Abstract
Neurofeedback and transcranial Direct Current Stimulation (tDCS) are promising techniques for neuroenhancement of attentional performance. As far as we know no study compared both techniques on attentional performance in healthy participants. We compared tDCS and neurofeedback in a randomized, single-blind, controlled experiment assessing both behavioral (accuracy and time reaction) and electrophysiological (N1, P1, and P3 components) data of participants responding to the Attention Network Task (ANT). Eighty volunteers volunteered for this study. We adopted standard protocols for both techniques, i.e., a Sensorimotor Rhythm (SMR) protocol for neurofeedback and the right DLPFC anodal stimulation for tDCS, applied over nine sessions (two weeks). We did not find significant differences between treatment groups on ANT, neither at the behavioral nor at the electrophysiological levels. However, we found that participants from both neuromodulation groups, irrespective of if active or sham, reported attentional improvements in response to the treatment on a subjective scale. Our study adds another null result to the neuromodulation literature, showing that neurofeedback and tDCS effects are more complex than previously suggested and associated with placebo effect. More studies in neuroenhancement literature are necessary to fully comprehend neuromodulation mechanisms.
Similar content being viewed by others
Introduction
Neuroenhancement can be defined as the procedure for improving cognitive performance in healthy subjects using non-invasive methods, such as pharmacological1 or non-invasive brain stimulation (NIBS) techniques. Along with the spread of pharmacological stimulants2, there is an increase in the use of neuromodulation devices such as neurofeedback3 and transcranial direct current stimulation (tDCS) for neuroenhancement4. Given its importance to daily life, academic and professional performance, attention has been a frequent target for neuroenhancement techniques5,6.
Neurofeedback is a technique where one aims to modulate the brain’s activity through a feedback signal derived from this ongoing activity provided by neuroimaging (e.g., functional magnetic resonance imaging) or electrophysiology (electro- or magnetoencephalography) techniques7. Neurofeedback studies in attention usually targeted theta, theta/beta ratio, sensory-motor rhythm (SMR), and gamma-band modulation8,9. While some reviews reported improvements in attentional performance8, other reviews indicated null effects9. Given the contradictory findings, a relevant issue in neurofeedback research is to disentangle the contribution of the placebo effect in positive findings10,11,12.
In tDCS, a low-intensity current (usually between 1 and 2 mA) is generated by a stimulator and applied through electrodes placed on the scalp, which modulates cortical excitability in brain areas beneath the electrodes and can modulate cognitive and behavioral performance. Two comprehensive reviews consistently showed enhancement of attentional performance in healthy subjects after tDCS over the dorsolateral prefrontal (DLPFC) or posterior parietal cortex (PPC)13,14. However, there is also controversy in tDCS findings, with some studies reporting positive effects15,16, while others found null16,17 or negative results (e.g., sustainable attention impairment of participants receiving tDCS while under high cognitive load)18. With mention that studies researching tDCS in other cognitive functions found that its effect is dependent on several parameters such as the participants’ performance at baseline19,20; cognitive load during stimulation18; or differences in positioning, orientation, and size of tDCS electrodes21.
To our knowledge, no study has yet compared both neurofeedback and tDCS on attentional performance in healthy participants. Thus, it would be relevant to research the use of standard protocols of both techniques in healthy samples to test their differential effects when compared to placebo on the Attentional Network Task (ANT). ANT is a well-validated paradigm to assess the efficiency of visual attentional components of alert, orienting, and executive control22. Additionally, it is also compatible with EEG, which allows the correlation of attentional electrophysiological components with behavioral measures such as accuracy and time reaction. There are several Event-related Potential (ERP) studies with ANT, typically focusing on attentional components such as N1, P1, and P322,23,24,25,26,27,28.
In this study, we aimed to research the effects of a two-week daily protocol of active and sham neurofeedback versus tDCS in healthy participants’ attentional performance. We ran a randomized, single-blind, controlled experiment to assess behaviorally (accuracy and time reaction) and electrophysiological (N1, P1, and P3 components) changes in response to the treatment. We decided on a two-week protocol with nine sessions based on neurofeedback studies on attention performance showing significant findings with ten sessions, one8 or two29 by week. We equalized number of sessions for tDCS to avoid any indirect effect associated with experiment duration between techniques. Standard protocols were adopted for both techniques: an SMR protocol for neurofeedback and the right DLPFC anodal stimulation for tDCS. Despite the existence of studies with anodal tDCS over left DLPFC, we choose to focus on right DLPFC based on previous studies pointing out its importance with phasic components of attention30, attentional focus31,32 and sustained attention33,34. Besides, meditation training usually leads to increased right DLPFC activity and greater attentional efficiency31,35. An SMR neurofeedback protocol was selected based on previous findings indicating that enhanced SMR (alone or associated with other protocols) improved attention in both healthy and clinical participants9,29,36.
Based on the studies previously presented, we believe that, regarding the behavioral and electrophysiological effects on the attentional task, neurofeedback will perform similarly to the sham neurofeedback and sham tDCS. In contrast, active tDCS will lead to attentional improvement compared to all other treatments. The attentional improvement detected will be expressed as a shorter reaction time and greater accuracy in the ANT and an enhanced ERP amplitude for attentional components (N1, P1, P3) in response to detecting the cues and targets on this task.
Method
Participants
Eighty healthy undergraduate students from Mackenzie Presbyterian University in São Paulo, Brazil, were recruited and randomly arranged in one of the four experimental groups: (1) active neurofeedback; (2) placebo neurofeedback; (3) active tDCS and (4) sham tDCS, with twenty participants each. We decided on twenty participants by group based on the literature indicating positive findings with tDCS or neurofeedback on attention performance of healthy participants with smaller sample sizes8,15,29,37. Following the criteria inclusion, the participants were between 18 and 35 years old, with normal or corrected-to-normal vision, and were right-handed (Edinburgh Handedness Inventory score > 80). Concerning the exclusion criteria, none of the participants reported a history of neurological or psychiatric disorder, metallic implants in the head, or were under the effect of psychoactive medication or drugs during the experiment. The study was approved by the Mackenzie Presbyterian University’s ethics committee and by the National Ethics Committee (SISNEP, Brazil; CAAE: 49534415.7.0000.0084) and was conducted according to the Declaration of Helsinki. All participants signed an informed consent and were informed that they could withdraw from the experiment at any moment.
Instruments
Edinburgh Handedness Inventory (EHI)38—It is a ten-item questionnaire, each describing a manual activity (e.g., writing, drawing, using scissors, sweeping the house) where the participant should report their hand preference. A final quotient hanging from − 100 (left-handed) to 100 (right-handed) indicates laterality.
Attention Network Task (ANT)—We used an adapted version of ANT39 programmed on E-Prime to evaluate attentional performance before and after neuromodulation treatments. Participants had to look at a fixation cross in the center of a white screen and respond to a target’s appearance, i.e., a group of five arrows above or below the fixation cross, indicating the central arrow’s direction (left or right) in a response box. The central arrow could be flanked by traces (neutral condition), same direction arrows (congruent condition), or opposite direction arrows (incongruent condition). In some trials an asterisk (cue) appeared, indicating where or when the arrows would appear. The cue could flash above or below the fixation cross, indicating that target would appear soon in that place (spatial condition); or it could flash below and above the fixing cross, indicating that the target would appear soon (double condition), or cue could not appear (absent condition). The classical ANT version adopts a fourth cue condition, the center cue, however, we decided to discard this condition for two reasons: (1) we preferred not to assess attentional network scores, but the raw accuracy and reaction time for each target and cue conditions, given some studies questioning the reliability of those scores40,41; and (2) it allowed us to increase the trial number of the other cues, enhancing its signal-to-noise ratio and making the task shorter to the participants, avoiding their fatigue. The test consisted of 216 trials, divided into six blocks of 36 trials, each block with four repetitions of target and cue conditions (3 targets × 3 cues × 4 rep.). The sequence of stimulus in a trial, showed in Fig. 1, consisted of the following screens: (1) fixation screen, with a black fixation cross in the center of a white window (random duration between 400 and 1600 ms); (2) cue screen, with double, spatial, or absent cue conditions (100 ms); (3) a second fixation screen (400 ms); (4) target screen, where arrows appeared, and the participant should respond (duration equal to response time and a maximum time of 1700 ms); (5) a final fixation screen, which lasted until the trial duration totalized 3500 ms.
Perceived Changes in Attentional Performance Scale (PCAPS)—Each participant reported at the end of the treatment how much they believe their attentional performance changed in response to the treatment, on a scale of “0” (“much worse”) to “10” (“much better”), where “5” was equivalent to no perceived changes. They should answer any perceived changes in their attention to the ANT task and in their day-to-day activities, such as improved attention in work or classes in the university.
Equipment
Neurofeedback—Neurofeedback intervention was conducted using a NeXus-4 EEG apparatus (Mindmedia Neuro and Biofeedback systems, Maasbracht, The Netherlands). For signal processing and neurofeedback training, we adopted the software Biotrace + from the same company. Participants did the neurofeedback protocol in a quiet room with a computer connected to two monitors, one for the researcher and a second monitor for the participant. A monopolar assembly was used for active and placebo protocols, with the active electrode placed on the vertex (Cz), the ground electrode placed on the right ear lobe (A2), and the reference electrode placed on the left earlobe (A1). The signal was corrected in the Biotrace + using the Automatic Artifact Rejection option, which rejected all the total power above 100μv and electromyography (EMG) artifacts (75–100 Hz) with power above 10μv. During the SMR protocol, the monitor presented to the participants presented several visual features: a bar graph reporting SMR amplitude; an image of a circle (similar to a mandala) that changed size according to SMR amplitude; and a bar chart showing the amplitude of EMG artifacts. Placebo neurofeedback followed the same protocol as the active, but the session exhibited to the participants in the monitor was a demonstration session, which were obtained from real EEG recordings, in this way identical to a real session. For each session, the participant did a baseline measure of SMR amplitude for 2 min. After this measure, participants performed the 20 min session, aiming to enhance SMR amplitude. During the session, participants were instructed to try to enhance their SMR activity by any means, while not enhancing the EMG signal (which indicated artifacts), and in case they reached the goal (increase SMR signal beyond 10% of the baseline) they would receive a visual reward, where the image on the screen would start moving. After each session, participants should report the occurrence of adverse effects during the session.
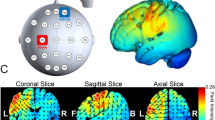
Transcranial Direct Current Stimulation (tDCS)—We applied the direct electrical current through rubber electrodes inside saline-soaked sponges fixed in the scalp by rubber bands, with the current delivered by a battery-driven stimulator. The tDCS stimulator was developed in our laboratory (São Paulo, Brazil; any technical aspect please contact psboggio@gmail,com). Anodal electrode was positioned over F4, according to the 10–20 positioning system, targeting the right DLPFC; and the reference electrode over the contralateral supraorbital region. The rubber electrodes were 35 cm2 (7 × 5 cm), and the stimulation intensity was 1.5 mA. Although most studies on this topic used 2.0 mA8,15, we decided to use 1.5 mA seeking greater comfort for the participant and reducing the total charge due to multiple sessions in the week. The safety aspects of the tDCS application followed the safety protocols suggested in the literature42,43,44. The sham tDCS followed the same protocol of the active, with the difference that the participant received the electric charge in the beginning and at the end of each session for only 30–15 s to ramp up to 1.5 mA and 15 s to ramp down. During the sham condition, the tDCS device showed the intensity (mA) in the display identical to the active condition to make it more believable. tDCS sessions lasted 20 min, and after each session, participants should report any side effects. We assessed the electrical current dispersion of the DLPFC montage through computational modeling on the SimNIBS, as shown in Fig. 2.
Electroencephalography (EEG)—To collect the electrophysiological data, we used an EEG system (Electrical Geodesics Inc., Amsterdam, The Netherlands), consisting of a Macintosh computer for signal registration, an EEG amplifier (EEG system 300), an articulated arm to hold the cap and Hydrocel caps of 128 channels of three different sizes. Each EEG electrode is connected to the scalp by a sponge, wetted with a mixture of warm water, neutral shampoo, and potassium chloride to maintain the impedance below 50 kilohms. The impedance was checked at least twice in the ANT, during the intervals between the blocks.
Procedure
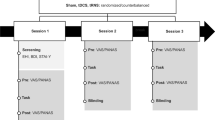
Participants were recruited via the internet and on the university campus, and those who showed interest were later contacted by email with detailed information about the experiment objectives, procedure, and inclusion/exclusion criteria. We randomly allocated the selected participants to one of the four experimental groups, and we scheduled the sessions for each one. The experiment lasted two weeks (ten days): (1) the first day with the ANT with EEG recording, and with the first 20-min neuromodulation session accordingly to their experimental group; (2) 8 days with 20-min treatment sessions (with an interval on the weekend); and (3) the tenth day with participants’ assessment in the ANT with EEG recording and the PCAPS. All the sessions occurred in Mackenzie's Social and Cognitive Neuroscience laboratory. The treatment sessions (tDCS or neurofeedback, active or sham) took place in a quiet room, with the participant at rest (for active or sham tDCS) or performing the neurofeedback task (for active or sham neurofeedback).
EEG data processing
Concerning the EEG data collected during ANT, we did a pre-processing with the following steps for ERP analysis: (1) data filtered by a 40 Hz low-pass filter; (2) segmentation of the data in − 100 to 500 ms relative to the start of each trial; (3) segments were averaged based on the type of cue and target, totaling nine categories (3 cues × 3 targets); (4) detection and marking of eye blink and movement artifact and noise (signal amplitude higher than 200 μV, blinking artifact with signal higher than 140 μV, eye movements artifact with signal higher than 80 μV); (5) exclusion of channels with more than 20% of noise and segments with more than ten noisy channels (from a total of 128), and with blink or eye movement artifacts. We inspected participants' EEG data to ascertain the number of good segments for each condition (9 conditions: 3 cues × 3 targets). Participants with losses greater than 30% (less than 50 good segments in each condition) in more than three categories (in a total of nine) were excluded. After pre-processing, we selected the time window and electrodes for the ERPs N1, P1, and P3, based on visual inspection. All the ERP were selected after target appearance, based on cue type (N1, P1, P3cue) or target type (P3). The electrodes and time window are the following: (1) P1 between 90 and 160 ms on parieto-occipital electrodes (geodesic net 61,62,78,77,67,71,76,75,72); (2) N1 between 160 and 220 ms on the same electrodes of P1; (3) P3cue—between 250 and 400 ms on the same electrodes of P1; (4) P3—between 300 and 450 ms on parietal (61,62,78,79,54,55,31,80) and occipital (71,75,76,72,67,77) electrodes. The electrode area for each ERP can be seen in Fig. 3. For each ERP component and participant, the peak amplitude was detected as the highest amplitude (positive or negative) inside the selected time window, and we extracted the mean amplitude of the peak considering a short time window around it (10 ms before and after).
Statistical analysis
We assessed behavioral and electrophysiological data of the ANT as the primary outcomes through a mixed analysis of variance (ANOVA) with the cue (absent, double, and spatial), target (neutral, congruent, and incongruent), and session (pre- vs. post-treatment ANT) as within-subject factors; and treatment (tDCS active, tDCS sham, Neurofeedback active, and Neurofeedback sham) as between-subject factor. For the behavioral data, we analyzed accuracy (ACC), i.e., the percentage of correct answers, and the averaged reaction time (RT) given cue or target condition, which was calculated only with the correct answers in the ANT. For the ERP components, we compared the averaged amplitude in microvolts (μV) for P1, N1 P3cue, and P3. For the P3 component, we also adopted region (parietal or occipital) as a within-subject factor, to identify differences between parietal and occipital electrodes in its amplitude. We also assessed perceived changes in attention performance for each treatment group employing a one-sample t-test comparing the averaged PCAPS score against 5 (i.e., no perceived changes in attention). We also compared PCAPS scores between treatment groups through a one-way ANOVA. Finally, we assessed if any electrophysiological component would be related to behavioral performance in ANT. For this analysis, we ran Pearson correlation tests between P1, N1, P3cue, and P3 amplitudes with the averaged reaction time in ANT, with separate analyses for each session (pre-and post-treatment). For all the tests mentioned above, we adopted Tukey post hoc tests when necessary, and we adopted a statistical significance of 5%. We ran all the statistical analysis on Jamovi software (Version 1.6).
Results
Statistical analyses were conducted with 73 participants (38 males, 35 females; 18–32 years old; mean age 23 ± 2.12). Of the total of 80 participants, four discontinued the treatment claiming conflicting schedules. One participant was excluded due to outlier data, with reaction time averages above three standard deviations in the ANT. Also, two participants were excluded due to noisy EEG data (few good segments in more than three categories, see data processing and statistical analysis). Participants reported no adverse effects in response to tDCS or neurofeedback and were naive to both techniques. Regarding the Neurofeedback sessions, we considered a session a success when the participant reached the goal of increasing the average SMR signal obtained by 10% beyond a baseline measure (obtained in the beginning of the same session). From the 19 participants in the active group: six participants have no success on increasing SMR in any session, seven participants have succeeded between one and three sessions; four participants succeeded between four and six sessions; and only one participant successfully increased SMR on more than six sessions, succeeding on all the nine sessions. The average number of successful sessions was 2.42 (SD: 2.47 sessions).
Behavioral data in ANT
Regarding accuracy, we detected ceiling effects for congruent and neutral targets, with accuracy above 98% and no variance in a few cases, and for incongruent targets, the overall accuracy was higher than 90%. On average, errors in accuracy were 2.61% for the pre-treatment session (where 3.16% of errors were due to omission) and 2.54% for the post-treatment session (where 8% of errors were due to omission). Given the large skewness and the heteroscedasticity of the accuracy data when split in cue and target conditions, we analyzed only the effects of treatment, session and treatment*session on the overall accuracy (Levene’s test p > 0.05), showing no significant differences for any factor: treatment [F(3,69) = 0.08, p = 0.97], session [F(1,69) = 0.08, p = 0.77], nor treatment*session [F(3,69) = 0.73, p = 0.54].
For the reaction time, we detected main effects for: cue [F(2,138) = 690.17, p < 0.001, η2p = 0.91], with significant differences between all conditions, where the shorter reaction time was for spatial and the longest for the absent condition; target [F(2,138) = 860.39, p = < 0.001, η2p = 0.93], with significant differences between all conditions, with shorter reaction times for neutral and the longest for incongruent targets; and session [F(1,69) = 22.49, p = < 0.001, η2p = 0.25], with a short reaction time in the post-treatment compared to pretreatment ANT. We also detected interaction effects between: cue*target [F(4,276) = 11.07, p = 0.14, η2p = 0.14], with significant differences between all conditions, crossing the main effects found for cue and target, as shown in Fig. 4; and target*session [F(2,138) = 23.57, p = < 0.001, η2p = 0.25], showing differences between pre and post-treatment sessions for all target conditions, with shorter reaction time in the latter. We did not find any significant main effect or interaction associated with treatment, indicating no significant neuromodulation effect.
ERP components in ANT
Concerning the P1 component, we detected main effects for cue [F(2,138) = 125.39, p = < 0.001, η2p = 0.65], with significant differences between all conditions, with larger amplitude for absent and the smaller for double conditions; and session [F(1,69) = 8.54, p = 0.005, η2p = 0.11], with enhanced amplitude of P1 component in post-treatment ANT compared to pretreatment; but no effect for treatment [F(3,69) = 0.07, p = 0.98].
For the N1 component, we detected main effects for cue [F(2,138) = 172.22, p < 0.001, η2p = 0.71], with post hoc showing significant difference between all cue levels, but no significant differences between sessions or treatments. Post-hoc analysis showed increased N1 amplitude for double cues, diminishing spatial cues, and the smaller N1 in response to absent cues.
Regarding the P3cue, we found significant effects for cue [F(2,138) = 49.57, p < 0.001, η2p = 0.42], with significant differences between all conditions, with larger amplitude for absent cue and smaller for spatial cue; session [F(1,69) = 11.14, p = 0.001, η2p = 0.14], with enhanced amplitude for P3cue in post- compared to pretreatment ANT; and interactions between cue*session [F(2,138) = 5.43, p = 0.005, η2p = 0.07], and cue*session*treatment [F(6,138) = 2.16, p = 0.05, η2p = 0.08]. The post-hoc for cue*session showed significant difference between all cue conditions in both pre- and post-treatment sessions, similar to the main effect detected for cue (larger amplitude for absent and smaller for spatial), and a significant difference between session pre- and post-treatment only for double cues, with no differences for spatial or absent cues. However, this difference for double cues between pre- and post-treatment was detected only for sham tDCS group, as indicated by the interaction cue*session*treatment.
Finally, the analysis for P3 component in response to targets showed main effects for target [F(2,138) = 118.91, p = 0.001, η2p = 0.63], with significant differences between all conditions, where neutral targets elicited the largest P3 amplitude and the incongruent the smallest amplitude; session [F(1,69) = 7.87, p = 0.007, η2p = 0.1], with larger P3 amplitude for post- compared to pretreatment ANT; region [F(1,69) = 6.86, p = 0.01, η2p = 0.09], with larger P3 amplitude for parietal compared to occipital electrodes; and the interaction target*region*treatment [F(6,138) = 2.2, p = 0.05, η2p = 0.09]. Despite this interaction reached significance level, we searched only for post-hoc comparisons of interest, i.e., differences in P3 amplitude between treatments for a same target and region, or differences between regions for a same target and treatment, but no differences were identified for those comparisons (in all cases we detected p > 0.1). The ERP results here presented can be visualized in Fig. 5.
Perceived Changes in Attentional Performance Scale (PCAPS)
One sample t-tests indicated that all participants in all the four treatment groups perceived an enhancement in their attentional performance in response to the treatment, as indicated in Table 1. Despite few participants reporting no attentional improvement in response to the treatment (number of participants—active tDCS: 1; sham tDCS: 4; active neurofeedback: 3; sham neurofeedback: 3), no participant reported worsened performance. We ran an one-way ANOVA to test differences between treatment groups in PCAPS score, showing no significant differences [F(3,38) = 0.98, p = 0.41].
Correlation between ERP and reaction time
We tested for correlation between ERP (P1, N1, P3cue, and P3) and reaction time in each ANT session separately, finding a significant negative correlation between P3cue and RT, as well as P3 and RT for both sessions (pre- and post-treatment), as shown on Table 2.
Complementary analyses
We ran the following complementary (post hoc) statistical analyses that were suggested during the manuscript review: (1) a mixed ANOVA with the attentional network scores (alert, orientation, and executive control) as dependent variables and with session and treatment as factors, where those scores were calculated following the method by Wang et al.45; (2) and a mixed ANOVA with exponential-gaussian parameters for reaction time (mu, sigma, and tau) as dependent variables and target, session, and treatment as factors; (3) ANCOVA models considering accuracy and RT as dependent variables, similar to the ANOVA models for both variables (presented on “Behavioral data in ANT”), however with perceived changes (PCAPS score) as a covariate. All analyses showed no significant main effect or interaction considering the treatment factor, which is the factor of our interest. In the supplementary information, we described in detail the results of the three analyses.
Discussion
The present study assessed attentional neuroenhancement through two neuromodulatory techniques, tDCS, and Neurofeedback. As our primary outcome, we compared behavioral and electrophysiological performance of participants in ANT pre-and post-treatment, but we did not find significant differences given the treatment group. However, we find that participants from the four treatment groups reported, through a subjective scale, attentional improvement in response to the treatment. The lack of any effect for active treatment goes against part of the literature, which shows in some cases a positive effect of neuromodulation on healthy participants’ performance on the ANT task, both for neurofeedback46,47 and tDCS15,16.
As for the neurofeedback, we did not find any effect on the visual attentional performance of healthy participants in the ANT, contrary to some studies46,47. Some differences regarding the neurofeedback application may explain this distinction between our null findings and the studies that obtained positive results in ANT. For example, those studies used distinct frequency bands, modulating theta frequency or an SMR/theta protocol, aiming to increase SMR power while inhibiting theta46,47. Other studies that focused on improving healthy participants' attentional performance by SMR, but with other attentional tasks, also involved training other frequencies, such as beta1 or theta48,49. In this way, it is possible that adopting only SMR training was not so efficient compared to controlling SMR along with beta or theta frequencies. Another possible explanation for our null result is the low success rate of our participants in reaching the neurofeedback goal, where one-third failed in all sessions, and only three succeeded in more than half of the sessions. This proportion of non-respondent is in line with the literature50,51,52, indicating that a considerable part of participants (around 50%) are non-respondents (or non-learners) to neurofeedback, having no success in modulating their brain activity through this technique. Future studies must assess the neuroenhancement effectiveness of neurofeedback, focusing on participants who are respondents to the neurofeedback.
Regarding the tDCS, despite some literature showing positive results, there are also mixed findings, with studies stimulating proximal cortical areas and finding contradictory effects on attention15,16. For example, Miler et al.15 found that anodal tDCS (2 mA) over the left dorsolateral cortex enhanced executive attention compared to sham, while Roy et al.16 found no effect of anodal tDCS (1.5 mA) over the same area in an attentional network. Similarly, Coffman et al.13 found enhanced alerting effect after anodal tDCS (2 mA) over right inferior gyrus compared to sham, where Trumbo et al.17 detected an inverse effect, with an increased alerting effect for the sham group compared to anodal tDCS (2 mA) over right inferior gyrus. In this way, our finding is in line with Trumbo et al.17, showing no significant effect for active tDCS compared to sham.
Some issues could be associated with this null effect in tDCS. First, it is essential to highlight that several other tDCS studies have been finding null effects on attention53 and in other cognitive functions, such as working memory54, or physiological processes, such as substance craving55,56. Several studies have been discussing the contradictory effects of tDCS57, which could be becoming more common due to the recognition of publishing bias, such as the file drawer effect58,59,60; and also related to complex mechanisms associated with tDCS parameters that can modulate its effects, such as: dose61,62, participants’ baseline performance19,20; timing of tDCS application, i.e., if before or during a cognitive or behavioral task63,64; anatomical differences between subjects65; or even their arousal level during the neuromodulation66. Because of the complex effects of tDCS, future studies must investigate the interaction between these effects, identifying the individual parameters or factors that optimize the application of this technique in healthy participants. Second, several tDCS studies in the literature did not evaluate how their tDCS montage would properly stimulate the target area. To test this, we assessed if the tDCS montage we adopted (a common montage in cognitive literature) would efficiently target the DLPFC by means of SimNIBS67, a toolbox that models the electrical current dissipation in the brain. This modeling showed that DLPFC montage (F4 and reference over contralateral supraorbital area) is not properly targeting the DLPFC, but it is most oriented to the dorsomedial prefrontal region. Given this, we also suggest properly assessing tDCS montages using computational models or neuro-navigational methods to guarantee electrical current focus on the brain target area.
Another critical issue behind the contradictory effects in tDCS and neurofeedback is the influence of placebo effects. In our study, although we did not identify any behavioral or electrophysiological effects on ANT in response to the treatment, we detected a significant effect on the Perceived Changes in Attentional Performance Scale for all the treatment groups (active and sham), indicating that, on average, participants in all groups reported improvement in attention in response to the treatment and with a similar average score. Since sham and active groups had similar behavioral and electrophysiological performance and a similar perception of attentional improvement, it is most likely that the detected improvement was due to a placebo effect. The placebo effect in neuromodulation techniques has recently been discussed for neurofeedback11,68 and tDCS69,70, and our findings support this literature. One possibility for the placebo effect in our study is the learning effect on the task, which may have influenced participants' perception regarding their attentional improvement. We found an interaction effect between target and session, with shorter RT times for all targets in post-treatment ANT session (more expressively to the incongruent targets) and not dependent on treatment group, indicating a learning effect of participants in the task, what as previously described in the literature71. However, it is essential to emphasize that several participants reported that such improvements were noticeable in their daily lives, such as at work or in class performance.
Regarding the behavioral data, we found similar patterns of accuracy and reaction time to the literature: with lower accuracy for incongruent targets22,25,28,72, and a similar RT pattern for the target (shorter RT for neutral and congruent, longer RT for incongruent targets) and the cue (shorter RT for spatial cues, increasing for double/central cues and the longest RT for absent cues)22,25,28,39,71,72. As for the electrophysiological data, we detected significant effects for all ERP components concerning cues or target detection.
For the P1, we identified higher amplitudes in response to absent cues, diminishing for double cues and smaller for space cues. Thus, P1 seems to index automatic visual attentional demand level to the target surging accordingly to the informational level of the cue. This result is similar to two other studies, showing significant differences between absent and double cues, and between spatial and absent cues26,28. For the component N1, we found increased amplitude in response to double cues compared to absent cues, according to the literature24,26,27,28. We also found enhanced N1 amplitude for spatial compared to absent cues, which was detected by Neuhaus et al.24, and go in line with previous findings showing increased N1 amplitude during detection of visual stimuli in attended- compared to unattended-locations73,74. As for P3cue, we did not find P3 analysis in the target window given cue type in the literature. For P3pt, we identified an identical pattern to P1, with the amplitude decreasing for absent, double, and smaller for spatial cues. The hypothesis for this effect is similar to that presented for P1, i.e. P3cue indexed cognitive demand during target processing in response to the cue informational level. Regarding the P3 findings in response to the target type, we found decreased amplitude for incongruent, increasing for congruent and the largest for neutral targets, which goes in line with the literature, usually reporting decreased amplitude for incongruent due to response inhibition mechanisms after detecting incongruent targets24,25,26,27.
We also found learning effects for P1, P3cue, and P3 in the ANT, with greater amplitude for those components in the post-treatment session. There are no similar descriptions in the literature since we have not identified studies evaluating electrophysiological components in the ANT on different sessions. Some studies that investigate ERP in other attentional tests on two different moments detected greater amplitudes for component P3 post-intervention, usually related to enhanced attentional performance in the last moment75,76. Given this, a possible hypothesis is that the increased P1, P3cue, and P3 on post-treatment ANT is indexing enhanced efficiency in automatic and controlled attentional processes, which could explain the learning effect detected in the RT. It seems to be the case, at least for P3cue and P3 components, since we found a negative correlation between those components and RT. In addition, increased P3 components have been previously associated with faster RT in the literature77,78,79, similar to our findings for target conditions, where increasing P3 amplitude between conditions (incongruent, congruent and neutral) was related to decreasing RT on them.
Finally, it is essential to discuss the limitations of our study. As a first limitation, we sought to evaluate the two neuromodulation techniques based on montages suggested in the literature; however, there are other montages and parameters that we did not assess and could have enhanced attentional performance. Given this, future studies should further investigate other tDCS montages, such as targeting parietal lobe or left DLPFC; or also test other parameters, such as applying tDCS online (i.e., while the volunteer performs the attentional task). Also, our montage (F4/contralateral supraorbital area) could have facilitated current shunting80, despite it being suggested to happen when electrodes are 5 cm of distance or less81. The same could be said for neurofeedback, i.e., we recommend future studies to assess other parameters that could be more effective. such as testing quantitative EEG, dividing each session in multiple blocks to enhance learning, applying more sessions, or aiming to modulate other signals, such as SMR/theta ratio. Also, it is relevant to highlight that we decided on a small sample size in our study (twenty by group). Although other studies with tDCS15,37 and neurofeedback8,29 of attention performance in healthy subjects had significant findings with smaller sample sizes (between nine and fifteen), our sample size could have underpowered our study, given the suggested small effect size for tDCS in the recent literature82. Thus, our small sample size does not allow us to conclude about the effectiveness of both techniques in neuroenhancement. We suggest that future studies adopt larger sample sizes to guarantee sufficient power to the experiment. Another limitation is that we did not fully assess all the distinct attentional domains (e.g., concentration) or modalities (e.g., auditory attention) or other cognitive domains, such as memory, motivation, etc., which could have explained the improvement reported by the participants. Also, in our study we assessed only college students, who are expected to represent a specific subpopulation with cognitive performance above average83, and who are also young. In this way, our sample does not allow us to generalize our findings to other populations with other educational levels and ages. Finally, our study was only single-blind, which can be considered a source of bias in comparison to a double-blind study. Despite all the limitations, our study points to the relevance of possible placebo effects along with both techniques, which was previously suggested in the literature and deserves to be considered in future neuroenhancement studies.
Conclusions
The present study endorses the literature indicating null effects of neuromodulatory techniques for neuroenhancement, suggesting the urgency for more studies in this area to understand which factors may be crucial for the effectiveness of such techniques, as well as studies properly controlling placebo effect, such as expectations related to neuromodulation or subjective perception of improving due to the treatment. In line with the literature in this topic, our study indicates how complex neuromodulation mechanisms are, and that placebo could be more relevant to its effects than previous thought. Given this, literature in this area should advertise for cautiousness84, mainly due to the emergence of clinics or DIY culture offering neuroenhancement as a "scientifically based" treatment with positive effects.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Repantis, D., Schlattmann, P., Laisney, O. & Heuser, I. Modafinil and methylphenidate for neuroenhancement in healthy individuals: A systematic review. Pharmacol. Res. 62, 187–206 (2010).
Maier, L. J., Haug, S. & Schaub, M. P. The importance of stress, self-efficacy, and self-medication for pharmacological neuroenhancement among employees and students. Drug Alcohol Depend. 156, 221–227 (2015).
Wexler, A. & Thibault, R. Mind-reading or misleading? Assessing direct-to-consumer electroencephalography (EEG) devices marketed for wellness and their ethical and regulatory implications. J. Cogn. Enhanc. 3, 131–137 (2019).
Wexler, A. Who uses direct-to-consumer brain stimulation products, and why? A study of home users of tDCS devices. J. Cogn. Enhanc. 2, 114–134 (2018).
Dubljević, V., McCall, I. C. & Illes, J. Neuroenhancement at work: Addressing the ethical, legal, and social implications. Adv. Neuroethics 2020, 87–103. https://doi.org/10.1007/978-3-030-27177-0_7 (2020).
Fisicaro, F., Lanza, G., Bella, R. & Pennisi, M. ‘Self-Neuroenhancement’: The last frontier of noninvasive brain stimulation?. J. Clin. Neurol. 16, 158 (2020).
Enriquez-Geppert, S., Huster, R. J. & Herrmann, C. S. EEG-neurofeedback as a tool to modulate cognition and behavior: A review tutorial. Front. Hum. Neurosci. 11, 158 (2017).
Gruzelier, J. H. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci. Biobehav. Rev. 44, 124–141 (2014).
Rogala, J. et al. The do’s and don’ts of neurofeedback training: A review of the controlled studies using healthy adults. Front. Hum. Neurosci. 10, 301 (2016).
Thibault, R. T., Lifshitz, M. & Raz, A. The climate of neurofeedback: Scientific rigour and the perils of ideology. Brain 141, e11 (2018).
Schabus, M. et al. Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia. Brain 140, 1041–1052 (2017).
Schönenberg, M. et al. Neurofeedback, sham neurofeedback, and cognitive-behavioural group therapy in adults with attention-deficit hyperactivity disorder: A triple-blind, randomised, controlled trial. Lancet Psychiatry 4, 673–684 (2017).
Coffman, B. A., Clark, V. P. & Parasuraman, R. Battery powered thought: Enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage 85, 895–908 (2014).
Yadollahpour, A., Asl, H. M. & Rashidi, S. Transcranial direct current stimulation as a non-medication modality for attention enhancement: A review of the literature. Res. J. Pharm. Technol. 10, 311 (2017).
Miler, J. A., Meron, D., Baldwin, D. S. & Garner, M. The effect of prefrontal transcranial direct current stimulation on attention network function in healthy volunteers. Neuromodulation 21, 355–361 (2018).
Roy, L. B., Sparing, R., Fink, G. R. & Hesse, M. D. Modulation of attention functions by anodal tDCS on right PPC. Neuropsychologia 74, 96–107 (2015).
Trumbo, M. C., Trumbo, M. C., Coffman, B. A. & Clark, V. P. The effect of transcranial direct current stimulation (tDCS) on the attention network task (ANT): Contextualizing prior research. Brain Stimul. 7, e8 (2014).
Roe, J. M. et al. The effects of tDCS upon sustained visual attention are dependent on cognitive load. Neuropsychologia 80, 1–8 (2016).
Benwell, C. S. Y., Learmonth, G., Miniussi, C., Harvey, M. & Thut, G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: Evidence from biparietal tDCS influence on lateralized attention bias. Cortex 69, 152–165 (2015).
Learmonth, G., Thut, G., Benwell, C. S. Y. & Harvey, M. The implications of state-dependent tDCS effects in aging: Behavioural response is determined by baseline performance. Neuropsychologia 74, 108–119 (2015).
Giordano, J. et al. Mechanisms and effects of transcranial direct current stimulation. Dose Response 15, 1559325816685467 (2017).
Fan, J., McCandliss, B. D., Sommer, T., Raz, A. & Posner, M. I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 14, 340–347 (2002).
Fan, J. et al. The relation of brain oscillations to attentional networks. J. Neurosci. 27, 6197–6206 (2007).
Neuhaus, A. H. et al. Event-related potentials associated with Attention Network Test. Int. J. Psychophysiol. 76, 72–79 (2010).
Galvao-Carmona, A. et al. Disentangling the attention network test: Behavioral, event related potentials, and neural source analyses. Front. Hum. Neurosci. 8, 813 (2014).
Williams, R. S. et al. Age differences in the Attention Network Test: Evidence from behavior and event-related potentials. Brain Cogn. 102, 65–79 (2016).
Kaufman, D. A. S., Sozda, C. N., Dotson, V. M. & Perlstein, W. M. An event-related potential investigation of the effects of age on alerting, orienting, and executive function. Front. Aging Neurosci. 8, 99 (2016).
Gonçalves, Ó. F. et al. Mind wandering and task-focused attention: ERP correlates. Sci. Rep. 8, 7608 (2018).
Kober, S. E. et al. Shutting down sensorimotor interference unblocks the networks for stimulus processing: An SMR neurofeedback training study. Clin. Neurophysiol. 126, 82–95 (2015).
Mannarelli, D. et al. The role of the right dorsolateral prefrontal cortex in phasic alertness: Evidence from a contingent negative variation and repetitive transcranial magnetic stimulation study. Neural Plast. 2015, 410785 (2015).
Deepeshwar, S., Vinchurkar, S. A., Visweswaraiah, N. K. & Nagendra, H. R. Hemodynamic responses on prefrontal cortex related to meditation and attentional task. Front. Syst. Neurosci. 8, 252 (2014).
Brosnan, M. B. et al. Prefrontal modulation of visual processing and sustained attention in aging, a tDCS-EEG coregistration approach. J. Cogn. Neurosci. 30, 1630–1645 (2018).
Vendrell, P. et al. The role of prefrontal regions in the Stroop task. Neuropsychologia 33, 341–352 (1995).
Wood, J. N. & Grafman, J. Human prefrontal cortex: Processing and representational perspectives. Nat. Rev. Neurosci. 4, 139–147 (2003).
Tomasino, B. & Fabbro, F. Increases in the right dorsolateral prefrontal cortex and decreases the rostral prefrontal cortex activation after-8 weeks of focused attention based mindfulness meditation. Brain Cogn. 102, 46–54 (2016).
Marzbani, H., Marateb, H. R. & Mansourian, M. Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 7, 143–158 (2016).
Coffman, B. A., Trumbo, M. C. & Clark, V. P. Enhancement of object detection with transcranial direct current stimulation is associated with increased attention. BMC Neurosci. 13(1), 1–8 (2012).
Oldfield, R. C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9, 97–113 (1971).
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I. & Posner, M. I. The activation of attentional networks. Neuroimage 26, 471–479 (2005).
McConnell, M. M. & Shore, D. I. Mixing measures: Testing an assumption of the Attention Network Test. Atten. Percept. Psychophys. 73, 1096–1107 (2011).
MacLeod, J. W. et al. Appraising the ANT: Psychometric and theoretical considerations of the Attention Network Test. Neuropsychology 24, 637–651 (2010).
Poreisz, C., Boros, K., Antal, A. & Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 72, 208–214 (2007).
Bikson, M., Datta, A. & Elwassif, M. Establishing safety limits for transcranial direct current stimulation. Clin. Neurophysiol. 120, 1033–1034 (2009).
Bikson, M. et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 9, 641–661 (2016).
Wang, Y. F. et al. A new method for computing attention network scores and relationships between attention networks. PLoS ONE 9(3), e89733 (2014).
Barnea, A., Rassis, A., Raz, A., Othmer, S. & Zaidel, E. Effects of neurofeedback on hemispheric attention networks. Brain Cogn. https://doi.org/10.1016/j.bandc.2004.08.013 (2004).
Wang, J.-R. & Hsieh, S. Neurofeedback training improves attention and working memory performance. Clin. Neurophysiol. 124, 2406–2420 (2013).
Gruzelier, J., Egner, T. & Vernon, D. Validating the efficacy of neurofeedback for optimising performance. Progress Brain Res. 2006, 421–431. https://doi.org/10.1016/s0079-6123(06)59027-2 (2006).
Vernon, D. et al. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int. J. Psychophysiol. 47, 75–85 (2003).
Doehnert, M., Brandeis, D., Straub, M., Steinhausen, H. C. & Drechsler, R. Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: Is there neurophysiological evidence for specific effects?. J. Neural Transm. 115(10), 1445–1456 (2008).
Hanslmayr, S., Sauseng, P., Doppelmayr, M., Schabus, M. & Klimesch, W. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl. Psychophysiol. Biofeedback 30(1), 1–10 (2005).
Weber, E., Köberl, A., Frank, S. & Doppelmayr, M. Predicting successful learning of SMR neurofeedback in healthy participants: Methodological considerations. Appl. Psychophysiol. Biofeedback 36(1), 37–45 (2011).
Jacoby, N. & Lavidor, M. Null tDCS effects in a sustained attention task: The modulating role of learning. Front. Psychol. 2018, 9 (2018).
Westwood, S. J. & Romani, C. Null effects on working memory and verbal fluency tasks when applying anodal tDCS to the inferior frontal gyrus of healthy participants. Front. Neurosci. 12, 166 (2018).
Claus, E. D., Klimaj, S. D., Chavez, R., Martinez, A. D. & Clark, V. P. A randomized trial of combined tDCS over right inferior frontal cortex and cognitive bias modification: Null effects on drinking and alcohol approach bias. Alcohol. Clin. Exp. Res. 43, 1591–1599 (2019).
Verveer, I., Remmerswaal, D., Jongerling, J., van der Veen, F. M. & Franken, I. H. A. No effect of repetitive tDCS on daily smoking behaviour in light smokers: A placebo controlled EMA study. PLoS ONE 15, e0233414 (2020).
Filmer, H. L., Mattingley, J. B. & Dux, P. E. Modulating brain activity and behaviour with tDCS: Rumours of its death have been greatly exaggerated. Cortex 123, 141–151 (2020).
Ferguson, C. J. & Heene, M. A vast graveyard of undead theories. Perspect. Psychol. Sci. 7, 555–561 (2012).
Franco, A., Malhotra, N. & Simonovits, G. Social science. Publication bias in the social sciences: Unlocking the file drawer. Science 345, 1502–1505 (2014).
Medina, J. & Cason, S. No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 94, 131–141 (2017).
Esmaeilpour, Z. et al. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 11, 310–321 (2018).
Jamil, A. et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiol. 595, 1273–1288 (2017).
Martin, D. M., Liu, R., Alonzo, A., Green, M. & Loo, C. K. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: Effect of timing of stimulation. Exp. Brain Res. 232, 3345–3351 (2014).
Oldrati, V., Colombo, B. & Antonietti, A. Combination of a short cognitive training and tDCS to enhance visuospatial skills: A comparison between online and offline neuromodulation. Brain Res. 1678, 32–39 (2018).
Kim, J.-H. et al. Inconsistent outcomes of transcranial direct current stimulation may originate from anatomical differences among individuals: Electric field simulation using individual MRI data. Neurosci. Lett. 564, 6–10 (2014).
Esposito, M., Ferrari, C., Fracassi, C., Miniussi, C. & Brignani, D. Arousal levels explain inter-subject variability of neuromodulation effects. bioRxiv https://doi.org/10.1101/2020.05.08.083717 (2020).
Thielscher, A., Antunes, A. & Saturnino, G. B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS?. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 222–225 (2015).
Thibault, R. T. & Raz, A. The psychology of neurofeedback: Clinical intervention even if applied placebo. Am. Psychol. 72, 679–688 (2017).
Fassi, L. & Kadosh, R. C. Is it all in our head? When subjective beliefs about receiving an intervention are better predictors of experimental results than the intervention itself. bioRxiv https://doi.org/10.1101/2020.12.06.411850 (2020).
Dawood, A. B. et al. Investigating the effects of tDCS on Visual orientation discrimination task performance: ‘The Possible Influence of Placebo’. J. Cogn. Enhanc. 4, 235–249 (2020).
Ishigami, Y. & Klein, R. M. Repeated measurement of the components of attention using two versions of the Attention Network Test (ANT): Stability, isolability, robustness, and reliability. J. Neurosci. Methods 190, 117–128 (2010).
Wang, Y.-F. et al. A new method for computing attention network scores and relationships between attention networks. PLoS ONE 9, e89733 (2014).
Luck, S. J. & Hillyard, S. A. The role of attention in feature detection and conjunction discrimination: An electrophysiological analysis. Int. J. Neurosci. 80, 281–297 (1995).
Luck, S. J. et al. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. J. Exp. Psychol. Hum. Percept. Perform. 20, 887–904 (1994).
Wu, S., Ji, H., Won, J., Liu, X. & Park, J.-J. Effects of acute visual stimulation exercise on attention processes: An ERP study. Int. J. Environ. Res. Public Health 2021, 18 (2021).
O’Toole, L. & Dennis, T. A. Attention training and the threat bias: An ERP study. Brain Cogn. 78, 63–73 (2012).
Friedman, D. P300 and slow wave: The effects of reaction time quartile. Biol. Psychol. 18, 49–71 (1984).
Roth, W. T., Ford, J. M. & Kopell, B. S. Long-latency evoked potentials and reaction time. Psychophysiology 15, 17–23 (1978).
Antonova, I. et al. Reaction time in a visual 4-choice reaction time task: ERP effects of motor preparation and hemispheric involvement. Brain Topogr. 29, 491–505 (2016).
Wagner, T. et al. Transcranial direct current stimulation: A computer-based human model study. Neuroimage 35, 1113–1124. https://doi.org/10.1016/j.neuroimage.2007.01.027 (2007).
Redfearn, J. W. T., Lippold, O. C. J. & Costain, R. Preliminary account of the clinical effects of polarizing the brain in certain psychiatric disorders. Br. J. Psychiatry 110, 773–785. https://doi.org/10.1192/bjp.110.469.773 (1964).
Dedoncker, J., Brunoni, A. R., Baeken, C. & Vanderhasselt, M. A. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimul. 9(4), 501–517 (2016).
Guerra-Carrillo, B., Katovich, K. & Bunge, S. A. Does higher education hone cognitive functioning and learning efficacy? Findings from a large and diverse sample. PLoS ONE 12, e0182276 (2017).
Wurzman, R., Hamilton, R. H., Pascual-Leone, A. & Fox, M. D. An open letter concerning do-it-yourself users of transcranial direct current stimulation. Ann. Neurol. 80, 1–4 (2016).
Funding
GGR was supported by a doctoral scholarship (nº 2015/18713-9) from São Paulo Research Foundation (FAPESP). OFG was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) as a Special Visiting Researcher of the Science Without Borders program (401143/2014-7). PSB was funded by a CNPq research fellowship (304164/2012-7).
Author information
Authors and Affiliations
Contributions
G.G.R., O.F.G., and P.S.B. developed the study concept and design. G.G.R. performed data collection, and G.G.R. and P.S.B. performed data analysis. All authors contributed to data interpretation, manuscript writing and all approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rêgo, G.G., Gonçalves, Ó.F. & Boggio, P.S. Attention neuroenhancement through tDCS or neurofeedback: a randomized, single-blind, controlled trial. Sci Rep 12, 17613 (2022). https://doi.org/10.1038/s41598-022-22245-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22245-6
This article is cited by
-
The Effect of Neurofeedback Training on Executive Control Network of Attention and Dart-Throwing Performance in Individuals with Trait Anxiety
Applied Psychophysiology and Biofeedback (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.