Abstract
Glycemic variability (GV) is a risk factor for depression in patients with diabetes. However, whether it is also a predictor of incident depression in people without diabetes remains unclear. We aimed to investigate the association between visit-to-visit variability in fasting serum glucose (FSG) levels and the incidence of depression among Koreans without diabetes. This retrospective cohort study included data of people without diabetes who did not have depression at baseline and had at least three FSG measurements (n = 264,480) extracted from the 2002–2007 Korean National Health Insurance Service–National Health Screening Cohort. GV was calculated as the average successive variability of FSG. Among 264,480 participants, 198,267 were observed during 2008–2013 and their hazard ratios (HR) of incident depression were calculated. Participants with the highest GV showed a higher risk of depression in fully adjusted models than those with the lowest GV (HR, 1.09; 95% CI, 1.02–1.16). The risk of incident depression heightened with increasing GV (p for trend < 0.001). Greater visit-to-visit GV may be associated with the risk of developing depression in people without diabetes. Conversely, maintaining steady FSG levels may reduce the risk of incident depression in people without diabetes.
Similar content being viewed by others
Introduction
Glycemic variability (GV) refers to fluctuations in blood glucose levels over a short (within- or between-day variability)- or long-term period (months or years) and is an integral component of glucose homeostasis1,2,3. While HbA1c has long been considered as the gold standard for measuring glycemic control, GV is increasingly gaining its clinical significance as a more meaningful measure of glycemic control compared to HbA1c2. Despite the controversial findings, a few studies have demonstrated that GV is associated with adverse clinical outcomes such as cardiovascular events, hypoglycemia, micro- and macrovascular complications, and mortality rates2,4,5,6. Besides, GV is reported to be correlated with psychiatric diseases such as depression and cognitive disorder2,7,8,9,10. In fact, a certain degree of GV can be observed in all groups, from people without diabetes to those with impaired glycemic levels or diabetes11,12. For example, exposure to variability in blood glucose levels was found to be more harmful than an episode of acute or chronic stable hyperglycemia in both normal and people with type 2 diabetes11,13. Based on the aim of treating diabetes to restore glycemia to that of persons without diabetes, examining the distribution of GV and its impact should begin among general populations without diabetes14. However, to the best of our knowledge, there have been no studies that investigated the impact of GV on the onset of depression in people without diabetes. When glucose fluctuates, excessive activation of oxidative stress and vascular damage are induced, all of which can contribute to developing depression10,13. Based on a prior study that found an adverse effect of GV on cardiovascular complications and mortality risk among people without diabetes like that of among patients with diabetes11, we expect that greater GV may further deteriorate the risk of incident depression among healthy people without diabetes. Thus, given that depression is one of the leading causes of disability and imposes a remarkable burden worldwide, understanding the factors that increase the risk of its incidence is a major public health interest10,15. While depression has long been recognized as such a common complication of diabetes16, it is more prevalent and severe in patients with greater glycemic instability7,10,17. Therefore, we aimed to investigate the association between variability in visit-to-visit fasting serum glucose (FSG) levels and the incidence of depression in healthy Asian populations without diabetes using a nationally representative cohort from South Korea.
Methods
Study population
We extracted data from the Korean National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) database between January 1, 2002, and December 31, 2013. The NHIS has offered health insurance for all Korean citizens since 198918 and provided biennial health screening examinations for all citizens aged ≥ 40 years old. During the examination, participants undergo urine and blood tests and basic physical measurements including height, weight, and blood pressure. Additionally, participants are required to fill out self-reported questionnaires regarding their health behavior, such as smoking, drinking, physical activity, and personal and family history of diseases. These collected data is then combined with sociodemographic information, hospital usage, and death register information, which would made into a subset dataset, called the NHIS-HEALS by a simple random sampling method. The NHIS-HEALS dataset offers a unique opportunity to explore the Korean population’s health-related characteristics19.
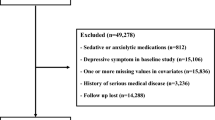
In total, 264,480 participants who underwent at least three health screening visits among the first (2002–2003), second (2004–2005), and third (2006–2007) health examinations and had FSG values were selected during screening. Among these, we excluded 22,119 participants diagnosed with diabetes and 6198 participants diagnosed with depression before the index date (January 1, 2008); 18,909 participants whose baseline FSG levels were ≥ 126.0 mg/dL in any of their health examinations; 519 participants who died before the index date; and 18,468 participants for missing data on covariates. Finally, our study population comprised 198,267 participants. The flowchart of our study is shown in Fig. 1.
Study variables
GV was calculated as the average successive variability (ASV) of the FSG values9. Specifically, GV was driven by calculating the average absolute values of the differences in FSG between the successive examinations20. The study population was then distributed into five groups according to the extent of GV. The first quintile represented the lowest GV, whereas the fifth quintile represented the highest GV. Depression was defined according to codes from the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) pertaining to depressive symptoms during the follow-up duration. ICD-10 is a standardized code for medical conditions and procedures established by the World Health Organization21. We considered incident depression as diagnosed with F32 (all mild to severe depressive episodes) or F33 (all recurrent depressive disorders) based on the ICD-10 code, along with the prescription of antidepressants.
Statistical analysis
The clinical course of the participants was followed up from January 1, 2008, until the date of depression diagnoses, death, or December 31, 2013, whichever came first. Moreover, we censored any patients diagnosed with diabetes during the follow-up period, mainly to preclude diabetes as a risk factor for depression and to solely determine the effect of GV as a predictor of depression. The risk of depression due to GV was determined by calculating the hazard ratios (HR) and 95% confidence intervals (CIs) using Cox proportional hazards regression analysis. In all our analyses, the first quintile of GV, which indicated the lowest variability, was used as the reference group. We then calculated p for trend values to detect any trend between GV and depression risk.
We adjusted for the following potential confounding covariates: age (continuous; years), sex (categorical; male or female), baseline FSG level (continuous; mg/dL), change in FSG level (continuous; mg/dL), household income (categorical; first, second, third, and fourth quartiles), body mass index (continuous; kg/m2), smoking (categorical; never, past, and current), alcohol consumption (categorical; 0, < 1, 1–2, 3–4, and ≥ 5 times per week), physical activity (categorical; 0, 1–2, 3–4, 5–6, and ≥ 7 times per week), blood pressure (continuous; mmHg), total cholesterol level (continuous; mg/dL), and the Charlson comorbidity index (continuous). Change in FSG, which meant to consider the direction of glucose change, was calculated as the difference in FSG values between the third and first health examinations. We used insurance premium as the proxy for household income and calculated the Charlson comorbidity index using the same algorithms as those reported in another study22.
Stratified analyses were conducted to further investigate any differences in the outcome according to subgroups. The subgroups included sex, impaired FSG status, direction of FSG change, household income, body mass index, smoking, physical activity, alcohol consumption, and the Charlson comorbidity index. Furthermore, sensitivity analyses were conducted after excluding the incidence of depression in the first 1–5 years from the index date. In addition, the coefficient of variation (CV) of the FSG values was calculated as an alternative metric to support the primary findings. CV was calculated by dividing the standard deviation of the FSG values by the mean values and is one of the most commonly used metrics for measuring the degree of variability23.
Data collection was conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA), and statistical analyses were performed using STATA version 13.0 (StataCorp, College Station, TX, USA). We used the chi-squared test to analyze categorical variables and analysis of variance for continuous variables to compare baseline characteristics according to the GV. Statistical significance was defined as a two-sided p value < 0.05.
Ethical considerations
This study was approved by the Seoul National University Hospital Institutional Review Board (IRB number, X-1701/378–902). The requirement for informed consent from the participants was waived by the Institutional Review Board of Seoul National University Hospital on account of the retrospective nature of this study, and the NHIS-HEALS database was anonymized according to strict confidentiality guidelines prior to distribution. All the study methods were carried out in accordance with relevant guidelines and regulations.
Results
A total of 264,480 people underwent the first (2002–2003), second (2004–2005), and third (2006–2007) health checkups, and 198,267 of them underwent follow-up for 6 years. We excluded patients who were diagnosed with diabetes (n = 22,119) and depression (n = 6198) before the index date. Participants with an FSG level ≥ 126 mg/dL (n = 18,909), who died before the index date (n = 519), and those with missing covariates (n = 18,468) during the study period were excluded.
Table 1 depicts the baseline characteristics of the study population according to GV. Among the total included participants, 20.95%, 20.45%, 20.68%, 18.13%, and 19.79% of participants were grouped into the first, second, third, fourth, and fifth quintiles of GV, respectively. The mean values of GV for each quintile are 3.35, 6.75, 9.92, 13.79, and 22.21 mg/dL, respectively. Compared with people with the lowest GV, those with the highest GV were more likely to be older, male, have lower household income, be current smokers, consume alcohol, engage in lower physical activity, and have more comorbidities.
During the 6-year follow-up, 9244 participants developed depression. The results of the main analyses are shown in Table 2. People in the fifth quintile (highest) of GV had a significantly higher risk of incident depression than those in the first quintile (lowest) of GV (HR, 1.09; 95% CI, 1.02–1.16). Furthermore, the risk of developing depression increased upon greater extent of GV (p for trend < 0.001). All results were significant after adjusting for the covariates. Furthermore, we analyzed Kaplan–Meier curves according to GV quintiles. Likewise, the Kaplan–Meier cumulative risks for depression were higher in the fifth quintile group than the first quintile group of GV. The result is included in Fig. 2.
We further conducted stratified analyses according to subgroups of sex, impaired FSG status, direction of change in FSG, household income, body mass index, smoking, physical activity, alcohol consumption, and the Charlson comorbidity index. For the subgroups of sex, we re-calculated the GV and its quintiles according to sex on account of slight unbalanced sex ratio of the study population (56.69% male and 42.31% female). No significant interaction was noted between GV and the onset of depression for any of these variables. The risk of developing depression appeared to be significantly higher upon greater GV in both sexes, people with normal FSG level (< 100 mg/dL), with any direction of FSG change, people within upper half household income level, any level of body mass index, people who never smoke, who ever do physical activities per week, who never drink, and whom with any comorbidities. The results are summarized in Table 3.
Furthermore, sensitivity analyses were conducted to evaluate the robustness of the results. First, we excluded the incidence of depression within 1–5 years of follow-up to solely examine the effect of GV as a potential risk factor for depression, and not as a prognostic symptom of its incidence. The results were consistent with the main results in which people with the highest GV showed a higher risk of incident depression. Moreover, the risk of developing depression appeared to be significantly higher upon greater GV (p for trend < 0.05) in all analyses. The results are summarized in Table 4 and Fig. 3. Second, the exact same Cox proportional hazards regression analyses were carried out using CV to define the GV. People in the fifth quintile (highest) of GV had a significantly higher risk of incident depression than those in the first quintile (lowest) of GV (HR, 1.10; 95% CI, 1.03–1.17), which is consistent with our findings based on ASV to define GV. Furthermore, the risk of developing depression increased with greater GV extent (p for trend = 0.004). Additionally, the results remained consistent even after excluding the incidence of depression within 1–5 years of follow-up. The results of using an alternative measure of variability are shown in Supplementary Tables S1 and S2.
Summarized hazard ratios of sensitivity analysis. Sensitivity analysis of the effect of GV on depression after excluding participants with events occurring within the first 1–5 years of follow-up. The hazard ratio calculated by Cox proportional hazards regression analysis after adjustments for age, sex, initial FSG, change in FSG, household income, body mass index, smoking, alcohol consumption, physical activity, systolic blood pressure, total cholesterol, and the Charlson comorbidity index. HR, hazard ratio; CI, confidence interval; GV, glucose variability; Q2, second quintile; Q3, third quintile; Q4, fourth quintile; Q5, fifth quintile.
Discussion
In this retrospective cohort study, we examined the association between visit-to-visit variability of FSG and the risk of depression among 198,267 Koreans without diabetes. The results revealed that GV is a significant predictor of the incidence of depression in people without diabetes. Among individuals with a greater GV, the risk of developing depression was significantly higher than those with the lowest GV after adjusting for confounding factors. To the best of our knowledge, this is the first study to report the greater GV as a risk factor for incident depression among people without diabetes.
According to earlier studies, people with normal glucose tolerance can also experience some degree of GV, and such oscillating glucose has greater detrimental effects on endothelial function and oxidative stress than constant high glucose in both people with and without diabetes13. An elevated risk of depression is linked to these biological changes. Along with the fact that GV significantly increases with age in the general adult population, evaluating the effect of GV in people without diabetes is crucial11. In addition, understanding the distribution and impact of GV in general populations is important for interpreting reference values. Given the goal of diabetes treatment is to restore glycemia to that of people without diabetes, the investigation of potential harm following GV should begin with examinations of those without diabetes, allowing for the determination of the point at which GV becomes pathologically significant14. Therefore, GV may also be a risk factor that should be monitored in a normal population without diabetes.
Consistent with our results, several studies have confirmed the association between visit-to-visit GV and depression, although their samples consisted of people with diabetes and relied on other glucose parameters rather than FSG7,10. For example, a recent study which investigated the effect of fasting plasma glucose coefficient of variation (FPG-CV) on the development of depression found that patients with type 2 diabetes and the highest 1-year FPG-CV had an elevated risk of depression10. Another study revealed that an increased variability in HbA1c is associated with higher risk of complications such as depression, though the study population was limited to elderly patients with type 2 diabetes7.
Several mechanisms may possibly underly the association between GV and incidence of depression. Given that visit-to-visit GV correlates with the mean concentration of blood glucose or HbA1c, it reflects the hyperglycemia status to a certain extent. While similar mechanisms may engage in the development of depression upon greater GV24, GV induces greater oxidative stress15,25,26 and endothelial dysfunction13,27 compared to single abnormal glycemic level11,28. These two mechanisms are commonly discussed as pathways that activate the pathogenesis of abnormalities in the neuroendocrine10,29,30 caused by GV13,31. Specifically, oscillating glycemic induces reactive oxygen species (ROS), leading to the activation of protein kinase C32,33, which, in turn, triggers apoptosis in endothelial cells32,34. In addition, through a transcription factor called nuclear factor kappa B (NF-κB), GV may fuel the concentration of pro-inflammatory cytokines and expression of adhesion molecules in endothelial cells32,33, which promote the incident depression31,35. Furthermore, the enhanced oxidative stress is further related to greater telomere shortening according to in vitro models36,37, which is often observed in neuropsychiatric disorders, including depression38. However, because most studies have been conducted on people with diabetes or in in vitro settings, it is unknown whether the same mechanisms of action can be applied to people without diabetes11.
The following limitations must be considered when interpreting our results. First, the retrospective nature of our study makes it difficult to fully control for all the factors that may affect GV and depression. For example, factors regarding dietary, behavioral, and socioeconomic characteristics may incur GV or depression39. According to independent studies dealt with glycemic control and depression respectively, factors like high-carbohydrate diet40, low physical activity41, consumption of alcohol42, smoking43, or higher income level41 can increase FSG. Moreover, depression is more prevalent among people who smoke, lack physical activity, consume excessive alcohol44, or those who are obese45,46, with lower income level47, or with chronic diseases45,47. Though we adjusted for as many confounding factors as possible including smoking, physical activity, alcohol intake, body mass index, household income, and the Charlson comorbidity index, other factors may further engage in the association between GV and incident depression. Therefore, dedicated studies based on larger individual-level information would be needed as to elucidate the link between GV and incident depression among people without diabetes. Second, the population in our sample is restricted to those who have undergone at least three health examinations, are probably in a certain socioeconomic group, or are more concerned with their own health. Additionally, close to 28% of our sample had three or more comorbidities, indicating that they were not in good health. These may reduce the generalizability of this study, which would mean that the findings of this study might not be applicable to populations with better health23. The Charlson comorbidity index and household income adjustments were attempts to address this issue, but external validation is still required before we can generalize our findings. Third, association surveys continue to serve as the foundation for hypotheses that are tested through more rigorous prospective research, typically randomized clinical trials48. Therefore, despite the fact that our study's large sample size from a representative cohort of Koreans dwarfs that of earlier studies and offers compelling evidence for the significance of GV in predicting future depression risk, the possibility of reversibility between the two factors should be considered. Additional study is needed to elucidate the causal relationship between GV and depression risk. Last but not least, while we used ASV of FSG among other GV indices, several other ways can be used to assess GV. Though there is no gold standard for defining GV11, the association between GV and incident depression may vary according to the measure taken for calculating GV1,49. Further studies based on other GV measures are warranted to enhance the generalizability of our results.
Despite the aforementioned limitations, our study is with some contributions. To the best of our knowledge, this is the first large-scale study to discover the relationship between GV and the risk of developing depression in people without diabetes. Our study has crucial clinical implications for people without diabetes who have generally been neglected in the past. Given the findings of our study, individuals without diabetes may develop depression when they have greater variability in fasting glycemic levels. Therefore, carefully monitoring an individual’s visit-to-visit GV could contribute to the prevention and reduction of the risk of developing depression as well as diabetes. Furthermore, we could also emphasize the importance of maintaining a certain level of stability of depression risk factors, as other studies have reported increased depression risk following greater variability of its other metabolic risk factors. Short-term systolic blood pressure variability, for example, has been widely linked to an increased risk of depression onset50. Also, depressed people have lower heart rate variability values than healthy people, indicating that this parameter could be used as a diagnostic and predictive biomarker of depression51. Hence, this study highlights the need for additional research into the impact of variability in depression risk factors.
In conclusion, greater visit-to-visit GV may increase the risk of developing depression even in people without diabetes. The association held true after considering people’s physical, psychological, and environmental factors. Maintaining a steady FSG level should be recommended to lower the risk of incident depression as well as diabetes. Larger studies are warranted to verify the causality between GV and depression among people without diabetes.
Data availability
The data used in this study are available from the Korean NHIS through a formal online proposal at https://nhiss.nhis.or.kr.
Abbreviations
- GV:
-
Glycemic variability
- NHIS-HEALS:
-
National Health Insurance Service–National Health Screening Cohort
- FSG:
-
Fasting serum glucose
- ASV:
-
Average successive variability
- ICD-10:
-
10th revision of the International Statistical Classification of Diseases and Related Health Problems
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- FPG-CV:
-
Fasting plasma glucose coefficient of variation
References
Satya Krishna, S. V., Kota, S. K. & Modi, K. D. Glycemic variability: Clinical implications. Indian J. Endocrinol. Metab. 17, 611–619. https://doi.org/10.4103/2230-8210.113751 (2013).
Zhou, Z., Sun, B., Huang, S., Zhu, C. & Bian, M. Glycemic variability: Adverse clinical outcomes and how to improve it?. Cardiovasc. Diabetol. 19, 102. https://doi.org/10.1186/s12933-020-01085-6 (2020).
Papachristoforou, E., Lambadiari, V., Maratou, E. & Makrilakis, K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 7489795. https://doi.org/10.1155/2020/7489795 (2020).
Hirakawa, Y. et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: The ADVANCE trial. Diabetes Care 37, 2359–2365 (2014).
Bruginski, D., Précoma, D. B., Sabbag, A. & Olandowski, M. Impact of glycemic variability and hypoglycemia on the mortality and length of hospital stay among elderly patients in Brazil. Curr. Diabetes Rev. 16, 171–180 (2020).
Gorst, C. et al. Long-term glycemic variability and risk of adverse outcomes: A systematic review and meta-analysis. Diabetes Care 38, 2354–2369 (2015).
Ravona-Springer, R. et al. Hemoglobin A1c variability predicts symptoms of depression in elderly individuals with type 2 diabetes. Diabetes Care 40, 1187–1193. https://doi.org/10.2337/dc16-2754 (2017).
Li, T.-C. et al. Visit-to-visit variations in fasting plasma glucose and HbA1c associated with an increased risk of Alzheimer disease: Taiwan Diabetes Study. Diabetes Care 40, 1210–1217 (2017).
Bancks, M. P. et al. Fasting glucose variability in young adulthood and cognitive function in middle age: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes Care 41, 2579–2585 (2018).
Li, C. I. et al. Competing risk analysis on visit-to-visit glucose variations and risk of depression: The Taiwan Diabetes Study. Diabetes Metab 46, 223–229. https://doi.org/10.1016/j.diabet.2019.08.003 (2020).
Yu, J. H. et al. Effects of long-term glycemic variability on incident cardiovascular disease and mortality in subjects without diabetes: A nationwide population-based study. Medicine (Baltimore) 98, e16317. https://doi.org/10.1097/MD.0000000000016317 (2019).
Wang, C. et al. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin. Endocrinol. (Oxf.) 76, 810–815. https://doi.org/10.1111/j.1365-2265.2011.04205.x (2012).
Ceriello, A. et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57, 1349–1354. https://doi.org/10.2337/db08-0063 (2008).
Gude, F. et al. Glycemic variability and its association with demographics and lifestyles in a general adult population. J. Diabetes Sci. Technol. 11, 780–790. https://doi.org/10.1177/1932296816682031 (2017).
Ceretta, L. B. et al. Increased prevalence of mood disorders and suicidal ideation in type 2 diabetic patients. Acta Diabetol 49(Suppl 1), S227-234. https://doi.org/10.1007/s00592-012-0435-9 (2012).
Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care 24, 1069–1078. https://doi.org/10.2337/diacare.24.6.1069 (2001).
Penckofer, S. et al. Does glycemic variability impact mood and quality of life?. Diabetes Technol. Ther. 14, 303–310. https://doi.org/10.1089/dia.2011.0191 (2012).
Cheol Seong, S. et al. Data resource profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 46, 799–800. https://doi.org/10.1093/ije/dyw253 (2017).
Seong, S. C. et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 7, e016640. https://doi.org/10.1136/bmjopen-2017-016640 (2017).
Echouffo-Tcheugui, J. B. et al. Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: The ALLHAT study. Diabetes Care 42, 486–493. https://doi.org/10.2337/dc18-1430 (2019).
Mccarthy M, B. K. ICD-10 Compliance: Process Improvement and Maintenance for Long-Term Care. 318 (HCPro a division of BLR, 2015).
Sundararajan, V. et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 57, 1288–1294. https://doi.org/10.1016/j.jclinepi.2004.03.012 (2004).
Zhao, M. J., Prentice, J. C., Mohr, D. C. & Conlin, P. R. Association between hemoglobin A1c variability and hypoglycemia-related hospitalizations in veterans with diabetes mellitus. BMJ Open Diabetes Res. Care 9, e001797 (2021).
Lachin, J. M. et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care 40, 777–783 (2017).
Ohara, M. et al. Relationship between daily and day-to-day glycemic variability and increased oxidative stress in type 2 diabetes. Diabetes Res. Clin. Pract. 122, 62–70. https://doi.org/10.1016/j.diabres.2016.09.025 (2016).
Chang, C. M., Hsieh, C. J., Huang, J. C. & Huang, I. C. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 49(Suppl 1), S171-177. https://doi.org/10.1007/s00592-012-0398-x (2012).
Thomas, A. J., Kalaria, R. N. & O’Brien, J. T. Depression and vascular disease: What is the relationship?. J. Affect. Disord 79, 81–95. https://doi.org/10.1016/S0165-0327(02)00349-X (2004).
Folli, F. et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro-and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 7, 313–324 (2011).
Esposito, K. et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 106, 2067–2072. https://doi.org/10.1161/01.cir.0000034509.14906.ae (2002).
Lustman, P. J. & Clouse, R. E. Depression in diabetic patients: The relationship between mood and glycemic control. J. Diabetes Complicat. 19, 113–122. https://doi.org/10.1016/j.jdiacomp.2004.01.002 (2005).
Vavakova, M., Durackova, Z. & Trebaticka, J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015, 898393. https://doi.org/10.1155/2015/898393 (2015).
Quagliaro, L. et al. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: The distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 183, 259–267. https://doi.org/10.1016/j.atherosclerosis.2005.03.015 (2005).
Hirsch, I. B. Glycemic variability: It’s not just about A1C anymore!. Diabetes Technol. Ther. 7, 780–783. https://doi.org/10.1089/dia.2005.7.780 (2005).
Risso, A., Mercuri, F., Quagliaro, L., Damante, G. & Ceriello, A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am. J. Physiol. Endocrinol. Metab. 281, E924-930. https://doi.org/10.1152/ajpendo.2001.281.5.E924 (2001).
Jeon, S. W. & Kim, Y. K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness?. World J. Psychiatry 6, 283–293. https://doi.org/10.5498/wjp.v6.i3.283 (2016).
Kawanishi, S. & Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 1019, 278–284. https://doi.org/10.1196/annals.1297.047 (2004).
von Zglinicki, T. Role of oxidative stress in telomere length regulation and replicative senescence. Ann. N. Y. Acad. Sci. 908, 99–110. https://doi.org/10.1111/j.1749-6632.2000.tb06639.x (2000).
Simon, N. M. et al. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry 60, 432–435. https://doi.org/10.1016/j.biopsych.2006.02.004 (2006).
Lin, L. Y. et al. Dietary patterns in relation to components of dyslipidemia and fasting plasma glucose in adults with dyslipidemia and elevated fasting plasma glucose in Taiwan. Nutrients. https://doi.org/10.3390/nu11040845 (2019).
Mukherjee, S., Thakur, G., Kumar, B. D., Mitra, A. & Chakraborty, C. Long-term effects of a carbohydrate-rich diet on fasting blood sugar, lipid profile, and serum insulin values in rural Bengalis. J. Diabetes 1, 288–295. https://doi.org/10.1111/j.1753-0407.2009.00050.x (2009).
Walatara, K. N., Athiththan, L. V., Hettiaratchi, U. K. & Perera, P. R. Effect of demographic status and lifestyle habits on glycaemic levels in apparently healthy subjects: A cross-sectional study. J. Diabetes Res. 2016, 5240503. https://doi.org/10.1155/2016/5240503 (2016).
Lim, J., Lee, J. A. & Cho, H. J. Association of alcohol drinking patterns with presence of impaired fasting glucose and diabetes mellitus among South Korean adults. J. Epidemiol. 28, 117–124. https://doi.org/10.2188/jea.JE20170021 (2018).
Sultana, R. et al. Fasting serum glucose level in male cigarette smoker. Mymensingh Med. J. 28, 808–810 (2019).
Emerson, N. D. et al. Behavioral risk factors for self-reported depression across the lifespan. Mental Health Prevent. 12, 36–41. https://doi.org/10.1016/j.mhp.2018.09.002 (2018).
Strine, T. W. et al. Depression and anxiety in the United States: Findings from the 2006 Behavioral Risk Factor Surveillance System. Psychiatr. Serv. 59, 1383–1390. https://doi.org/10.1176/appi.ps.59.12.1383 (2008).
Gigantesco, A., Ferrante, G., Baldissera, S., Masocco, M. & Group, P. C. Depressive symptoms and behavior-related risk factors, Italian population-based surveillance system, 2013. Prev. Chronic Dis 12, E183. https://doi.org/10.5888/pcd12.150154 (2015).
Turgunova, L. et al. The incidence of depression among the population of Central Kazakhstan and its relationship with sociodemographic characteristics. Behav. Neurol. 2017, 2584187. https://doi.org/10.1155/2017/2584187 (2017).
Krakoff, L. R. & Phillips, R. A. Blood pressure variability: insights from “Big Data”. J Am Coll Cardiol. 68, 1387–1388 (2016).
Ajjan, R., Slattery, D. & Wright, E. Continuous glucose monitoring: A brief review for primary care practitioners. Adv. Ther. 36, 579–596. https://doi.org/10.1007/s12325-019-0870-x (2019).
Tully, P. J., Debette, S. & Tzourio, C. The association between systolic blood pressure variability with depression, cognitive decline and white matter hyperintensities: The 3C Dijon MRI study. Psychol. Med. 48, 1444–1453 (2018).
Brunoni, A. R. et al. Heart rate variability is a trait marker of major depressive disorder: Evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int. J. Neuropsychopharmacol. 16, 1937–1949 (2013).
Author information
Authors and Affiliations
Contributions
H.J.K. and S.M.P. conceptualized and designed the study. S.M.P. had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. H.J.K. conducted the statistical analysis. All authors engaged in the analysis and interpretation of data. H.J.K. and S.M.P. wrote the draft of the manuscript. S.C. acquired data. All authors critically revised the manuscript to express important intellectual content in a clearer manner. S.M.K. provided administrative, technical, or material support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, H.J., Kim, S.M., Lee, G. et al. Association between visit-to-visit fasting glycemic variability and depression: a retrospective cohort study in a representative Korean population without diabetes. Sci Rep 12, 18692 (2022). https://doi.org/10.1038/s41598-022-22302-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22302-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.