shown significant correlations in depressive episodes between WM structures and peripheral inflammation10,34,38. In a volumetric MRI study by Frodl et al., participants with depression who were not drug-naïve showed smaller hippocampal volumes but higher IL-6 concentrations39. Two further DTI studies showed negative correlations between the FA values of various WM fiber tracts and peripheral inflammatory cytokines (i.e. IL-1β and TNF-⍺), as well as with acute-phase inflammatory proteins (i.e. CRP) in patients with a first episode or drug-naïve depressed patients28,34,38.
This literature shows that peripheral inflammation and changes in WM microstructure are associated with depression28,34,38. It would be interesting to see this in drug-naïve first-episode depression and in people in outpatient treatment to define endophenotypes in depression40. Therefore, the main aim of this study was to investigate the relationship between WM integrity as measured by FA and peripheral inflammation in a community sample with and without depression. A negative association between peripheral inflammation and fractional anisotropy in depression is expected. However, no a priori assumptions were made about the regional distribution of these changes.
Materials and methods
This work is part of a study on depression and also fatigue, which included the investigation with (sMRI, fMRI, DTI, EEG) in different samples. Results on cytokine concentrations, depressive symptoms and fatigue in the total sample of this project were published elsewhere by Pedraz-Petrozzi et al.35.
Study participants
Fifty-one participants were recruited between June and September 2019. Two participants revoked their consent to have blood drawn while the experiment was being carried out and were therefore excluded from participation. In the end, one group consisted of 24 participants with a depressive episode (DE group) and another group of 25 participants without depression (healthy control group or HC group). The groups were frequency-matched according to age and gender. The main characteristics of the DE and HC groups are reported in Table 1. Participants in the DE group had received medical, psychotherapeutic, or both types of treatment for at least 6 months before this study. As inclusion criterion, participants of the DE group had to meet the ICD-10 criteria (World Health Organization, 10th version of the International Classification of Diseases) for a depressive episode. The rating was carried out by clinical experts from the Department of Psychiatry at the University Hospital of Giessen35,41. People with psychotic disorders, current psychotic episodes, or any medical condition were not included in the study; however, participants with a comorbid psychiatric disorder, such as personality or anxiety disorders, were included if they had a predominant depressive episode in the past 6 months. Individuals could not participate if they suffered from acute or chronic disease or illness, particularly infection-related, except depression.
No participant in the HC group was taking any psychotropic drugs. In the group of depressed participants (DE), 18 of 24 received pharmacotherapy, 14 of whom received monotherapy with antidepressants (4 with escitalopram, 1 with sertraline, 2 with citalopram, 1 with paroxetine, 2 with fluoxetine, 1 with opipramol, 1 with duloxetine, and 1 with venlafaxine) or quetiapine (1 participant). Another 3 participants were treated with 2 antidepressants (sertraline + amitriptyline, venlafaxine + mirtazapine, and citalopram + bupropion), and one participant was treated with a combination of duloxetine and prothipendyl. Finally, 1 participant received diazepam pro re nata (PRN). The remaining participants indicated that they had been without pharmacological treatment for at least 6 months.
Insufficient knowledge of German and severe somatic restrictions, such as severe visual and hearing impairments, or age over 65 years, were considered as exclusion criteria. Other exclusion criteria corresponding to the safety instructions of the MRI scanner manufacturer were also considered in this study.
The estimated power of the total sample was estimated using G*Power 3.1 (N = 49, power = 0.95) and is above the accepted minimum power threshold (1 − β = 0.80), which indicates that the sample size for this study design is sufficient to meet the study objectives to reach.
Evaluation of depression symptoms: BDI-FS
The German validated version of the Beck Depression Inventory—fast screening (BDI-FS) was used to assess the symptom burden of the depression for each participant35,42. The BDI-FS is a validated short version of the BDI-II, which evaluates self-criticism, self-aversion, past failure, pessimism, anhedonia, sadness, and suicidal behavior (i.e., thoughts or wishes). The BDI-FS consists of 7 items, and the scores ranged between 0 and 21 points. A higher score reflects a higher symptom burden of a depressive episode. The scores can be summarized in four different categories (minimal, mild, moderate, and severe) based on the total number of points (minimal: 0–3 points; mild: 4–8 points; moderate: 9–12 points; severe: 13–21 points)35.
Peripheral inflammatory markers—cytokines
Between 8:00 am and 12:00 pm, peripheral venous blood samples were collected with potassium Ethylenediaminetetraacetic acid sample tubes (K-EDTA, SARSTEDT AG & Co. KG, Nümbrecht, Germany). Blood samples were recollected after 12 h of fasting. After recollection, the blood samples were centrifuged for 15 min at 4 °C at 1100×g, and the collected plasma was immediately stored at – 20 °C. The plasma tubes were delivered to the clinical immunology research facilities of the Justus Liebig University Giessen, within a maximum of 4 weeks and stored at – 80 °C. Four different cytokines (interleukin 6 or IL-6, interleukin 1 beta or IL-1β, tumor necrosis factor alpha or TNF-α, and interferon-gamma or IFN-γ) were measured using Quantikine ELISA kits (R&D Systems Inc., Minneapolis, Minnesota, United States of America). Intra- and inter-precision values were < 10%. Any values below the minimum detectable dose were considered zero and included in the analysis. The selected cytokines’ concentration was estimated using Tecan Reader with the Magellan Reader Software (Tecan Group Ltd., Männedorf, Switzerland). Parameters were calculated using Marquardt’s 4-parameter estimation method.
Diffusion tensor imaging
Data acquisition
All participants were subjected to a DTI imaging protocol with a SIEMENS MAGNETROM Prisma 3.0 Tesla MRI scanner and a 64-channel head coil (Bender Institute for Neuroimaging, Faculty of Psychology, Justus-Liebig University Giessen).
The DTI protocol consisted of two measurements, anterior–posterior (AP) and posterior–anterior (PA). The AP DTI image protocol was measured with the following settings: TR/TE = 6200 ms/67 ms, 60 slices, slice thickness = 2 mm, field of view (FoV) = 232 mm, number of excitations = 1, and spatial resolution = 2 × 2 × 2 mm, diffusion gradient (b value) = 2000 s/mm2, duration = 7 min and 40 s. Regarding the diffusion gradient, an MDDW diffusion mode (Siemens Multidirectional Diffusion Imaging) with 64 directions and 2 weightings was used. The PA DTI image protocol was used with settings similar to the AP protocol: TR/TE = 6200 ms/67 ms, 60 slices, slice thickness = 2 mm, field of view (FoV) = 232 mm, number of excitations = 1 and spatial resolution = 2 × 2 × 2 mm, diffusion gradient (b-value) = 2000 s/mm2, duration = 1 min and 41 s. Similar diffusion gradient settings were used in the PA imaging protocol, namely an MDDW diffusion mode with 6 directions and 2 weightings.
Image processing
Fractional anisotropy (FA) was chosen as the preferred measure in this study because it is the most summary measure of microstructural integrity, which has been extensively shown to be very sensitive to different types of microstructural changes. However, it should be noted that other DTI measurements, including axial or radial diffusion or free water analysis, have recently been used in inflammation and MDD research and may represent promising approaches43,44.
FA was computed using the FMRIB Software Library (FSL)45 FDT-processing pipeline. No settings had to be adjusted. Dicom data were converted into NIFTY, vector orientation was checked, susceptibility-induced distortion correction (fieldmap estimation) was performed using topup46, brain extraction applied BET47 on the output of topup. Distortion correction (eddy currents, susceptibility-induced distortions, and subject’s motion) was conducted using eddy48 with a fieldmap estimated by topup. Further, diffusion tensors were fitted on the eddy-corrected data using dtifit. For voxel-wise statistical analyses, data were processed applying the standard FSL pipeline for TBSS49. Finally, the whole-brain FA was calculated as the average of all non-zero values of each FA-skeleton from the participants.
Statistical analysis
General information, including clinical data and peripheral cytokine concentrations, are displayed as tables. Continuous variables are presented using measures of central tendency (mean, standard deviation) and dichotomous categorical variables using frequency and count data. T-tests (continuous variables) or Fisher’s exact tests (dichotomous categorical variables) were applied for differences between groups. The means were considered different if they had a two-sided p value ≤ 0.05. In this case, homogeneity between the groups was assumed if the p value was greater than 0.05.
To evaluate the relationship between WM integrity and peripheral inflammation, correlations between the mean whole-brain FA values, the four cytokines, and the BDI-FS were tested first for each group separately, following the recommendations of previous studies50,51. Furthermore, the correlations were partialized and controlled for age and BMI since these variables are frequently confounding factors in studies involving FA values4,52 and cytokines53,54. Regarding the multiple testing issue, the p values were adjusted following the number of pro-inflammatory markers and BDI-FS scores, defining significance finally for these correlations as pBONF = 0.01 and presenting these results in Table 2. The obtained correlation matrix also gives relevant information for the voxelwise analysis procedure described below.
In addition, differences between BDI-FS scores and pro-inflammatory cytokines on the mean whole-brain FA were analyzed using a general linear model (GLM) with ANOVA omnibus tests. This model consisted of the factors group status (i.e., DE and HC group) and the dichotomized cytokine concentrations using the median split method. Other variables, such as age and BDI-FS scores, were included in this model as covariates. A separate ANOVA was conducted for each cytokine, and the resulting p values were adjusted using Bonferroni correction for multiple tests (pBONF = 0.05/4 = 0.0125). Moreover, effect sizes were calculated using partial eta-squared (η2p) values. The effects for both partial ES formulae were defined as following: very small (η2p < 0.01), small (0.01 ≤ η2p < 0.06), moderate (0.06 ≤ η2p < 0.14) and large (η2p ≥ 0.14)55,56. The results of this model are presented in Fig. 1.
(a) Results of the GLM on the whole-brain FA with factors group status (HC; DE) and dichotomized cytokine concentration (median split: lower; higher). Age was included as a covariate. The interaction plots of group x cytokine concentration are displayed. Bar height represents the mean FA values, error bars indicate the CI95+. IL-1β showed a main effect (p = 0.002) but no interaction effect (p = 0.061). TNF-ɑ showed no main effect (p = 0.286), but an interaction effect (p = 0.002). Both effects survive Bonferroni adjusted threshold of pBONF = 0.0125. IL-6 and IFN-ɣ showed no effects over threshold. (b) Violin plots representing the distribution of whole-brain FA within groups HC and DE.
Both correlation and analysis of variance were performed using SPSS version 26.0 (International Business Machines Corporation, New York, United States of America) or jamovi 2.0.057 together with the toolbox GAMLj58.
Finally, voxel-wise statistical analyses were performed using a GLM to determine possible relationships with inflammatory parameters in particular WM structures. The design matrix consisted of regressors for the group status, age, gender, body mass index (BMI in kg/m2), BDI-FS values, and the within-covariates for the peripheral cytokine concentrations. This model estimation was performed using the FSL randomize tool using a sample of 5000 permutations combined with the Threshold-Free Cluster Enhancement59. Contrast calculation included the mean group differences, the effects of the covariates, and the group differences of the slope of the regression of FA on the peripheral cytokines. In addition, F-contrasts were calculated to examine the effect of the combination of all cytokines. Result maps are presented in Fig. 2 and thresholded at pFWE ≤ 0.05 (corrected for multiple comparisons across space).
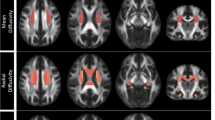
FA results for the interaction effect of group status (DE, HC) * IL-1β. Each row shows the sagittal, coronal, and axial views aligned with the coordinates of the respective cluster maximum (radiological display convention). Each slice consists of an FA template (grey), the mean FA skeleton of this study (green), overlaid by the age effect (blue; pFWE ≤ 0.05) and by the thickened threshold statistic with pFWE ≤ 0.05 (red-yellow), representing more positive slopes in the HC group. The clusters refer to the descriptions in Table 3: Cluster 1—Genu Corpus Callosum; Cluster 2—Superior longitudinal fasciculus L; Cluster 3 & 6—Splenium Corpus Callosum; Cluster 4—Posterior Corona Radiata R; Cluster 5—Anterior Corona Radiata R. R/L right/left hemisphere, P/A posterior/anterior, I/S inferior/superior.
Ethical approval and admission to participation
Each participant or its legal authorized representative was fully informed of this study and gave their written consent to participate. This study was approved by the ethics committee of the Medical Faculty of the JLU (file number AZ 81/18) and carried out in accordance with the Helsinki Declaration and the ethical standards of the APA. This study is part of a project to investigate inflammatory factors and fatigue in patients with depression and multiple sclerosis. Declarations of consent in the original language (German) are available on request.
Results
Sample description
Regarding age (t = − 1.62, p = 0.113), gender (χ2 = 0.03, df = 1, p = 0.858), smoking behavior (χ2 = 0.27, df = 1, p = 0.667) and BMI (t = 0.56, p = 0.581) no differences were found between DE and HC. However, the BDI-FS values were higher in DE than in HC (t = − 4.78, p < 0.001). The IL-6 concentrations were higher in DE compared to HC (t = − 2.07, p = 0.045), no differences between the two groups were found with regard to the remaining peripheral cytokines (Table 1).
Fractional anisotropy (FA)
Mean whole-brain FA analysis
Whole-brain FA correlations with cytokine correlation and BDI-FS values within each group are shown in Table 2. This analysis found significant correlations for FA values with IL-1β and TNF-ɑ in the DE group. However, only IL-1β survived the Bonferroni-adjusted threshold. Both correlations were negatively signed, indicating less FA with higher cytokine concentration.
The GLM analysis revealed a main effect of IL-1β on the whole-brain FA (F1,46 = 10.40, p = 0.002, η2p = 0.21), showing lower FA in the subgroup having higher IL-1β concentration (Fig. 1). Similar effects were not seen for the other cytokines: IL-6 (F1,46 = 0.41, p = 0.525, η2p = 0.01), TNF-ɑ (F1,46 = 1.17, p = 0.286, η2p = 0.03), IFN-γ (F1,46 = 0.00, p = 0.98, η2p = 0.00). However, TNF-ɑ concentrations showed an interaction effect with group status, showing only differences in the DE group (F1,46 = 11.13, p = 0.002, η2p = 0.21). Once more, higher cytokine concentrations were associated with lower FA. Both results also survive the Bonferroni adjusted threshold. The results of this analysis are summarized in Fig. 1.
Voxelwise FA analysis
Voxelwise FA analysis did not show effects of group, sex, BMI, or BDI-FS values. However, the covariable age explained a substantial portion of variance (Fig. 2). Comparing the slopes of the regression of FA on the peripheral cytokines, a significant effect was found in the analysis including the IL-1β concentrations (Fig. 2). Here, the slope for the HC group was significantly more positive than the slope in the DE group. The detailed statistics, including t and p values for the clusters and voxel-by-voxel statistical parametric threshold maps, are presented in Fig. 2 and Table 3. Concerning the presented results, three clusters cover the genu of corpus callosum and splenium of the corpus callosum. The remaining three clusters contain the superior longitudinal fasciculus, the anterior and the posterior corona radiata. A similar effect as described for IL-1β was not found for the other three cytokines (IL-6, TNF-α and IFN-γ). A combination of the contrasts for all four cytokines, as can be tested using F-contrasts, did not show an effect above the threshold.
Discussion
The main result of this study showed an altered association of IL-1β with fractional anisotropy, as can be measured using diffusion tensor imaging, in depression. The most intriguing fact is thereby, that this result was not found in the comparison of healthy with severely depressed or unmedicated first episode people34 but it was found in a community sample where the people suffering from depression were outpatients mostly under pharmacological treatment. The “healthy controls” were also part of a community sample that reported fewer symptoms of depression. It was shown that the IL-1β concentration correlated negatively with the wholebrain FA in the group with depression. Correlation of age and BMI with FA was always taken into account. In the group without depression this relationship did not occur. That means that in people with depression FA values were less when the cytokine concentration was higher. Whether the participants had a depression or not, in average the FA was less with higher IL-1β cytokine concentration.
Concretizing the localisatory aspect of this result, the voxelwise FA analysis showed that that the slopes of the regression of FA on IL-1β was different in the groups. That means that the IL-1β concentration correlated more negatively in people with depression than without depression. This effect predominantly occurred in the big commissural fibers of the brain, the genu and splenium of the corpus callosum. The same effects occurred also in the superior longitudinal fasciculus, the anterior and the posterior corona radiata. Similar effects could not be shown for the other three cytokines. A basic association between microstructural changes (lower FA) and major depression has been reported for all of these structures34,60,61.
Many studies have shown that IL-1β in particular has a negative impact on myelination and WM microstructure and that its pharmacological blockade could reduce apoptosis, microgliosis, and inflammation27,62,63,64. Those changes have also been found in animal models for depression65,66. It is therefore to be expected that FA and IL-1β correlate more negatively in people with depression than without depression. Likewise, human studies have shown negative correlations between markers of peripheral inflammation and mean whole brain FA in other diseases with a neuroinflammatory component67,68. With regard to depression, only one human study reported a similar result with regard to peripheral IL-1β and WM integrity34 and showed between-group effects of IL-1β levels in the inferior fronto-occipital fasciculus (IFOF) and in the genu of the corpus callosum (GCC) first episode of treatment-naïve depression. This was partly shown in the current study with depressed outpatients. An interaction effect of the group * covariate of the IL-1β level was found in the GCC, but not in the inferior fronto-occipital fasciculus (Fig. 2). Instead, an effect was found in the superior longitudinal fasciculus, the anterior and the posterior corona radiata. However, due to inconsistencies, the published coordinates are not always easy to assess, so overlaps cannot be ruled out. Since both studies showed a similar interaction effect in samples with different properties, first episode treatment-naïve34 vs. non-drug-naïve treated outpatients, at least the correlation between corpus callosum and peripheral inflammation may be a characteristic of depression regardless of treatment.
Higher TNF-α levels were also associated with lower whole-brain FA levels in the depressed group when groups with higher and lower cytokine concentrations were compared. In the voxelwise analysis, however, this cytokine with pFWEmax = 0.088 just missed the voxel threshold of pFWE = 0.05 and was therefore not considered relevant for the interpretation. A DTI-based meta-analysis suggested the influence of inflammatory cytokines (i.e., IL-1β, IL-6, and TNF-α) on changes in the microstructural integrity of the WM10. However, this could only be shown, probably due to variance issues, when the TNF-α distribution was dichotomized by a median split, although TNF-α and IL-1β shared 31% of the variance in the group with depression. Another probable reason might be group-related differences between depression participants. There is only one study with the voxelwise approach that showed a connection between TNF-α and FA in depression38, which, however, differs from the results of the current study and the study cited above34. To speculate, individual differences could account for the different outcomes, i.e., macrophages, for example, produce cytokines, IL-1β and TNF-α, which have an inflammatory effect69. Nevertheless, in some groups with depression one cytokine predominates over the other34,38. That could be an interesting starting point for further studies.
The influence of the medication in the depressive group on the results is difficult to classify. There are some recent studies that make the problem clear. In drug-naïve MDD patients, higher levels of FA were found in some white matter structures but not in the corpus callosum after treatment with venlafaxine plus benzodiazepines PRN70. IL-1ß effects were not observed, but an inverse correlation between the left posterior limb of the inner capsule and the highly sensitive C-reactive protein was reported, regardless of treatment. Another recent study examined the relationship between the IL-8/IL-10 ratio and free water before and after treatment with ketamine44. The antidepressant medication of the patient group was very similar to the current study. The results were not comparable to the current study. Ketamine infusion had no effect on WM microstructure but reduced the IL-8/IL-10 ratio. In a combined DTI and immunological study of bipolar disorder, including a sample of depressed participants, effects occurred in bipolar patients and no IL-1β effects were reported71. Sugimoto et al., studying drug-naïve MDD patients, found an association between IL-1ß levels and the genu corpus callosum34, as did the current study of participants taking antidepressants. Lim al. found higher TNF-α and IL-8 and lower FA in the corpus callosum, anterior corona radiate, superior corona radiate, and superior longitudinal fasciculus in untreated but not drug-naïve participants with MDD compared to an HC group. IL-1β levels were quite lower than in our study, other cytokine levels were equal to or lower than in our study38. Although there was no correlation with IL-1β, the highlighted MW structures are very similar to our study. The clusters we found are smaller, the sample size was about the same, as was the difference in depressiveness between groups. Given the results of these more recent studies, it seems unlikely that an association of IL-1β and FA in the corpus callosum, the superior longitudinal fasciculus, and the anterior corona radiata is primarily due to drug effects. In addition to medication, the influence of psychotherapy on the hypothalamic–pituitary–adrenal axis (HPA) and immune regulation and their possible connection should not be neglected. In participants with depression, Doolin et al. found a negative association between IL-1β, mRNA and morning cortisol72. Effects of non-drug therapies or treatments such as psychotherapy or even exercise, both of which have at least low evidence effects on the HPA axis and immune regulation, were not considered in any of the cited studies and would be an important topic for future depression research.
The strength of this study was to examine a sample of outpatients diagnosed with a depressive episode, who were medicated and who were not first-episode patients, showing an effect of the proinflammatory cytokine IL-1β on DTI measures. This allows the findings to be extended to a broader population of people with depression. Nevertheless, some limitations should be considered. (1) IL-1β concentrations were close to the lowest limit of detection, as shown in Table 1. The work of Sugimoto et al.34, which shared similarities with this study, also reported low IL-1β concentrations near the lowest limit of detection. (2) The results of this study cannot say anything about causality, of whether depression triggers inflammation or vice versa. (3) The heterogeneity of the depressed patients represented a limitation in this study, since participants with comorbidity (personality and anxiety disorders) were included in this study. However, these participants reported a predominant depressive episode in the last 6 months and thus met the inclusion requirements. (4) In our view, it is not a problem that there were more female participants than male participants, as this ratio is consistent with statistical and epidemiological data on depression worldwide.
In conclusion, proinflammatory IL-1β and TNF-α showed to be more negatively correlated with white matter FA of the brain in outpatients with depression, particularly for IL-1β in the corpus callosum. Future studies should consider further examining the correlations between IL-1β and FA levels of WM structures at different stages of depressive disorder (e.g., healthy controls, treated depressed patients, and untreated first-episode depressed patients) or in different types of depression (e.g., atypical depression, depression with somatic symptoms, bipolar vs. unipolar depression). Longitudinal methods could help to decipher the time course of inflammation, microstructural changes and depression. Finally, fractional anisotropy (FA) as a summary measure of microstructural integrity, which has been extensively shown to be very sensitive to different types of microstructural changes, seemed to be an appropriate measure in the context of this study. However, it is recommended to include other DTI measures such as axial and radial diffusion or free water others to learn about the nature of the change.
Data availability
The data that support the results of this study are not publicly available due to the applicable national data protection law, but can be requested from the respective author upon justified request.
References
Grieve, S. M., Williams, L. M., Paul, R. H., Clark, C. R. & Gordon, E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. Am. J. Neuroradiol. 28, 226–235 (2007).
Alba-Ferrara, L. M. & de Erausquin, G. A. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front. Integr. Neurosci. 7, 1–5 (2013).
Minati, L. & Węglarz, W. P. Physical foundations, models, and methods of diffusion magnetic resonance imaging of the brain: A review. Concepts Magn. Reson. Part A 30A, 278–307 (2007).
Bettcher, B. M. et al. Body mass and white matter integrity: The influence of vascular and inflammatory markers. PLoS One 8, e77741 (2013).
Bettcher, B. M. et al. Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol. Aging 36, 948–954 (2015).
Wersching, H. et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74, 1022–1029 (2010).
Kelly, S. et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 23, 1261–1269 (2018).
Phan, K. L. et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol. Psychiatry 66, 691–694 (2009).
Versace, A. et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch. Gen. Psychiatry 65, 1041 (2008).
Murphy, M. L. & Frodl, T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol. Mood Anxiety Disord. 1, 3 (2011).
Hermesdorf, M. et al. Reduced fractional anisotropy in patients with major depressive disorder and associations with vascular stiffness. NeuroImage Clin. 14, 151–155 (2017).
Carballedo, A. et al. Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 537–548 (2012).
Repple, J. et al. Severity of current depression and remission status are associated with structural connectome alterations in major depressive disorder. Mol. Psychiatry 25, 1550–1558 (2020).
Coloigner, J. et al. White matter abnormalities in depression: A categorical and phenotypic diffusion MRI study. NeuroImage Clin. 22, 101710 (2019).
Osoba, A. et al. Disease severity is correlated to tract specific changes of fractional anisotropy in MD and CM thalamus—a DTI study in major depressive disorder. J. Affect. Disord. 149, 116–128 (2013).
Poletti, S. et al. Impact of early and recent stress on white matter microstructure in major depressive disorder. J. Affect. Disord. 225, 289–297 (2018).
Won, E. et al. Association between reduced white matter integrity in the corpus callosum and serotonin transporter gene DNA methylation in medication-naive patients with major depressive disorder. Transl. Psychiatry 6, e866–e866 (2016).
Liu, X. et al. Relationship between white matter integrity and serum cortisol levels in drug-naive patients with major depressive disorder: Diffusion tensor imaging study using tract-based spatial statistics. Br. J. Psychiatry 208, 585–590 (2016).
Guo, W. et al. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci. Lett. 522, 139–144 (2012).
Ma, N. et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am. J. Psychiatry 164, 823–826 (2007).
Yuan, Y. et al. White matter integrity of the whole brain is disrupted in first-episode remitted geriatric depression. NeuroReport 18, 25 (2007).
Taylor, W. D. et al. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One 3, e3267 (2008).
Wang, T. et al. Early-stage psychotherapy produces elevated frontal white matter integrity in adult major depressive disorder. PLoS ONE 8, e63081 (2013).
Yang, X. et al. White matter microstructural abnormalities and their association with anticipatory anhedonia in depression. Psychiatry Res. Neuroimaging 264, 29–34 (2017).
Pfarr, J. et al. Brain structural connectivity, anhedonia, and phenotypes of major depressive disorder: A structural equation model approach. Hum. Brain Mapp. 42, 5063–5074 (2021).
Boroujeni, M. E. et al. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem. Neurosci. 12, 2143–2150 (2021).
Kelly, S. B. et al. Interleukin-1 blockade attenuates white matter inflammation and oligodendrocyte loss after progressive systemic lipopolysaccharide exposure in near-term fetal sheep. J. Neuroinflamm. 18, 189 (2021).
Thomas, M. et al. Elevated systemic inflammation is associated with reduced corticolimbic white matter integrity in depression. Life 12, 43 (2021).
Menard, C. et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760 (2017).
Dudek, K. A. et al. Molecular adaptations of the blood–brain barrier promote stress resilience vs depression. Proc. Natl. Acad. Sci. 117, 3326–3336 (2020).
Köhler, C. A. et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 135, 373–387 (2017).
Hiles, S. A., Baker, A. L., de Malmanche, T. & Attia, J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: Exploring the causes of heterogeneity. Brain. Behav. Immun. 26, 1180–1188 (2012).
Maes, M., Mihaylova, I., Kubera, M. & Ringel, K. Activation of cell-mediated immunity in depression: Association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 36, 169–175 (2012).
Sugimoto, K. et al. Relationship between white matter integrity and serum inflammatory cytokine levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Transl. Psychiatry 8, 141 (2018).
Pedraz-Petrozzi, B., Neumann, E. & Sammer, G. Pro-inflammatory markers and fatigue in patients with depression: A case–control study. Sci. Rep. 10, 9494 (2020).
Dowlati, Y. et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457 (2010).
Howren, M. B., Lamkin, D. M. & Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 71, 171–186 (2009).
Lim, J., Sohn, H., Kwon, M.-S. & Kim, B. White matter alterations associated with pro-inflammatory cytokines in patients with major depressive disorder. Clin. Psychopharmacol. Neurosci. 19, 449–458 (2021).
Frodl, T. et al. Reduced expression of glucocorticoid-inducible genes GILZ and SGK-1: high IL-6 levels are associated with reduced hippocampal volumes in major depressive disorder. Transl. Psychiatry 2, e88–e88 (2012).
Zobel, A. & Maier, W. Endophaenotypen—ein neues Konzept zur biologischen Charakterisierung psychischer Stoerungen. Nervenarzt 75, 205–214 (2004).
World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research (1993).
Kliem, S., Mößle, T., Zenger, M. & Brähler, E. Reliability and validity of the beck depression inventory-fast screen for medical patients in the general German population. J. Affect. Disord. https://doi.org/10.1016/j.jad.2013.11.024 (2014).
Bergamino, M., Pasternak, O., Farmer, M., Shenton, M. E. & Paul Hamilton, J. Applying a free-water correction to diffusion imaging data uncovers stress-related neural pathology in depression. NeuroImage Clin. 10, 336–342 (2016).
Langhein, M. et al. Association between peripheral inflammation and free-water imaging in Major Depressive Disorder before and after ketamine treatment—a pilot study. J. Affect. Disord. 314, 78–85 (2022).
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
Andersson, J. L. R., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20, 870–888 (2003).
Smith, S. M. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143–155 (2002).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078 (2016).
Smith, S. M. et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 (2006).
Marzban, C., Illian, P. R., Morison, D. & Mourad, P. D. Within-group and between-group correlation: Illustration on noninvasive estimation of intracranial pressure (2013).
Ranganathan, P., Pramesh, C. & Aggarwal, R. Common pitfalls in statistical analysis: Logistic regression. Perspect. Clin. Res. https://doi.org/10.4103/picr.PICR_87_17 (2017).
Nenonen, M. et al. Possible confounding factors on cerebral diffusion tensor imaging measurements. Acta Radiol. Open 4, 204798161454679 (2015).
Azizian, M. et al. Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender. Ann. Clin. Biochem. 6, 25 (2016).
El-Mikkawy, D. M. E., El-Sadek, M. A., El-Badawy, M. A. & Samaha, D. Circulating level of interleukin-6 in relation to body mass indices and lipid profile in Egyptian adults with overweight and obesity. Egypt. Rheumatol. Rehabil. 47, 7 (2020).
Morris, P. E. & Fritz, C. O. Effect sizes in memory research. Memory https://doi.org/10.1080/09658211.2013.763984 (2013).
Cambridge, U. of. Rules of thumb on magnitudes of effect sizes. 2019 http://imaging.mrc-cbu.cam.ac.uk/statswiki/FAQ/effectSize.
Love, J. et al. The jamovi project. https://www.jamovi.org (2020).
Gallucci, M. GAMLJ—General Analyses for Linear Models. https://www.jamovi.org/library.html (2019).
Smith, S. & Nichols, T. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44, 83–98 (2009).
Pisner, D. A., Shumake, J., Beevers, C. G. & Schnyer, D. M. The superior longitudinal fasciculus and its functional triple-network mechanisms in brooding. NeuroImage Clin. 24, 101935 (2019).
Choi, S. et al. Association of brain-derived neurotrophic factor DNA methylation and reduced white matter integrity in the anterior corona radiata in major depression. J. Affect. Disord. 172, 74–80 (2015).
Boato, F. et al. Absence of IL-1β positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J. Neuroinflamm. 10, 792 (2013).
Prins, M. et al. Interleukin-1β and interleukin-1 receptor antagonist appear in grey matter additionally to white matter lesions during experimental multiple sclerosis. PLoS One 8, e83835 (2013).
Cai, Z., Lin, S., Pang, Y. & Rhodes, P. G. Brain injury induced by intracerebral injection of interleukin-1beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr. Res. 56, 377–384 (2004).
Grippo, A. J., Francis, J., Beltz, T. G., Felder, R. B. & Johnson, A. K. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol. Behav. 84, 697–706 (2005).
Xu, H. et al. Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatr. Dis. Treat. 11, 597 (2015).
Swardfager, W. et al. Peripheral inflammatory markers indicate microstructural damage within periventricular white matter hyperintensities in Alzheimer’s disease: A preliminary report. Alzheimers Dement. Diagn. Assess. Dis. Monit. 7, 56–60 (2017).
Chiang, P.-L. et al. White matter damage and systemic inflammation in Parkinson’s disease. BMC Neurosci. 18, 48 (2017).
Baer, M. et al. Tumor necrosis factor alpha transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-B p50. Mol. Cell Biol. 18, 12 (1998).
Chen, L., Zeng, X., Zhou, S., Gu, Z. & Pan, J. Correlation between serum high-sensitivity C-reactive protein, tumor necrosis factor-alpha, serum interleukin-6 and white matter integrity before and after the treatment of drug-naïve patients with major depressive disorder. Front. Neurosci. 16, 948637 (2022).
Magioncalda, P. et al. White matter microstructure alterations correlate with terminally differentiated CD8+ effector T cell depletion in the peripheral blood in mania: Combined DTI and immunological investigation in the different phases of bipolar disorder. Brain. Behav. Immun. 73, 192–204 (2018).
Doolin, K. et al. Diurnal hypothalamic–pituitary–adrenal axis measures and inflammatory marker correlates in major depressive disorder. Int. J. Mol. Sci. 18(10), 2226. https://doi.org/10.3390/ijms18102226 (2017).
Acknowledgements
This project was financially supported by the German Academic Exchange Service (DAAD; 2017-57320205) and the “Immunität und Seele” foundation, Marchioninistr. 15, DE-BY 81377). Thanks also go to the Bender Institute for Neuroimaging at JLU and its team, as well as Ms. Carina Scheiyäck and Ms. Mona Arnold from the Laboratory for Internal Medicine and Rheumatology at JLU.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study is part of a project entitled: “Correlations between systemic inflammation, brain endophenotypes and fatigue in participants with depression”. This project was financially supported by the German Academic Exchange Service (DAAD), consisting of a grant for BPP and material costs. (Graduate School Scholarship Programme, 2017 (57320205)). The “Immunität und Seele” foundation (Chair: Prof. Dr. Norbert Müller; Marchioninistr. 15, DE-BY 81377) financially supported part of the material costs. Both supporters have no influence on the design, data collection, analysis, interpretation of the results, or any other aspect of the study. The authors of this study were not hired by any organization that may gain or lose financially through this publication. With exception of point “Funding”, for this study the authors did not receive any compensation (monetary, shares, etc.) from companies, consultation fees or other forms of remuneration from any organizations.
Author information
Authors and Affiliations
Contributions
G.S. and B.P.P. developed the idea for the study. G.S. wrote the methods and results section, carried out the data analysis, supervised the methods (especially on topics related to depression and neuroimaging). B.P.P. supervised the data collection and performed the analysis of the blood samples, wrote the introduction, participated in the MR data analysis, contributed to the methods part, wrote the discussion part. E.N. participated in data analysis, supervised methods (especially in molecular biology matters). C.B. contributed to the method part in particular with questions about the technical implementation of magnetic resonance imaging and questions about the analysis pipeline for the diffusion tensor imaging data. Finally, all authors worked on all parts of the manuscript, proofreading and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sammer, G., Neumann, E., Blecker, C. et al. Fractional anisotropy and peripheral cytokine concentrations in outpatients with depressive episode: a diffusion tensor imaging observational study. Sci Rep 12, 17450 (2022). https://doi.org/10.1038/s41598-022-22437-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22437-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.