Abstract
We repeatedly measured isotopic compositions of noble gases and CO2 in volcanic gases sampled at six fumaroles around the Kusatsu-Shirane volcano (Japan) between 2014 and 2021 to detect variations reflecting recent volcanic activity. The synchronous increases in 3He/4He at some fumaroles suggest an increase in magmatic gas supply since 2018. The increase in magmatic gas supply is also supported by the temporal variations in 3He/CO2 ratios and carbon isotopic ratios of CO2. The 3He/40Ar* ratios (40Ar*: magmatic 40Ar) show significant increases in the period of high 3He/4He ratios. The temporal variation in 3He/40Ar* ratios may reflect changes in magma vesicularity. Therefore, the 3He/40Ar* ratio of fumarolic gases is a useful parameter to monitor the current state of degassing magma, which is essential for understanding the deep process of volcanic unrest and may contribute to identifying precursors of a future eruption. These results provide additional validation for the use of noble gas and carbon isotopic compositions of fumarolic gases for monitoring magmatic–hydrothermal systems.
Similar content being viewed by others
Introduction
The compositions of volcanic gases reflect deep to subsurface volcanic processes such as degassing from magma, incorporation of shallower components, and vapor–liquid separation1. Hence, volcanic gases provide direct information about a magmatic‒hydrothermal system beneath a volcano.
The 3He/4He ratios of volcanic gases (up to ~ 8 RA in Japan2, where RA denotes the atmospheric 3He/4He ratio of 1.4 × 10−6; ref.3) are sometimes lower than the 3He/4He ratio in the mantle (7–9 RA4) due to the incorporation of crustal He with a low 3He/4He ratio of ~ 0.01–0.02 RA5. The low crustal 3He/4He ratio is due to 4He production by the decay of U and Th. Since the proportion of magmatic and crustal He in volcanic gases may vary in response to magmatic gas flux, 3He/4He ratios might be useful for monitoring volcanic activity. For example, pre-eruptive 3He/4He ratio increases have been reported at some volcanoes, suggesting that an increase in magmatic He contribution could precede the eruptions6,7,8.
Some fractions of CO2 in fumarolic gases might be added by shallow processes after degassing from magma, such as decarbonation of crustal limestone9,10. The relative contributions of major CO2 sources in fumarolic gases can be estimated by combining the 3He/CO2 ratios and carbon isotopic ratios of CO211. Although some physicochemical processes at shallow depths can modify both parameters, the spatial-temporal variations in 3He/CO2 ratios and carbon isotope ratios of CO2 have been used to monitor volcanic activities and understand the structures of magmatic‒hydrothermal systems12,13,14,15,16,17.
Some temporal variations in the relative abundances of volatile species in magmatic gases have been attributed to solubility-controlled fractionation that correlates with the vesicularity of degassing magma13,15,16,18,19,20. Since noble gases such as He and Ar are chemically inert, their abundance ratios are not affected by any chemical process. Thus, the temporal variations in magmatic 3He/40Ar* ratios (40Ar*: 40Ar corrected for the atmospheric contribution) in volcanic gases may reflect a change in the state of degassing magma.
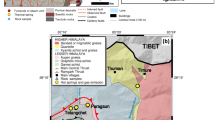
To investigate the applicability of these isotopic parameters to monitoring the magmatic‒hydrothermal system of the Kusatsu-Shirane volcano, we have repeatedly measured the concentrations and isotopic compositions of noble gases and CO2 in fumarolic gases since 2014. Kusatsu-Shirane is an active stratovolcano that consists of three pyroclastic cones: Mt. Shirane, Mt. Ainomine, and Mt. Motoshirane, where phreatic eruptions frequently occur21. The presence of an electrically conductive structure at depths of 1–3.5 km broadly extending from beneath Mt. Shirane to Mt. Motoshirane has been detected by magnetotelluric surveys (Fig. 1), and this structure has been interpreted as a fluid reservoir supplying magmatic‒hydrothermal fluid to fumaroles and hot springs22,23,24. Because of its frequent eruptions and long-term multiparameter observations of volcanic activity21,25,26,27,28, Kusatsu-Shirane is one of the best case study fields for monitoring a magmatic‒hydrothermal system.
Map of the Kusatsu-Shirane volcano. White circles are the locations of fumaroles where samples were collected for this study. A white star indicates the location of the phreatic eruption on 23 January 2018. Yellow circles show the Kitagawa geothermal area located on the northern flank of Mt. Shirane. The red area indicates the horizontal extent of electrical conductor “C2”, which is probably a hydrothermal fluid reservoir beneath the Kusatsu-Shirane volcano23. The base map is from the website of the Geospatial Information Authority of Japan (https://maps.gsi.go.jp/).
Fumarolic gases were repeatedly sampled at three fumaroles in the Kitagawa geothermal area (Kitagawa fumaroles W, C, and E), the Sesshogawara fumarole (S) on the eastern side of the volcano, the Manza fumarole (M) on the western side of the volcano, and the Motoshirane fumarole (MS) on the eastern side of Mt. Motoshirane (Fig. 1 and Supplementary Fig. S1). The sampling periods are between July 2015 and April 2021 for the W and E fumaroles, between October 2016 and April 2021 for the C fumarole, between October 2014 and April 2021 for the Sesshogawara fumarole, and between March 2018 and April 2021 for the Manza fumarole. The Motoshirane fumarolic gas was sampled on 11 August 2020.
In this paper, we discuss following topics based on the fumarolic gas compositions at the Kusatsu-Shirane volcano: (1) temporal variations in magmatic gas supply based on 3He/4He ratios; (2) CO2 sources feeding fumaroles based on spatial–temporal variations in 3He/CO2 ratios and carbon isotopic compositions; and (3) temporal variations in magma vesicularity based on 3He/40Ar* ratios.
Results
Temporal variations in 3He/4He ratios
The noble gas and CO2 concentrations, He, Ne, and Ar isotopic ratios, and 13C/12C ratios of CO2 in the fumarolic gas samples are listed in Supplementary Table S1. The 13C/12C ratios are shown in the delta (δ) notation as parts per thousand deviations (per mil, ‰) from the international Pee Dee Belemnite (PDB) standard. Five samples were collected as residual gases (R-gases) in Giggenbach-type bottles (see “Methods” section and Supplementary Table S1). Since noble gas concentrations in the residual gases increase due to the adsorption of acidic gases (such as CO2 and H2S) into the KOH solution in the bottles, those samples are excluded from the discussion about noble gas concentrations.
All measured 3He/4He ratios (2.5–8.1 RA) are significantly higher than the atmospheric value, indicating the presence of mantle He. The (3He/4He)corr ratios (air-corrected 3He/4He) are calculated using the 4He/20Ne ratios (Eqs. 1 and 2). The (3He/4He)corr ratios for Kitagawa fumaroles W (6.7–8.0 RA), C (6.2–8.1 RA), and E (7.1–8.0 RA) are generally higher than those for the Sesshogawara (6.4–7.8 RA) and Manza (5.3–7.4 RA) fumaroles (Supplementary Table S1). The (3He/4He)corr ratio of the Motoshirane fumarolic gas (8.5 ± 2.1 RA) may be similar to those of the other fumaroles, although it has a large error due to the correction for significant atmospheric contamination. Because of the significant atmospheric contamination, the Motoshirane sample is excluded from the following discussions unless otherwise noted. According to Eq. 3, 3Heair contributes less than 9% of the sample 3He. Since the high (3He/4He)corr ratios indicate crustal 3He is negligible, the 3He of most samples is dominantly derived from magma.
Figure 2 shows the temporal variations in the (3He/4He)corr ratios for the Kitagawa, Sesshogawara, and Manza fumaroles, along with the monthly numbers of earthquakes at the Kusatsu-Shirane volcano. The dotted lines in Fig. 2a–c indicate a phreatic eruption that occurred at Mt. Motoshirane on 23 January 201829. The temporal (3He/4He)corr variation patterns for the three Kitagawa fumaroles W, C, and E are roughly synchronized. The (3He/4He)corr ratios increased in May 2018, decreased in October 2018, and then progressively increased. We separated the study period since July 2015 into four intervals based on the averages of (3He/4He)corr ratios for the three Kitagawa fumaroles (Fig. 2b). In periods I (July 2015–November 2017) and III (October 2018–August 2018), the average (3He/4He)corr ratios were lower than the arbitrarily determined value of 7.8 RA. In periods II (May 2018–August 2018) and IV (October 2019–April 2021), the average (3He/4He)corr ratios were higher than 7.8 RA. Period IV might have continued after the most recent sampling in April 2021.
Temporal variations in air-corrected 3He/4He ((3He/4He)corr) ratios of fumarolic gases and monthly numbers of volcanic earthquakes at Kusatsu-Shirane volcano. (a) (3He/4He)corr ratios measured at the W, C, and E fumaroles in the Kitagawa geothermal area. The Roman numerals above the panel are periods defined by the average (3He/4He)corr ratios of the Kitagawa fumarolic gases. High average (3He/4He)corr ratios (> 7.8 RA) were measured in periods II and IV. (b) (3He/4He)corr ratios measured at the Sesshogawara (S) and Manza (M) fumaroles, and the averages of W, C, and E fumaroles. (c) Numbers of volcanic earthquakes per month in all areas of Kusatsu-Shirane (white) and near Mt. Motoshirane (red). The earthquake data are provided by the Japan Meteorological Agency. Dotted lines indicate 23 January 2018, when a phreatic eruption occurred at Mt. Motoshirane.
The range of temporal variations for the Sesshogawara fumarole is smaller than that for the Kitagawa fumaroles except for the episodic drop in February 2018, three weeks after the 2018 eruption. The volcanic gases before the eruption were not sampled at the Manza fumarole. The (3He/4He)corr ratios for the Manza fumarole progressively increased after the eruption until June 2018. In June 2019, the (3He/4He)corr ratio episodically decreased and recovered in the next month.
Temporal variations in carbon isotopic ratios and 3He/CO2 ratios
The δ13C-CO2 values for the Kitagawa fumaroles W, C, and E varied in the ranges of − 4.4 to − 1.4‰, − 4.9 to − 1.6‰, and − 4.2‰ to − 1.8‰, respectively. Those for the Sesshogawara and Manza fumaroles varied in the ranges of − 4.5 to − 0.8‰ and − 3.7 to − 0.5‰, respectively.
The 3He/CO2 ratios for the Kitagawa fumaroles W, C, and E significantly varied in the ranges of (0.3–1.6) × 10−10, (0.5–1.7) × 10−10, and (0.4–1.5) × 10−10, respectively. Those for the Sesshogawara and Manza fumaroles show relatively minor variations that are in the ranges of (0.3–0.7) × 10−10 and (0.3–0.5) × 10−10, respectively. The 3He/CO2 ratios of the W, C, and E samples collected on the same date are similar, and high in the periods II and IV when the (3He/4He)corr ratios were high (Supplementary Fig. S2c).
Figure 3 shows 3He/CO2 versus δ13C-CO2 diagrams. All data points are plotted within the region defined by the three-component mixing of the MORB-type mantle11,30, limestone-derived CO211, and organic-derived CO211 (Fig. 3). The 3He/CO2 ratios and the δ13C-CO2 values for the three Kitagawa fumaroles show a negative correlation (Fig. 3b). On the other hand, the 3He/CO2 ratios and the δ13C-CO2 values for the Sesshogawara and Manza fumaroles are poorly correlated (Fig. 3c).
3He/CO2 versus δ13C-CO2 diagrams for the fumarolic gas samples. (a) Gas compositions for the W, C, and E fumaroles in the Kitagawa geothermal area and the Sesshogawara (S) and Manza (M) fumaroles. A dotted square indicates the areas of panels (b) and (c). (b) Gas compositions for the Kitagawa W, C, and E fumaroles. (c) Gas compositions for the S and M fumaroles. The mantle 3He/CO2 ratio of (5.99 ± 0.75) × 10−10 is after ref.30. The ranges of δ13C-CO2 values for mantle (− 4‰ to − 9‰), organic carbon (− 20‰ to − 40‰), and limestone (− 2‰ to 2‰) are after ref.11. The estimated δ13C-CO2 value (1.1‰) for Kusatsu-Shirane crustal CO210 is also shown. Black lines are the mixing lines between mantle composition and organic-derived CO2, and mantle composition and limestone-derived CO2.
Temporal variations in 3He/40Ar* ratios
Figure 4 shows the 40Ar/36Ar versus 38Ar/36Ar diagrams. The black line is a mass fractionation line for the atmospheric Ar3. Most data points are plotted above the atmospheric mass fractionation line, indicating that those fumarolic gases contain excess 40Ar (i.e., 40Ar*), that may originate in the mantle or crust where radiogenic 40Ar has been produced by the decay of 40 K. The sample 38Ar/36Ar ratios show negative anomalies up to ~ 1.6% relative to the air. The anomalies reflect mass fractionation because the mantle 38Ar/36Ar ratio is almost indistinguishable from the atmospheric value3. Because such isotopic shifts should be parallel to the atmospheric mass fractionation line, the 40Ar* concentrations are calculated from the vertical deviations from the atmospheric mass fractionation line using Eqs. 4 and 5. Some samples have large 40Ar* errors or no resolvable 40Ar excess due to significant contributions of atmospheric Ar.
Ar three-isotope diagrams for the fumarolic gas samples collected at the W, C, and E fumaroles in the Kitagawa geothermal area, and the Sesshogawara (S) and Manza (M) fumaroles. The air composition of (38Ar/36Ar, 40Ar/36Ar) = (0.188, 296) and a mass fractionation line for the atmospheric Ar are also shown3.
The 40Ar* concentrations in the Kitagawa samples are higher than ~ 0.8 ppm (Supplementary Table S1). Assuming that the crustal 4He/40Ar ratio is ~ 6 (a typical production ratio in the crust5), the crustal 40Ar concentrations in the samples are estimated from the crustal 4He concentrations. Assuming that the (3He/4He)corr ratios reflect a simple two-component mixing of the crustal and magmatic He with 3He/4He ratios of 0.02 RA4 and 8.1 RA (the highest (3He/4He)corr ratio in this study), respectively, crustal 4He concentrations in all the samples are estimated. Accordingly, the crustal 40Ar in the samples should be less than 9% of their total 40Ar*. Therefore, the most 40Ar* of the Kitagawa samples are derived from the magma containing mantle Ar. Some Sesshogawara and Manza samples also contain detectable 40Ar*. The crustal 40Ar in these samples contributes less than 7% of their total 40Ar*.
In summary, both 3He and 40Ar* in the samples are mostly magmatic in origin. Because of their inertness, the 3He/40Ar* variations of the fumarolic gases may directly reflect the 3He/40Ar* variations of magmatic gas. Some 3He/40Ar* ratios have large errors due to the large uncertainties of 40Ar*. In order to examine the 3He/40Ar* variation properly, we used the 3He/40Ar* ratios with < 70% errors in the following discussion. These are 35 of the 63 samples for which Ar isotopic compositions were measured.
Figure 5 shows temporal variations in the 3He/40Ar* ratios for the Kitagawa, Sesshogawara, and Manza fumaroles along with the 3He/40Ar* ratios of the fumarolic gases collected at the Kitagawa geothermal area in 2000–2001 ((0.1–0.3) × 10−4; calculated from the literature data31). The 3He/40Ar* ratios for the three Kitagawa fumaroles synchronously varied in the range of (0.2–1) × 10−4, and the 3He/40Ar* ratios were high during the high (3He/4He)corr periods II and IV (Fig. 5). The 3He/40Ar* ratios for the Sesshogawara fumarole were almost stable in the range of (0.1–0.3) × 10−4. The 3He/40Ar* ratios for the Manza fumarole were (0.02–0.1) × 10−4.
Temporal variations in the 3He/40Ar* ratios of fumarolic gases. (a) 3He/40Ar* ratios measured at the W, C, and E fumaroles in the Kitagawa geothermal area and those of fumarolic gases collected in the same area in 2000–200131. Gray areas highlight the periods of high magma vesicularity, which are suggested by increased 3He/40Ar* ratios. (b) 3He/40Ar* ratios measured at the Sesshogawara (S) and Manza (M) fumaroles. The Roman numerals above the panels are periods defined by the average (3He/4He)corr ratios of the Kitagawa fumarolic gases (see text for details). Samples with uncertainties of more than 70% in the 3He/40Ar* ratios are not shown. Dotted lines indicate 23 January 2018, when a phreatic eruption occurred at Mt. Motoshirane.
Discussion
As previously mentioned, the (3He/4He)corr ratios of the fumarolic gases reflect the mixing of magmatic and crustal He. The synchronous temporal variations in the (3He/4He)corr ratios for the three Kitagawa fumaroles suggest that they are fed by a common volcanic gas reservoir. The highest observed (3He/4He)corr ratio of 8.1 RA indicates that the 3He/4He ratio of the Kusatsu-Shirane magma is ~ 8.1 RA or higher. This is in the range of the 3He/4He ratios of mid-ocean ridge basalts (7–9 RA4). Therefore, the Kusatsu-Shirane magma is almost unaffected by crustal He. The (3He/4He)corr ratios for the Sesshogawara and Manza fumaroles are lower than those for the Kitagawa fumaroles, reflecting a greater influence of crustal He.
The average (3He/4He)corr ratio of the Kitagawa fumarolic gases since May 2018 (~ 7.8 RA) is higher than that before November 2017 (~ 7.2 RA). The increase in (3He/4He)corr ratios can be explained by an increase in magmatic He supply or a decrease in crustal He supply. For the Kitagawa fumaroles, the former is more likely because this is consistent with the enrichments in magmatic gas species (such as CO2 and He) in the fumarolic gases since May 201828. Thus, the magmatic gas supply may have become more substantial since sometime between November 2017 and May 2018. This view is supported by previous reports of shallow inflation and more volcanic earthquakes around Mt. Shirane since April 2018, three months after the 2018 eruption21,25 (Fig. 2c). The enhanced volcanic activity may reflect the supply of magmatic fluid from a magma chamber, a potential pressure source for deep inflation at a depth of ~ 4 km since 201821,26.
It should be noted that some previous studies proposed a mixing of two magmatic components with different 3He/4He ratios to explain (3He/4He)corr variations of volcanic gases15,32,33,34. In this case, one component with a higher 3He/4He ratio may originate from a primitive magma. The other component with a lower 3He/4He ratio may originate from an aged magma, which might have experienced crustal contamination and magma aging that can lower the 3He/4He ratio15. However, this may not be the case for the Kusatsu-Shirane volcano, where the 3He/40Ar* and 3He/CO2 ratios are low when the (3He/4He)corr ratio is low (Fig. 5 and supplementary Fig. S2c). This relationship is opposite to the expected composition of aged (more degassed) magma, which should have higher 3He/40Ar* and 3He/CO2 ratios because the solubilities of Ar and CO2 in silicate melt are lower than He35,36.
The magmatic gas supply might have been especially large during periods II (May 2018–August 2018) and IV (October 2019–April 2021) when relatively high (3He/4He)corr ratios are observed. This is supported by the recent variation in the Cl concentration of the Yugama crater lake, suggesting an increase in the supply of magmatic fluid in 2018 and 202027,37. This result confirms that the (3He/4He)corr ratio is an excellent parameter to monitor the temporal variations in magmatic gas supply at a volcano with a well-developed hydrothermal system that may interfere with the compositions of other chemically reactive magmatic gas species, as has been previously proposed by the studies at the other volcanoes6,7,8.
At the Sesshogawara and Manza fumaroles, significantly low (3He/4He)corr ratios were measured after the phreatic eruption in 2018 (Fig. 2), suggesting a decrease in the magmatic/crustal He ratios in response to the eruption. The synchronous responses may reflect that both fumaroles are connected to the hydrothermal fluid reservoir that caused the phreatic eruption at Mt. Motoshirane21. This is consistent with a magnetotelluric study23 that suggested the presence of a hydrothermal fluid reservoir (the C2 conductor in Fig. 1) broadly spread beneath the Kusatsu-Shirane volcano.
During the 2018 eruption, fluid injection from the hydrothermal fluid reservoir to shallower depths was suggested from ground deformation recorded by a borehole tiltmeter network at the Kusatsu-Shirane volcano21. The low (3He/4He)corr ratios after the eruption might indicate that the hydrothermal fluid in the deeper part of the fluid reservoir is more influenced by crustal He than the shallower part, which constantly supplies fluid to the fumaroles. In this case, the fluid with a low 3He/4He ratio might have been temporarily supplied from the deeper part to the fumaroles. Another process that would lower the (3He/4He)corr ratios is a decrease in the hydrothermal fluid supply to the fumaroles. A previous study reported a gradual decrease in the (3He/4He)corr ratios of fumarolic and hot spring gases with distance from the center of volcanic activity at the Kusatsu-Shirane volcano (i.e., the Yugama crater lake), indicating the continuous addition of crustal He during the transport of hydrothermal fluid10. Since the hydrothermal fluid is the carrier of magmatic He with a high 3He/4He ratio, the 3He/4He ratios of fumarolic gases would become lower when the fluid supply decreases.
The low (3He/4He)corr ratio was measured at the Manza fumarole alone in June 2019. In this case, the scenario of low-3He/4He fluid injection is less likely because it would also result in a low (3He/4He)corr ratio at the Sesshogawara fumarole. Therefore, the low (3He/4He)corr ratio in June 2019 suggests a decrease in the hydrothermal fluid supply to the Manza fumarole.
Some physicochemical reactions such as (1) vapor–liquid separation, (2) pH-related CO2-HCO3− equilibrium, and (3) carbonate precipitation can modify 3He/CO2 and δ13C-CO2 values of volcanic gases at a magmatic‒hydrothermal system2,10,38,39. For our samples, (2) and (3) are ruled out because both require medium to high-pH conditions, although the magmatic‒hydrothermal fluid of the Kusatsu-Shirane volcano is strongly acidic (pH < 3.2)10,27,40. The (1) vapor–liquid separation may cause the 3He/CO2 fractionation due to the lower solubility of He than CO2 in aqueous fluid41. However, because the bulk of the volatiles partition into the gas phase, the 3He/CO2 ratios of the sampled gas phase are expected to approach that of the fluid before vapor–liquid separation2. Accordingly, the 3He/CO2 and δ13C-CO2 variations may not be attributable to the fractionation due to the above reactions.
The Kitagawa fumarolic gas compositions show a negative correlation in the 3He/CO2 versus δ13C-CO2 diagram, and the data points with lower 3He/CO2 ratios distribute toward the limestone-derived CO2 composition (Fig. 3b). A previous study reported that δ13C-CO2 values of volcanic gases from the Kusatsu-Shirane volcano increase with distance from the Yugama crater, suggesting the shallow assimilation of crustal CO2 produced by decarbonation of the limestones with a δ13C-CO2 value of 1.1‰ around the Kusatsu-Shirane volcano10. Therefore, the negative correlations most likely reflect the mixing of the magmatic gas and the crustal CO2 with various proportions (Fig. 3b). This view is supported by the fact that the high 3He/CO2 ratios were observed in the periods II and IV when the (3He/4He)corr ratios were high, reflecting large magmatic gas contribution (Supplementary Fig. S2). Note that the 3He/CO2 and δ13C-CO2 values of the magmatic gas are probably fractionated during magmatic degassing as discussed later.
For the Sesshogawara and Manza fumaroles, the lower 3He/CO2 ratios than the Kitagawa fumaroles suggest smaller magmatic CO2 contributions. This is consistent with the lower (3He/4He)corr ratios for both fumaroles (Fig. 2), indicating smaller contributions of magmatic He. The δ13C-CO2 variations of more than a few ‰, independent of the 3He/CO2 ratios, may reflect the addition of various amounts of organic-derived CO2 at shallow depths. The soil CO2 from some parts of the Satsuma-Iwojima volcano mainly consists of biogenic CO2 with a low δ13C-CO2 of − 27‰42. Similar soil CO2 dissolved in meteoric water is a potential source of the organic-derived CO2 since the incorporation of meteoric water has been suggested from the elemental and isotopic compositions of the Sesshogawara and Manza fumarolic gases28.
The fractionation of 3He/40Ar* ratios in the gas phase during vapor–liquid separation should be minimal for our samples because the solubilities of He and Ar in water are similarly low, and the difference is much smaller than those of He and CO25,41. Therefore, the 3He/40Ar* ratios in the fumarolic gases are expected to be almost identical to the magmatic gas composition. The 3He/40Ar* ratios of the Kitagawa fumarolic gases have episodically increased since May 2018. The highest ratios were ~ 5 times the pre-eruptive value of ~ 3 × 10−5 in 2017 (Fig. 5). In addition, the 3He/40Ar* ratios of (1–3) × 10−5 in 2000–200131 were similar to the pre-eruptive value. Since the volcano had been calm between 1991 and 201443,44, these values indicate that the 3He/40Ar* ratios were low during low volcanic activity.
Volatiles in a gas phase separated from a silicate melt are fractionated as functions of solubilities and melt vesicularity. Volatiles with lower solubilities are more partitioned into the gas phase, and the degree of elemental fractionation decreases with increasing vesicularity1,45. Figure 6 shows the fractionations of He/Ar and He/CO2 ratios in the gas phase of an anhydrous basaltic magma as functions of magma vesicularity in a closed system (computed from Eq. (6) in “Methods” section). Since the solubility of Ar in a silicate melt is lower than He35, the He/Ar ratio in the gas phase is low when the magma vesicularity is small. Although the solubility difference between He and Ar may decrease when the H2O concentration in a silicate melt is higher, the solubility of He is always higher than Ar46. Therefore, the increase in 3He/40Ar* ratios is attributable to the enhanced vesicularity of the degassing magma. This inference is supported by the compositional trend for Kitagawa samples showing a linear correlation in the 3He/40Ar* versus 3He/CO2 diagram (Fig. 7) because a vesicularity-controlled fractionation trend is expected to be linear. For example, the vesicularity-controlled fractionation trend for the gas phase of a magma with a composition of “A”, calculated from Eq. 6 and the parameters used for Fig. 6, yields the straight line in Fig. 7. The enhanced magma vesicularity is consistent with an increase in the magmatic gas supply since 2018, inferred from the (3He/4He)corr ratios. Another process that would potentially change the 3He/40Ar* ratios is a mixing of two (or more) magmatic components with different compositions. However, this is less likely because two-component mixing yields a curved correlation in the 3He/40Ar* versus 3He/CO2 diagram, as demonstrated by the mixing curve between “A” and “B” in Fig. 7, which is different from the trend for the Kitagawa samples.
He/Ar and He/CO2 fractionations in the gas phase during closed system degassing of basaltic melt. Black lines show the fractionation lines computed using Eq. 6 with assumptions that the solubilities of He, Ar, and CO2 in the melt are 5.7 × 10−4 cm3STP g−1 bar−1, 6.4 × 10−5 cm3STP g−1 bar−1, and 2.9 × 10−4 cm3STP g−1 bar−1, respectively35,36. The initial He/Ar and He/CO2 ratios in magma are 1. See “Methods” section for details.
3He/40Ar* versus 3He/CO2 diagram for the fumarolic gas samples. Samples with uncertainties of more than 70% in the 3He/40Ar* ratios are not shown. The straight line shows a relationship between 3He/40Ar* and 3He/CO2 ratios in the gas phase of a magma with a composition of “A” as a function of magma vesicularity. The relationship is computed using Eq. 6 with assumptions that the solubilities of He, Ar, and CO2 in the magma are 5.7 × 10−4 cm3STP g−1 bar−1, 6.4 × 10−5 cm3STP g−1 bar−1, and 2.9 × 10−4 cm3STP g−1 bar−1, respectively35,36. The dashed curve represents a two-component mixing curve for gases with compositions of “A” and “B”. The points “A” and “B” are on the linear regression line for the Kitagawa fumarolic samples with the maximum (1.7 × 10−10) and minimum (6.8 × 10−11) 3He/CO2 ratios, respectively.
The linear correlation also indicates that the 3He/CO2 variations in the Kitagawa fumarolic gases during the periods of high 3He/CO2 ratios (> ~ 1 × 10−10) mainly reflect vesicularity-controlled fractionation, suggesting that the CO2 is mostly magmatic during these periods. The highest 3He/CO2 ratio (~ 1.7 × 10−10) is much lower than the MORB-type mantle value ~ 6 × 10−10 (ref.30) despite the most 3He is also magmatic in origin. The low 3He/CO2 ratios support the previous study that suggested the 3He/CO2 ratio of the Kusatsu-Shirane magma is lower than the MORB-type mantle value due to the addition of CO2 derived from subducted materials11. The δ13C-CO2 values of the Kitagawa samples with high 3He/CO2 ratios (> ~ 1 × 10−10) have a wide range from − 4.9 to − 2.0‰. It is known that the δ13C-CO2 exsolved from the magma is fractionated to a few ‰ heavier value than that in the melt47. The δ13C-CO2 variation may reflect a complex fractionation during magma degassing. However, it cannot be excluded that the variation is due to the assimilation of organic-derived CO2 at shallower depths like that proposed for the Sesshogawara and Manza fumaroles.
Noble gases are minor volatile species in magma and do not affect vesiculation. Instead, the enhancement in magma vesicularity occurs when the magma is depressurized or the concentrations of major volatile species in the magma (e.g., H2O and CO2) increase. The depressurization of magma may be caused by magma ascent or breakdown of a self-sealed zone (Fig. 8), which is mainly formed by the precipitation of silica at the brittle‒ductile transition zone (370–400 °C) around the magma and may induce overpressure27,28,48. On the other hand, the increase in the volatile concentration may be caused by an addition of volatile-rich magma or a decrease in the melt volume as a result of crystallization. However, the former process is less likely because the long-term decreasing trend of the SO4/Cl ratio of the Yugama crater lake since early 2000s is inconsistent with an intrusion of new magma that may cause an increase in the SO4/Cl ratio27. The latter process is inconsistent with the rapid enhancement in magma vesicularity because crystallization is a continuous process during the entire magma cooling history18. Therefore, depressurization due to magma ascent or breakdown of a self-sealed zone might have caused the enhancement in magma vesicularity in periods II and IV.
Here, we summarize a possible scenario for the temporal variations in the magmatic gas composition at the Kusatsu-Shirane volcano since 2014. The enhancement in magma vesicularity started in April 2018 and increased the 3He/40Ar* ratio of the gas exsolved from the magma. Then, magmatic gas with a high 3He/40Ar* ratio was supplied to the Kitagawa fumaroles, resulting in the episodic increases in the 3He/40Ar* ratios observed in period II (Fig. 5a). The enhanced magma vesicularity also increased the magma gas supply, resulting in high (3He/4He)corr ratios (Fig. 2a). The low 3He/40Ar* ratios in period III suggest a short-term decrease in magma vesicularity, possibly due to pressurization by magma descent or growth of a self-sealed zone27,28,48. The high 3He/40Ar* ratios in period IV indicate that the magma vesicularity increased again.
Figure 8 shows a schematic illustration of the magmatic–hydrothermal system at the Kusatsu-Shirane volcano that summarizes the discussion above. The hydrothermal fluid reservoir broadly extending beneath the Kusatsu-Shirane volcano23 may supply magmatic–hydrothermal fluid to the fumaroles around the volcano. The fluid is heterogeneously influenced by magmatic gas. The composition of fluid feeding the Kitagawa fumarolic gases is significantly affected by the magmatic gas, and the 3He/40Ar* variation due to the changes in magma vesicularity is detectable. The fluid compositions feeding the Sesshogawara fumarole and the Manza fumarole are also affected by the magmatic gas, but not sensitive to the temporal variations of magmatic gas composition.
Conclusion
We measured the concentrations and isotopic compositions of noble gases and CO2 in fumarolic gases collected repeatedly at the Kusatsu-Shirane volcano between October 2014 and April 2021. The variations in air-corrected 3He/4He ratios suggest that the magmatic gas supply increased a few months after the phreatic eruption at Mt. Motoshirane in January 2018, then decreased between October 2018 and August 2019, and then increased again after October 2019. The relationship between the 3He/CO2 ratios and δ13C-CO2 values also supports the temporal variations in magmatic gas contribution. Significant increases in 3He/40Ar* ratios were detected in the periods of large magmatic gas supply. The increase probably reflects enhancement in the magma vesicularity. The detection of the 3He/40Ar* variation strongly supports that the 3He/40Ar* ratio is useful to monitor the current state of degassing magma, which is essential for understanding the deep process of volcanic unrest and may contribute to identifying precursors of a future eruption. Since fumaroles are commonly observed at active volcanoes, the monitoring of a magmatic–hydrothermal system by noble gas and carbon isotopic compositions of fumarolic gases should be applicable to other volcanoes.
Methods
Gas sampling
Fumarolic gas samples were collected at six sites at the Kusatsu-Shirane volcano (Fig. 1). Three sites (W, C, E) are in the Kitagawa geothermal area located on the northern side of Mt. Shirane. The locations of W, C, E, Sesshogawara (S), and Manza (M) are the same as those of W, C, E, S, and M in ref.28, respectively. The temperatures of fumarolic gases were almost at the boiling points of water at the elevations of the sampling sites during the sampling period, except for the Motoshirane fumarolic gas. The temperature of the Motoshirane fumarolic gas was almost the same as the ambient temperature, and the gas was not accompanied by wet steam.
In most cases, the gas samples were collected in 50 ml glass sample containers with vacuum valves at both ends. The fumarolic gas was introduced into the container from a fumarolic vent using a titanium pipe and Tygon tubes through a 100 ml glass bottle with vacuum valves at both ends. The 100 ml glass bottle was cooled in ice water to condense the water vapor of the fumarolic gas. The “dry” gas sample from which water vapor was almost completely removed by condensation was collected after flushing the sample container several times with fumarolic gas. The pH values of the condensed water were lower than 4.4 for all samples, indicating that CO2 dissolution into the condensed water was negligible. Therefore, the δ13C values of CO2 in the “dry” gas samples were assumed to be the same as those in the fumarolic gases.
Some samples were collected in 120 ml Giggenbach-type glass bottles containing 5 molar 20 ml KOH solution, following the method described in ref.28. Since water and acidic gases are adsorbed into the KOH solution, noble gases reside in the headspace of the bottle as a residual gas (R-gas). The samples collected in the Giggenbach-type bottles are tagged as “yes” in the R-gas column of Supplementary Table S1.
Noble gas measurements
Noble gas analysis was carried out using a noble gas mass spectrometer (modified VG5400/MS-IV) at the University of Tokyo. Details on the mass spectrometric system and basic analytical procedure are the same as those described in refs.49,50. Air standards were frequently measured during the analysis to determine the mass discrimination factors and the sensitivities of the mass spectrometers for all noble gases except for helium isotopic ratios. The correction factor for the helium isotopic ratios was determined using an interlaboratory helium standard named HESJ. The recommended 3He/4He ratio of HESJ is 20.63 ± 0.10 RA51. The errors in the noble gas isotopic ratios are 1 SD, including statistical errors during sample analysis, errors in the discrimination factors, and the error in the He standard gas. Uncertainties of the concentrations are assumed to be 5% for He, Ne, and Ar and 10% for Kr and Xe, based on the reproducibility of standard gas measurements.
CO2 measurements
After noble gas analysis, the 13C/12C ratios (expressed as δ13CPDB values) and concentrations of CO2 in the gas samples were measured using a gas chromatography, combustion and mass spectrometry (GC/C/MS) system (Delta-S, Finnigan MAT instrument) at the University of Tokyo. The mass discrimination for δ13C and the sensitivity for CO2 of the GC/C/MS system were calibrated by standard gas measurements (CO2 > 99.95%, δ13C = − 30.90‰), which were intermittently carried out during sample analysis. Uncertainties of the CO2 concentrations and the δ13C-CO2 values are assumed to be 10% and 0.3‰, respectively, based on the reproducibility of standard gas measurements.
Calculation of (3He/4He)corr ratios
The (3He/4He)corr ratios (air-corrected 3He/4He) are calculated by the following equations assuming that the 4He/20Ne ratios of magmatic and crustal components are significantly higher than that of air52:
where (4He/20Ne)air of 0.318 is assumed3.
Calculation of 3Heair/3Hesample ratios
The fractions of 3Heair in the samples are calculated by the following equation assuming that all 20Ne is derived from the air:
Calculation of 40Ar* concentrations
The concentration of excess 40Ar (= 40Ar*) is given by:
where (40Ar/36Ar)atm is the 40Ar/36Ar ratio of atmospheric Ar in a sample. The (40Ar/36Ar)atm ratio is given by:
where 3031 and − 273.8 are the slope and intercept of the mass fractionation line for atmospheric Ar, respectively. The mass fractionation line is calculated assuming the Rayleigh process and the air Ar values of (38Ar/36Ar, 40Ar/36Ar) = (0.188, 296)3.
Fractionation of He/Ar and He/CO2 ratios in the gas phase of magma
Since growing bubbles in a silicate melt quickly reach and maintain chemical equilibrium with the surrounding liquid, nonequilibrium exsolution might be due only to dramatic depressurization occurring during explosive volcanic activity18. Therefore, the 3He/40Ar* variations were not likely to be produced by kinetic fractionation caused by the difference in He and Ar diffusivities.
The noble gases in a gas phase exsolved from a silicate melt are fractionated as functions of solubilities and melt vesicularity45:
where (Ci/Cj)gas is the abundance ratio of species i and j in the gas phase, (Ci/Cj)0 is the initial abundance ratio in the melt, V* is the vesicularity of the melt of density ρ, Si and Sj are the solubilities, Te is the equilibrium temperature, and T0 is 273 K.
According to Eq. 6, noble gases with lower solubilities are preferentially partitioned into the gas phase, and the degree of fractionation decreases with increasing vesicularity. The solubility of Ar in a silicate melt is lower than that of He by a factor of 6–11 (ref.35). Therefore, the increases in 3He/40Ar* ratios are attributable to the increased vesicularity of degassing magma. The fractionation of He/Ar in a gas phase of basaltic magma is computed by using Eq. 6 with assumptions that the solubilities for He and Ar are 5.7 × 10−4 cm3STP g−1 bar−1 and 6.4 × 10−5 cm3STP g−1 bar−1 (alkali olivine basalt at 1350°C35), respectively, ρ is 2.8 g cm−3, and TC is 1350 °C (Fig. 6a). Since the solubility of Ar is ~ 10 times lower than that of He, the He/Ar ratio in the gas phase can vary by a factor of ~ 10 at a maximum. Similarly, the fractionation of He/CO2 is also computed by assuming a CO2 solubility of 2.9 × 10−4 cm3STP g−1 bar−1 (0.567 ppm bar−1 in anhydrous silicate melts, ref.36) (Fig. 6b).
Data availability
All data obtained in this study are included in the Supplementary Data file.
References
Giggenbach, W. F. Chemical composition of volcanic gases. In Monitoring and Mitigation of Volcano Hazards (eds. Scarpa, R. & Tilling, R. I.) 221–256 (Springer Berlin, Heidelberg, 1996). https://doi.org/10.1007/978-3-642-80087-0_7.
Sano, Y. & Fischer, T. P. The analysis and interpretation of noble gases in modern hydrothermal systems. in Advances in Isotope Geochemistry (ed. Burnard, P.) 249–317 (Springer Berlin, Heidelberg, 2013). https://doi.org/10.1007/978-3-642-28836-4_10.
Ozima, M. & Podosek, F. A. Noble Gas Geochemistry 2nd edn. (Cambridge University Press, 2002).
Graham, D. W. Noble gas isotope geochemistry of mid-ocean ridge and ocean island basalts: Characterization of mantle source reservoirs. Rev. Mineral. Geochem. 47, 247–317 (2002).
Ballentine, C. J. & Burnard, P. G. Production, release and transport of noble gases in the continental crust. Rev. Mineral. Geochem. 47, 481–538 (2002).
Padrón, E. et al. Diffusive helium emissions as a precursory sign of volcanic unrest. Geology 41, 539–542 (2013).
Sano, Y. et al. Ten-year helium anomaly prior to the 2014 Mt Ontake eruption. Sci. Rep. 5, 1–7 (2015).
Paonita, A., Caracausi, A., Martelli, M. & Rizzo, A. L. Temporal variations of helium isotopes in volcanic gases quantify pre-eruptive refill and pressurization in magma reservoirs: The Mount Etna case. Geology 44, 499–502 (2016).
Mason, E., Edmonds, M. & Turchyn, A. V. Remobilization of crustal carbon may dominate volcanic arc emissions. Science (80) 357, 290–294 (2017).
Sano, Y., Hirabayashi, J. I., Oba, T. & Gamo, T. Carbon and helium isotopic ratios at Kusatsu-Shirane Volcano, Japan. Appl. Geochem. 9, 371–377 (1994).
Sano, Y. & Marty, B. Origin of carbon in fumarolic gas from island arcs. Chem. Geol. 119, 265–274 (1995).
Ray, M. C., Hilton, D. R., Muñoz, J., Fischer, T. P. & Shaw, A. M. The effects of volatile recycling, degassing and crustal contamination on the helium and carbon geochemistry of hydrothermal fluids from the Southern Volcanic Zone of Chile. Chem. Geol. 266, 38–49 (2009).
Caracausi, A., Italiano, F., Paonita, A., Rizzo, A. & Nuccio, P. M. Evidence of deep magma degassing and ascent by geochemistry of peripheral gas emissions at Mount Etna (Italy): Assessment of the magmatic reservoir pressure. J. Geophys. Res. Solid Earth https://doi.org/10.1029/2002JB002095 (2003).
Hilton, D. R. et al. Monitoring of temporal and spatial variations in fumarole helium and carbon dioxide characteristics at Poás and Turrialba Volcanoes, Costa Rica (2001–2009). Geochem. J. 44, 431–440 (2010).
Paonita, A., Caracausi, A., Iacono-Marziano, G., Martelli, M. & Rizzo, A. Geochemical evidence for mixing between fluids exsolved at different depths in the magmatic system of Mt Etna (Italy). Geochim. Cosmochim. Acta 84, 380–394 (2012).
Rizzo, A. et al. New insights into magma dynamics during last two eruptions of Mount Etna as inferred by geochemical monitoring from 2002 to 2005. Geochem. Geophys. Geosyst. https://doi.org/10.1029/2005GC001175 (2006).
Martelli, M., Caracausi, A., Paonita, A. & Rizzo, A. Geochemical variations of air-free crater fumaroles at Mt Etna: New inferences for forecasting shallow volcanic activity. Geophys. Res. Lett. 35, 21302 (2008).
Nuccio, P. M. & Paonita, A. Magmatic degassing of multicomponent vapors and assessment of magma depth: Application to Vulcano Island (Italy). Earth Planet. Sci. Lett. 193, 467–481 (2001).
Cogliati, S. et al. Tracking the behaviour of persistently degassing volcanoes using noble gas analysis of Pele’s hairs and tears: A case study of the Masaya volcano (Nicaragua). J. Volcanol. Geotherm. Res. 414, 107212 (2021).
Bini, G. et al. Nitrogen, helium, and argon reveal the magmatic signature of fumarole gases and episodes of outgassing from upper-crustal magma reservoirs: The case of the Nisyros caldera (Aegean Arc, Greece). Geochim. Cosmochim. Acta 335, 68–84 (2022).
Terada, A. et al. The 2018 phreatic eruption at Mt. Motoshirane of Kusatsu-Shirane volcano, Japan: Eruption and intrusion of hydrothermal fluid observed by a borehole tiltmeter network. Earth Planets Sp. 73, 1–17 (2021).
Nurhasan, et al. Two electrical conductors beneath Kusatsu-Shirane volcano, Japan, imaged by audiomagnetotellurics, and their implications for the hydrothermal system. Earth, Planets Sp. 58, 1053–1059 (2006).
Matsunaga, Y. et al. Magmatic hydrothermal system inferred from the resistivity structure of Kusatsu-Shirane Volcano. J. Volcanol. Geotherm. Res. 390, 106742 (2020).
Tseng, K. H. et al. Anatomy of active volcanic edifice at the Kusatsu-Shirane volcano, Japan, by magnetotellurics: hydrothermal implications for volcanic unrests. Earth, Planets Sp. 72, 161 (2020).
Yamada, T. et al. Locating hydrothermal fluid injection of the 2018 phreatic eruption at Kusatsu-Shirane volcano with volcanic tremor amplitude. Earth Planets Sp. 73, 14 (2021).
Munekane, H. Modeling long-term volcanic deformation at Kusatsu-Shirane and Asama volcanoes, Japan, using the GNSS coordinate time series. Earth Planets Sp. 73, 1–15 (2021).
Yaguchi, M., Ohba, T. & Terada, A. Groundwater interacting at depth with hot plastic magma triggers phreatic eruptions at Yugama Crater Lake of Kusatsu-Shirane Volcano (Japan). Front. Earth Sci. 9, 1056 (2021).
Ohba, T. et al. Time variation in the chemical and isotopic composition of fumarolic gasses at Kusatsu-Shirane Volcano, Japan. Front. Earth Sci. 7, 249 (2019).
Yaguchi, M., Ohba, T., Numanami, N. & Kawaguchi, R. Constituent mineral and water-soluble components of volcanic ash from the 2018 eruption of Mt Motoshirane of Kusatsu-Shirane Volcano, Japan. J. Disaster Res. 14, 991–995 (2019).
Tucker, J. M., Mukhopadhyay, S. & Gonnermann, H. M. Reconstructing mantle carbon and noble gas contents from degassed mid-ocean ridge basalts. Earth Planet. Sci. Lett. 496, 108–119 (2018).
Ohwada, M. Behavior of volatiles in volcanic hydrothermal systems inferred from noble gas abundances and isotopic ratios. Ph.D. dissertation, Tokyo Institute of Technology (2003).
Paonita, A., Caracausi, A., Martelli, M. & Rizzo, A. L. Temporal variations of helium isotopes in volcanic gases quantify pre-eruptive refill and pressurization in magma reservoirs: The Mount Etna case. Geology 44, 499–502 (2016).
Paonita, A. et al. Intense overpressurization at basaltic open-conduit volcanoes as inferred by geochemical signals: The case of the Mt. Etna December 2018 eruption. Sci. Adv. 7, eabg6297 (2021).
Kis, B. M. et al. Noble gas and carbon isotope systematics at the seemingly inactive Ciomadul Volcano (Eastern-Central Europe, Romania): Evidence for volcanic degassing. Geochem. Geophys. Geosyst. 20, 3019–3043 (2019).
Lux, G. The behavior of noble gases in silicate liquids: Solution, diffusion, bubbles and surface effects, with applications to natural samples. Geochim. Cosmochim. Acta 51, 1549–1560 (1987).
Ni, H. & Keppler, H. Carbon in silicate melts. Rev. Mineral. Geochem. 75, 251–287 (2013).
Terada, A., Yaguchi, M. & Ohba, T. Quantitative assessment of temporal changes in subaqueous hydrothermal activity in active Crater Lakes during unrest based on a time-series of lake water chemistry. Front. Earth Sci. 9, 1206 (2022).
Welhan, J. A., Poreda, R. J., Rison, W. & Craig, H. Helium isotopes in geothermal and volcanic gases of the Western United States, II. Long Valley Caldera. J. Volcanol. Geotherm. Res. 34, 201–209 (1988).
Marty, B., Jambon, A. & Sano, Y. Helium isotopes and CO2 in volcanic gases of Japan. Chem. Geol. 76, 25–40 (1989).
Ohba, T., Hirabayashi, J. & Nogami, K. Temporal changes in the chemistry of lake water within Yugama Crater, Kusatsu-Shirane Volcano, Japan: Implications for the evolution of the magmatic hydrothermal system. J. Volcanol. Geotherm. Res. 178, 131–144 (2008).
Wilhelm, E., Battino, R. & Wilcock, R. J. Low-pressure solubility of gases in liquid water. Chem. Rev. 77, 219–262 (1977).
Shimoike, Y., Kazahaya, K. & Shinohara, H. Soil gas emission of volcanic CO2 at Satsuma-Iwojima volcano, Japan. Earth Planets Sp. 54, 239–247 (2002).
Takahashi, K. & Fujii, I. Long-term thermal activity revealed by magnetic measurements at Kusatsu-Shirane volcano, Japan. J. Volcanol. Geoth. Res. 285, 180–194 (2014).
Terada, A. Kusatsu-Shirane volcano as a site of phreatic eruptions. J. Geol. Soc. Japan 124, 251–270 (2018).
Jambon, A., Weber, H. & Braun, O. Solubility of He, Ne, Ar, Kr and Xe in a basalt melt in the range 1250–1600°C. Geochemical implications. Geochim. Cosmochim. Acta 50, 401–408 (1986).
Nuccio, P. M. & Paonita, A. Investigation of the noble gas solubility in H2O-CO2 bearing silicate liquids at moderate pressure II: The extended ionic porosity (EIP) model. Earth Planet. Sci. Lett. 183, 499–512 (2000).
Holloway, J. R. & Blank, J. G. Chapter 6. Application of experimental results to C-O-H species in natural melts. Volatiles Magmas https://doi.org/10.1515/9781501509674-012 (1994).
Fournier, R. O. Hydrothermal processes related to movement of fluid from plastic into brittle rock in the magmatic-epithermal environment. Econ. Geol. 94, 1193–1211 (1999).
Sumino, H., Nagao, K. & Notsu, K. Highly sensitive and precise measurement of helium isotopes using a mass spectrometer with double collector system. J. Mass Spectrom. Soc. Jpn. 49, 61–68 (2001).
Sumino, H. et al. Noble gas and carbon isotopes of fumarolic gas from Iwojima volcano, Izu-Ogasawara arc, Japan: Implications for the origin of unusual arc magmatism. Chem. Geol. 209, 153–173 (2004).
Matsuda, J. et al. The 3He/4He ratio of the new internal He standard of Japan (HESJ). Geochem. J. 36, 191–195 (2002).
Sano, Y., Wakita, H., Ohsumi, T. & Kusakabe, M. Helium isotope evidence for magmatic gases in Lake Nyos, Cameroon. Geophys. Res. Lett. 14, 1039–1041 (1987).
Acknowledgements
We are grateful to the Japan Meteorological Agency for providing the number of volcanic earthquakes around the Kusatsu-Shirane volcano. We thank Springer Nature Author Services for English language editing. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, under its Integrated Program for Next Generation Volcano Research and Human Resource Development (Program Grant Number JPJ005391) and The Second Earthquake and Volcano Hazards Observation and Research Program (Earthquake and Volcano Hazard Reduction Research).
Author information
Authors and Affiliations
Contributions
T.Obase. and H.S. designed the study. All authors contributed to the gas sampling. T.Obase, H.S., K.T., K.K., and K.Y. conducted the sample analysis. T.Obase wrote the initial draft, and all authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Obase, T., Sumino, H., Toyama, K. et al. Monitoring of magmatic–hydrothermal system by noble gas and carbon isotopic compositions of fumarolic gases. Sci Rep 12, 17967 (2022). https://doi.org/10.1038/s41598-022-22280-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22280-3
This article is cited by
-
Volcanic soil gas 4He/CO2 ratio: a useful geochemical tool for real-time eruption forecasting
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.