Abstract
The present review systematically analyzed in vitro and in situ studies investigating physical diffusion barriers (sealants, desensitizer or adhesives) to prevent the development or the progression of root (dentin) demineralization. Three electronic databases (PubMed-Medline, CENTRAL, Ovid-EMBASE) were screened for studies from 1946 to 2022. Cross-referencing was used to identify further articles. Article selection and data abstraction were done in duplicate. Languages were not restricted. The type of outcome was not restricted, and their mean differences (MD) were calculated using fixed- or random-effects models. Risk of Bias was graded using Risk of Bias 2.0 tool. From 171 eligible studies, 34 were selected for full-text analysis evaluating 69 different materials, and 17 studies—still evaluating 36 different materials—were included (3 in situ and 14 in vitro). Ten studies evaluated desensitizers; 8 adhesives; and 1 infiltration. Meta-analyses were possible for all 17 studies. Meta-analyses revealed that lesion depth after no treatment was significantly higher than after the application of single-step adhesives (MD[95%CI] = − 49.82[− 69.34; − 30.30]) and multi-step adhesives (MD[95%CI]=–60.09 [–92.65, –27.54]). No significant differences in the lesion depth increase between single- and multi-step adhesives could be observed (MD[95%CI]=30.13 [–21.14, 81.39]). Furthermore, compared to no treatment the increase of the lesion depth was significantly hampered using desensitizers (MD[95%CI] = − 38.02[− 51.74; − 24.31]). Furthermore, the included studies presented unclear or high risk. A physical diffusion barrier can significantly hamper the increase of lesion depth under cariogenic conditions. Furthermore, multi-step adhesives seem not to be more effective than single-step adhesives. However, this conclusion is based on only few in vitro and in situ studies.
Similar content being viewed by others
Introduction
Life expectancy has gradually increased in many countries, bringing along many new health vulnerabilities, also regarding oral health. The elderly can present decreased motor skills1, resulting in difficulties to perform a proper oral hygiene, increasing the susceptibility to caries2. The prevalence of root caries is further propelled in this age group, since the elderly show higher indices of gingival recession and root exposure3, and also a reduced salivary secretion4. Consequently, several non-invasive approaches have been tested to prevent the development or to inactivate Root Caries Lesions (RCL)5,6, though not all were (completely) successful. Therefore, other micro-invasive strategies have been tested to further prevent RCL.
Dental sealants showed clinically significant results in reducing the incidence of pit and fissure caries7, proximal caries8 as well as the development of white spot lesions during orthodontic treatments with fixed appliances9. Sealants have also been tested on dentin in vitro10,11,12,13. Other micro-invasive strategies, such as the use of desensitizers10,14,15,16 or adhesives13,17,18, can also act as physical barriers that may prevent growth of biofilm by blocking nutrition. Nonetheless, these diffusion barriers have solely been tested in vitro or in situ, and no quantitative data synthesis (meta-analysis) focusing on the effect of various micro-invasive strategies to prevent the development and/or the progression of RCL has been published yet.
Thus, this systematic review was designed and caried out with the aim to critically summarize and evaluate results of in vitro and in situ studies investigating physical diffusion barriers (e.g. sealants, desensitizers or adhesives) to prevent the development or the progression of root (dentin) demineralization.
Methods
Review design
This review aimed at systematically retrieving and analyzing in vitro and in situ studies assessing physical diffusion barriers (e.g. sealants, desensitizer or adhesives) to reduce or arrest the development or the progression of root (dentin) demineralization. The review was conducted according to the guidelines by the Cochrane Collaboration19; reporting followed the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses) (please see Supplementary Material)20. Since this is an review on in vitro and in situ studies and since no study registration is necessary for this type of review it was not registered in e.g. prospero.
Inclusion and exclusion criteria
Based on the following PICOS (Participants, Intervention, Outcome, Study design) schema, in vitro and in situ studies assessing the effect of any kind of physical diffusion barrier on root (dentin) demineralization were included (Table 1).
The following inclusion criteria were adopted:
-
Controlled in vitro and in situ studies on dentin specimens undergoing a cariogenic challenge (no further specification regarding e.g. minimum follow-up period, minimum number of specimens, etc. were made)
-
assessment of different physical diffusion barriers (e.g. sealants, desensitizers or adhesives)
-
assessment of root (dentin) demineralization (development and/or regression)
The following exclusion criteria were adopted:
-
outcomes not assessing root (dentin) caries
-
‘single group studies’/studies without any control group
Literature sources
Two authors (TM, RJW) independently reviewed the title and abstract of articles retrieved following a defined search strategy (Supplementary Table 1). The reviewers were not blinded to journal names nor to article authors. No limitations concerning language or status were applied. Grey literature was not evaluated. The electronic search was conducted through PubMed-Medline, CENTRAL, Ovid-EMBASE for studies from 1946 to August 29th 2022 and the results of searches were cross-checked to eliminate duplicates. A detailed sequence of filtering search results to include relevant articles can be found in the supplementary document.
At first the titles and abstracts of the searched articles were examined independently by two authors (TM, RJW). Any disagreements in the eligibility criteria were solved by discussion and if no consensus was reached, a third author (SHN) was consulted. Then, selected studies were screened full-text. Cross-referencing was performed to identify further relevant articles that could fulfil the inclusion criteria.
Data extraction
Two authors (TM, RJW) extracted the data by means of predefined structured tables (Microsoft Excel, Microsoft Corporation, Redmond, USA)21,22. For each study, the following data were systematically extracted:
-
study type and setting
-
treatment and control groups
-
type of intervention: physical diffusion barrier (sealants, desensitizer, adhesive, etc.)
-
product brands
-
follow-up time/study duration
-
primary and secondary outcomes
-
lesion depth
-
mineral loss
-
dentinal tubule occlusion
-
antibacterial activity
-
etc.
-
-
number of participants and specimens being included
-
type of teeth used (bovine vs. human)
-
type of baseline condition (sound dentine or pre-demineralized dentin)
-
sample size
-
numeric and narrative main results
Risk of bias assessment
Two authors (TM and RJW) independently evaluated the risk of bias. Any disagreement between the reviewers was discussed until an agreement was reached and if needed, by consulting a third author (SHN). For risk of bias assessment, the guidelines by the Cochrane Collaboration19 were slightly adapted: risk of bias criteria being used in recent systematic reviews of in vitro studies were added23,24. Thus, risk of bias assessment included:
-
random sequence generation
-
allocation concealment
-
blinding of participants and personnel
-
blinding of outcome assessment
-
incomplete outcome data
-
selective outcome
-
description of sample size calculation
-
use of teeth with similar dimensions
-
use of caries lesions (artificial or natural) with similar dimensions
-
treatment performed by the same operator
-
materials used according to the manufacturers’ instructions
-
Anything else ideally prespecified (conflict of interest, sponsored by manufacturer)
Data analysis
The statistical analyses were conducted in Review Manager (RevMan version 5.4 software, Cochrane Collaboration, Copenhagen, Denmark, 2014)25. Fixed or random-effects meta-analyses were performed depending on heterogeneity (I2 < 35%: fixed-effects; I2 > 35%: random-effect)9,26. Statistical significance was defined as p value ≤ 0.05 (Z test) and heterogeneity was assessed with I2. Forest plots were created to illustrate the meta-analysis.
For continuous variables, the primary measures of effect between treatment and control groups were the mean differences (MD) for studies using the same outcome and standardized mean differences (SMD) for studies using the same construct but different scales21,27.
Assessment of reporting bias
In the presence of more than 10 studies in a meta-analysis, the possible presence of publication bias was investigated for the primary outcome. Publication bias was assessed by Funnel plots28.
Sensitivity analysis
We explored whether or not the analysis of studies stratified by (1) risk of bias yielded similar or different results. For this studies at high risk of bias were eliminated in a second/third analysis.
Statement of ethics
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
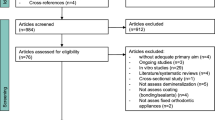
A total of 171 studies were initially identified, and after title and abstract screening, 34 studies analyzing 69 different materials were assessed for eligibility (Fig. 1). After full-text screening 17 studies had to be excluded (Supplementary Table 2) and 17 studies—still evaluating 36 different materials—were included10,11,14,15,29,30,31,32,33,34,35,36,37,38,39,40,41. Characteristics of the included studies are shown in Table 2. Three studies were in situ studies14,15,29 and 14 were in vitro studies.
Ten studies evaluated desensitizer10,11,14,15,29,30,31,32,33,34, 8 studies adhesive14,35,36,37,38,39,40,41, 1 sealants10 and 1 infiltrants39. The development of new dentin lesions was investigated in 13 studies10,11,14,15,29,30,31,32,33,35,36,38,40 whereas 4 studies34,37,39,41 analyzed the progression of artificial lesions. The outcomes were described by using lesion depth10,11,14,15,29,34,35,36,37,38,39,40,41 and mineral loss11,30,31,32,33,34,41 (Table 2).
Meta-analyses were performed for studies investigating similar interventions and outcomes in more than one study. Meta-analyses could have been performed comparing single-step adhesives versus untreated control14,35,36,37,38,40,41 and for multi-step adhesives versus untreated control14,18,35,36,37,39,40, single-step adhesives versus multi-step adhesives14,35,36,37,40 as well as desensitizers versus untreated control10,11,14,15,29,34. However, in some comparisons a few studies had to be excluded because no numeric results had been reported42,43, an erosive/abrasive challenge was made instead of a cariogenic one12 or the specimens were solely stored in remineralization solution without simulating any cariogenic challenge18,44.
From the meta-analyses, lesion depth after untreated control (no application of a physical barrier) was significantly higher than after the application of single-step adhesives (MD [95%CI] = − 49.82 [− 69.34; − 30.30])) and multi-step adhesives (MD[95%CI]=–60.09 [–92.65, –27.54]) (Fig. 2). Contrastingly, no significant differences in the increase of lesion depth between single- and multi-step adhesive was observed (MD[95%CI]=30.13 [–21.14, 81.39]). Furthermore, compared to no treatment, the increase of the lesion depth was significantly hampered using desensitizers (MD[95%CI] = − 38.02 [− 51.74; − 24.31]).
Quantitative meta-analyses for increase of the lesion depth comparing (A) single-step adhesives versus untreated control, (B) multi-step adhesives versus untreated control, (C) single-step versus multi-step adhesives (D) desensitizer versus untreated control; and (E) same as D, but for outcome. For each comparison MD, 95% CI, forest plots, heterogeneity parameter (I2) as well as overall statistics (Z, P) are shown.
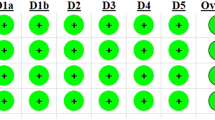
Risk of bias analysis
Risk of bias was assessed for all 17 studies included in the meta-analysis (Fig. 3). Regarding the performance and detection bias, all studies showed an unclear risk of bias (except two studies showing a high risk of performance bias15,29). Furthermore, 5 studies were sponsored by the manufactures of the tested products31,32,33,38,40. Since no further information was presented about the independence of the study, the domain “other bias” was rated as high risk of bias. Overall risk of bias was low for 15 and unclear for 2 studies.
Sensitivity analysis
When excluding studies at high risk the meta-analysis did not change.
Discussion
The present review investigated the caries-preventive effect of different sealants, desensitizers and adhesives. A total of 17 in vitro and in situ studies were extracted from the literature, which analyzed 36 materials investigating the prevention of development or progression of root (dentin) demineralization. This reflects that no gold standard therapy has been established yet, neither on in vitro nor in situ studies. All tested materials acting as physical barriers were able to significantly hamper cariogenic lesions.
The materials significantly decreased the development of artificial root (dentin) caries lesion when compared to their non-use. This is in line with previous reviews on clinical studies analyzing sealants7,8,9, which observed that at 24 months follow-up, the use of occlusal (resin) sealants significantly reduced the incidence of fissure caries ; after a mean follow-up of 25 months a superior efficacy of proximal sealants or infiltrants over non-invasive treatments (including dietary control, biofilm control or control of de- and remineralization) was also observed; and at a median follow-up time of more than one year (12.75 months) coating agents significantly reduced the incidence of post-orthodontic white spot lesions. However, it has to be mentioned that these three reviews7,8,9 analyzed clinical studies on enamel lesions, whereas the present study solely concentrates on dentin lesions in vitro and in situ. Nonetheless, all reviews indicate that physical diffusion barriers seem to be able to prevent the development or progression of caries lesions on both enamel and dentin, by blocking bacteria nutrition and by impeding acid diffusion into the hard tissue, thus preventing (further) mineral loss.
Desensitizers are mainly used on exposed dentin to reduce dentin hypersensitivity. Nevertheless, in 10 studies, they were also tested as agents to protect against dentin demineralization. A total of 11 different desensitizers were evaluated, including light curing materials, with or without fluoride release, and different active compounds. Noteworthy, the present meta-analyses showed that the use of desensitizers significantly hampered the progression of caries lesions on dentin when compared to no treatment. This is probably due to the formation of a physical diffusion barrier from the active ingredients and the presence of fluoride, the influence of these variables was not verified in the present meta-analyses.
Glass ionomer cements (GIC) are commonly used to restore (sometimes invasively) root caries lesions45,46. Annual failure rates had an impressive range differed between 2.4 and 44% and success rates were significantly lower than for composite restorations6. Interestingly, in one of the included studies18, GIC was also used as a micro-invasive strategy to provide a physical barrier, though it was difficult to apply in thin layers and virtually impossible to create a smooth surface. Nonetheless, GIC was included as positive control because of its ability to release fluoride and adhesion to tooth structures47. Under bacteria-free and solely remineralization conditions, specimens treated with GIC showed the highest mineral change, indicating remineralization, and the highest fluoride uptake. However, the mechanical stability or retention of the thin GIC layer and the surface roughness/smoothness was not analyzed. Thus, it remains unclear if GIC could be applied in thin layers to successfully provide a physical barrier in vivo.
In recent years resin infiltration—which was primarily developed to arrest approximal (enamel) caries lesions48—has also been shown to mask white spot lesions26. After polymerization the infiltrant occludes diffusion pathways for cariogenic acids and dissolved minerals, thus acting as a physical barrier that, hypothetically, can also be applied to dentin lesions. However, the pores of demineralized dentin are larger than those in demineralized enamel, which offer a path for facilitated transport of dentinal fluid49, thus affecting the resin infiltration process. So, theoretically, no capillary forces could arise in demineralized dentin. Nonetheless, resin infiltration has been used in one of the included studies as micro-invasive strategy to provide a physical barrier39, but instead of acting only on the surface (like in the case of the other materials), it acts inside the lesion body. Interestingly, the resin infiltration formed inhomogeneous penetration layers in demineralized dentin, though still significantly reducing the increase of lesion depth when compared to the untreated control group. Since human demineralized dentin was infiltrated in vitro, it might be speculated that, firstly, capillary forces might arise when dentin fluid is not simulated—as it was the case in the abovementioned study, and secondly, that hybridization by resin interdiffusion into the exposed dentinal collagen layer, combined with attachment of resin tags into the opened dentin tubules, cannot only be observed after the application of dentin adhesives50 but also after resin infiltration.
In the present study, the Cochrane Collaboration’s tool for assessing risk of bias was specifically adjusted for in vitro and in situ studies. For this, the criteria were complemented by relevant criteria being identified in previous systematic reviews of in vitro studies23,24, with the assessment consisting of eleven criteria. Overall risk of bias was unclear for two studies31,33 and low for the other 15 studies. Nonetheless, the included studies still presented unclear or high risk for several of the domains. Lack of information about blinding of personnel, outcome assessment and any conflict of interest were the main reasons for high/moderate risks and should be carefully considered in future studies.
There are several limitations in the present meta-analysis. Firstly, the results were obtained from in vitro and in situ studies, since until now no in vivo study has investigated physical diffusion barriers to reduce or arrest the development or the progression of root (dentin) demineralization. Secondly, in most of the studies, the control group consisted of a separated group, but in few studies, the control was a protected area within each specimen. Moreover, in most of the studies the test agents were applied on sound dentin surfaces, and sometimes on artificial dentin lesions. Thirdly, the pH-cycling conditions varied between the studies. Constant demineralizing conditions were mostly used, and intermittent demineralization conditions to simulate oral pH fluctuations were used only in a few studies.Within the limitations of this systematic review, it can be concluded that physical diffusion barriers can significantly hamper the development or the progression of root (dentin) demineralization on in vitro and in situ models. Furthermore, single-step adhesives seem not to be more effective than multi-step adhesives. Nonetheless, results should be interpreted with caution, due to the low numbers of in vitro and in situ studies.
Data availability
All data generated or analyzed during this study are included in this article [and/or] its supplementary material files. Further enquiries can be directed to the corresponding author.
References
Hahn, M., Wild-Wall, N. & Falkenstein, M. Age-related changes of neural control processes and their significance for driving performance. In Age-Differentiated Work Systems (eds Schlick, C. M. et al.) 299–317 (Springer, Berlin, 2013).
Idaira, Y. et al. Factors affecting the oral condition of patients with severe motor and intellectual disabilities. Oral. Dis. 14(5), 435–439 (2008).
Heasman, P. A. et al. Gingival recession and root caries in the ageing population: A critical evaluation of treatments. J. Clin. Periodontol. 44(Suppl 18), S178–S193 (2017).
Han, P., Suarez-Durall, P. & Mulligan, R. Dry mouth: A critical topic for older adult patients. J. Prosthodont. Res. 59(1), 6–19 (2015).
Wierichs, R. J. & Meyer-Lueckel, H. Systematic review on noninvasive treatment of root caries lesions. J. Dent. Res. 94(2), 261–271 (2015).
Meyer-Lueckel, H., Machiulskiene, V. & Giacaman, R. A. How to intervene in the root caries process? Systematic review and meta-analyses. Caries Res. 53(6), 599–608 (2019).
Ahovuo-Saloranta, A. et al. Pit and fissure sealants for preventing dental decay in permanent teeth. Cochrane Database Syst. Rev. 7(7), Cd001830 (2017).
Krois, J. et al. Sealing or infiltrating proximal carious lesions. J. Dent. 74, 15–22 (2018).
Kamber, R. et al. Efficacy of sealants and bonding materials during fixed orthodontic treatment to prevent enamel demineralization: A systematic review and meta-analysis. Sci. Rep. 11(1), 16556 (2021).
Gernhardt, C. R. et al. The effect of different desensitizing agents on initial demineralization of human root dentin. Quintessence Int. 36(9), 679–685 (2005).
Miyajima, H. et al. In vitro assessment of a calcium-fluoroaluminosilicate glass-based desensitizer for the prevention of root surface demineralization. Dent. Mater. J. 35(3), 399–407 (2016).
Zhao, X. et al. Protective effects of resin sealant and flowable composite coatings against erosive and abrasive wear of dental hard tissues. J. Dent. 49, 68–74 (2016).
Wegehaupt, F. J., Kummer, G. & Attin, T. Prevention of erosions by a surface sealant and adhesives under abrasive conditions. Swiss Dent. J. 127(9), 740–747 (2017).
Gernhardt, C. R., Bekes, K. & Schaller, H. G. Influence of three different sealants on root dentin demineralization in situ. Am. J. Dent. 20(6), 390–393 (2007).
Bekes, K. et al. The influence of application of different desensitisers on root dentine demineralisation in situ. Int. Dent. J. 59(3), 121–126 (2009).
Schupbach, P., Lutz, F. & Finger, W. J. Closing of dentinal tubules by Gluma desensitizer. Eur. J. Oral. Sci. 105(5 Pt 1), 414–421 (1997).
Zhang, N. et al. Development of a multifunctional adhesive system for prevention of root caries and secondary caries. Dent. Mater. 31(9), 1119–1131 (2015).
Okuyama, K. et al. Efficacy of a new filler-containing root coating material for dentin remineralization. Am. J. Dent. 29(4), 213–218 (2016).
Higgins, J. P. T., et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). 2020, Cochrane (2020).
Page, M. J. & Moher, D. Evaluations of the uptake and impact of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and extensions: A scoping review. Syst. Rev. 6(1), 263 (2017).
Wierichs, R. J. et al. Efficacy of nano-hydroxyapatite on caries prevention - a systematic review and meta-analysis. Clin. Oral. Investig. 26(4), 3373–3381 (2022).
Tennert, C. et al. Posterior ceramic versus metal restorations: A systematic review and meta-analysis. Dent. Mater. 38(10),1623–1632. (2022).
Soares, F. Z. M. et al. Bovine tooth is a substitute for human tooth on bond strength studies: A systematic review and meta-analysis of in vitro studies. Dent. Mater. 32(11), 1385–1393 (2016).
Nunez, C. C., et al. Mechanical properties of restored teeth after selective caries excavation: A systematic review and meta-analysis of in vitro studies. Int. J. Odontostomat. 15(1), 204–212 (2021).
Mantel, N. Chi-square tests with one degree of freedom, extensions of the Mantel–Haenszel procedure. J. Am. Stat. Assoc. 58(303), 690–700 (1963).
Bourouni, S. et al. Efficacy of resin infiltration to mask post-orthodontic or non-post-orthodontic white spot lesions or fluorosis—a systematic review and meta-analysis. Clin. Oral. Investig. 25(8), 4711–4719 (2021).
Wierichs, R. J., Carvalho, T. S. & Wolf, T. G. Efficacy of a self-assembling peptide to remineralize initial caries lesions—A systematic review and meta-analysis. J. Dent. 109, 103652 (2021).
Egger, M. et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Bekes, K. et al. The influence of different irradiation doses and desensitizer application on demineralization of human dentin. Oral. Oncol. 45(9), e80–e84 (2009).
Kawamura, K. et al. Anti-demineralization effect of desensitizer containing copolymer and sodium fluoride on root dentin—a transverse microradiographic study. Acta Biomater. Odontol. Scand. 5(1), 38–43 (2019).
Lodha, E. et al. Effect of different desensitizers on inhibition of bovine dentin demineralization: Micro-computed tomography assessment. Eur. J. Oral. Sci. 122(6), 404–410 (2014).
Obayashi, S. et al. Preventive effect of experimental polymer-based desensitizers with NaF on demineralization of root dentin-observed using micro-CT. Dent. Mater. J. 39(6), 1050–1056 (2020).
Oshima, M. et al. Effect of polymer-based desensitizer with sodium fluoride on prevention of root dentin demineralization. Am. J. Dent. 28(3), 123–127 (2015).
Saad, A. et al. Inhibitory effect of zinc-containing desensitizer on bacterial biofilm formation and root dentin demineralization. Dent. Mater. J. 38(6), 940–946 (2019).
Gernhardt, C. R. et al. The influence of dentin adhesives on the demineralization of irradiated and non-irradiated human root dentin. Oper. Dent. 29(4), 454–461 (2004).
Hahn, P. et al. Influence of two dentin bonding systems on the demineralization of the root surface. Oper. Dent. 24(6), 344–350 (1999).
Kuramoto, A. et al. Inhibition of root caries progression by an antibacterial adhesive. J. Dent. Res. 84(1), 89–93 (2005).
Walter, R. et al. Effect of resin adhesive systems on root caries formation in vitro. Quintessence Int. 39(1), 33–37 (2008).
Zhou, Y. et al. Evaluation of resin infiltration on demineralized root surface: An in vitro study. Dent. Mater. J. 36(2), 195–204 (2017).
Swift, E. J. et al. Prevention of root surface caries using a dental adhesive. J. Am. Dent. Assoc. 125(5), 571–576 (1994).
Tao, S. et al. Nano-calcium phosphate and dimethylaminohexadecyl methacrylate adhesive for dentin remineralization in a biofilm-challenged environment. Dent. Mater. 36(10), e316–e328 (2020).
Grogono, A. L. & Mayo, J. A. Prevention of root caries with dentin adhesives. Am. J. Dent. 7(2), 89–90 (1994).
Prabhakar, A. R., Dhanraj, K. & Sugandhan, S. Comparative evaluation in vitro of caries inhibition potential and microtensile bond strength of two fluoride releasing adhesive systems. Eur. Arch. Paediatr. Dent. 15(6), 385–391 (2014).
Kwong, S. M. et al. An ultrastructural study of the application of dentine adhesives to acid-conditioned sclerotic dentine. J. Dent. 28(7), 515–528 (2000).
Wierichs, R. J., Kramer, E. J. & Meyer-Lueckel, H. Risk factors for failure of class V restorations of carious cervical lesions in general dental practices. J. Dent. 77, 87–92 (2018).
Wierichs, R. J., Kramer, E. J. & Meyer-Lueckel, H. Risk factors for failure in the management of cervical caries lesions. Clin. Oral. Investig. 21(6), 2123–2131 (2017).
Mitra, S. B. Adhesion to dentin and physical properties of a light-cured glass-ionomer liner/base. J. Dent. Res. 70(1), 72–74 (1991).
Chatzimarkou, S., Koletsi, D. & Kavvadia, K. The effect of resin infiltration on proximal caries lesions in primary and permanent teeth. A systematic review and meta-analysis of clinical trials. J. Dent. 77, 8–17 (2018).
de Barros Pinto, L. et al. Natural enamel caries, dentine reactions, dentinal fluid and biofilm. Sci. Rep. 9(1), 2841 (2019).
Van Meerbeek, B. et al. Clinical status of ten dentin adhesive systems. J. Dent. Res. 73(11), 1690–1702 (1994).
Acknowledgements
This study was conducted as part of the master thesis of T.M.
Author information
Authors and Affiliations
Contributions
W.R.J.: contributed to conception, design, acquisition, analysis and interpretation, drafted and critically revised the manuscript. M.T.: contributed to design, acquisition and interpretation, drafted and critically revised the manuscript. C.G: contributed to interpretation and critically revised the manuscript. C.T.S.: contributed to design, interpretation and critically revised the manuscript. N.S.H.: contributed to conception, design, analysis and interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wierichs, R.J., Müller, T., Campus, G. et al. Systematic review and meta-analysis on physical barriers to prevent root dentin demineralization. Sci Rep 12, 18194 (2022). https://doi.org/10.1038/s41598-022-22132-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22132-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.