Abstract
Decreased pancreatic volume, increased pancreatic fat mass, and serrated pancreatic margins are characteristic morphological changes of the pancreas in subjects with type 2 diabetes mellitus. This retrospective study aimed to clarify the clinical significance of pancreatic morphological changes in subjects with type 2 diabetes mellitus who underwent abdominal magnetic resonance imaging. The mean age and HbA1c value were 59.1 ± 16.3 years old and 8.9 ± 2.3%, respectively. Pancreatic body mass corrected for body surface area (BSA) in subjects with diabetes mellitus was lower compared to those in normal glucose tolerance (49.4 ± 15.3 cm3 vs. 60.9 ± 7.8 cm3), although it did not reach a statistic significance. There was a negative correlation between BSA-corrected pancreatic volume and age, duration of diabetes, glycoalbumin, mean and max IMT, and there was a positive correlation between BSA-corrected pancreatic volume and HOMA2-β. Serration of the pancreatic limbus was more often observed in subjects with diabetes mellitus compared to those in normal glucose tolerance (74.1% vs. 14.3%). Subjects with serrated changes were older and had higher HbA1c, and visceral fat area was significantly larger in subjects with serrated changes. BSA-corrected pancreatic volume in subjects with serrated changes was significantly smaller, and mean IMT was significantly thicker in subjects with serrulation. Furthermore, advanced diabetic retinopathy and diabetic nephropathy were more often observed in subjects with serrated changes. Taken together, decreased BSA-corrected pancreatic volume and serrated changes were associated with the progression of vascular complications in subjects with type 2 diabetes mellitus.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus is a disease that causes β-cell dysfunction due to genetic and environmental factors; it is estimated that β-cell function is reduced by 50% at the onset of type 2 diabetes mellitus1. Previous studies using ultrasonography and computed tomography (CT) to evaluate pancreatic volume have shown a 7–22% decrease in pancreatic volume in subjects with type 2 diabetes compared to subjects without glucose intolerance2,3. Decreased β-cell counts and fat deposition and fibrosis in the pancreas have been postulated as reasons for the decreased pancreatic volume in type 2 diabetes4. Magnetic resonance imaging (MRI) has superior spatial resolution compared to CT, and it is more sensitive to the intra-abdominal fat than CT. The use of MRI for the safe evaluation of pancreatic morphology has led to studies in subjects with type 2 diabetes mellitus, and the results of these studies showed that the pancreas is a characteristic pancreatic organ in type 2 diabetes mellitus5. Imaging findings included decreased pancreatic volume, increased pancreatic fat content, and serrated pancreatic limbus. There was a significant correlation between pancreatic volume and homeostatic model assessment β-cell function (HOMA-β). Previous studies have suggested that morphological changes in the pancreas in type 2 diabetes mellitus may have some effect on endogenous insulin secretion6. Although there is concern about the possible association between morphological changes in the pancreas and diabetes-related complications, there have been no reports examining the relationship between diabetes-related complications and changes in pancreatic morphology. This study aims to clarify the clinical significance of pancreatic morphological changes on diabetes-related complications and pathological progression in subjects with type 2 diabetes who underwent abdominal MRI.
Materials and methods
Study subjects
This retrospective study conducted at the Department of Diabetes, Metabolism, and Endocrinology, Kawasaki Medical School, Japan, included adult subjects aged 20 years or older who underwent abdominal MRI at our department between December 1, 2018, and October 31, 2021. The study was approved by the Ethics Committee of Kawasaki Medical School and its affiliated hospitals (No. 5500-00).
A total of 95 adult subjects were screened; those with type 1 diabetes mellitus (n = 4), cases complicated with malignancy (n = 20), those taking steroids (n = 3), cases in which insulin secretory capacity was not evaluated (n = 1), and cases in which pancreatic volume could not be calculated from the imaged images (n = 2) were excluded from the study. A total of 65 subjects, 58 diabetic and 7 non-diabetic, were finally included in the analysis. In non-diabetic patients who underwent MRI, 6 cases had non-functioning adrenal adenomas and 1 case experienced hypoglycemia.
Methods
Pancreatic volume measurements and serrated changes of the pancreatic limbus were evaluated by two physicians trained by a radiologist and a radiology technologist. The pancreas was reconstructed into a 3D image using SYNAPSE VINCENT (FUJIFILM, Tokyo, Japan) from 3 to 5 mm thick T1 fat suppression images obtained by MRI. Pancreatic volume was corrected for body surface area (BSA), which was calculated using the formula: body weight 0.425 × height 0.725 × 0.0071847. The relationship between the obtained changes in pancreatic volume and pancreatic limbus and diabetes-related parameters at MRI imaging, physical examination, smoking history, alcohol consumption history, and carotid ultrasound was evaluated. Carotid IMT was assessed by an ultrasound technician with ultrasonographic equipment, Aplio series (CANON MEDICAL SYSTEMS, Tochigi, Japan). The carotid IMT was evaluated in the common carotid artery wall 10 mm centrally from the carotid sinus. 2 IMT points were measured within a 10 mm area, and the mean value was defined as mean IMT. The maximum diameter in the same measurement range was defined as max IMT. Data about diabetic retinopathy and nephropathy evaluated within 1 year before and after the MRI were included in the analysis. The Davis classification was used to evaluate diabetic retinopathy, which was grouped into non-diabetic retinopathy (NDR), simple diabetic retinopathy (SDR), pre-proliferative retinopathy (PPDR), and proliferative diabetic retinopathy (PDR). Diabetic nephropathy was evaluated by dividing the patients into 5 stages based on eGFR and albuminuria: Stage 1 was defined as eGFR > 30 mL/min/1.73 m2 and urinary albumin < 30 mg/gCr; Stage 2 was defined as eGFR > 30 mL/min/1.73 m2 and urinary albumin Stage 3: eGFR ≥ 30 mL/min/1.73 m2 and urinary albumin ≥ 300 mg/gCr, Stage 4: eGFR < 30 mL/min/1.73 m2, and Stage 5: patients on dialysis therapy. Urinary albumin was natural logarithmized when calculating the correlation coefficient with pancreatic volume. To assess endogenous insulin secretory capacity, HOMA2-β was calculated using the HOMA2 Calculator for Mac OS X Catalina and Linux (Diabetes Trials Unit, Oxford, England). Application program interface was downloaded from http://www.dtu.ox.ac.uk/homacalculator/index.php. All methods were carried out in accordance with relevant guidelines and regulations.
Statistical analysis
Clinical characteristics of the subjects used in the analysis were age, laboratory findings at MRI imaging, endogenous insulin secretory capacity, and carotid ultrasound. Student's t-test was used to compare normal glucose tolerance cases with diabetic cases, and correlation analysis and multiple regression analysis were used to evaluate pancreatic volume corrected for BSA and each parameter. The changes in the pancreatic limbus and each parameter were evaluated using the chi-square test. Multiple regression analysis was performed to evaluate the impact of BSA-corrected pancreatic volume on HOMA2-β and mean IMT. The objective variables were HOMA2-β and mean IMT, and the explanatory variables were as follows: (model 1) age, sex, BMI, duration of type 2 diabetes, HbA1c, and BSA-corrected pancreatic volume; (model 2) age, sex, systolic blood pressure, LDL-cholesterol, HbA1c, and BSA-corrected pancreatic volume; (model 3) age, sex, BMI, Brinkmann’s index, HbA1c, and BSA-corrected pancreatic volume. Statistical software used were Excel Statistics for Mac version 16.54 (Social Research Information, Tokyo, Japan) and JMP version 16.0.1 (SAS Institute Inc.).
Informed consent
Informed consent was obtained from all subjects in this study.
Results
Clinical characteristics
The clinical characteristics of the subjects with type 2 diabetes are shown in Table 1. The mean age was 59.1 ± 16.3 years, and 72.4% of the participants were male. HbA1c (National Glycohemoglobin Standardization Program [NGSP]) was 8.9 ± 2.3%. Duration of diabetes mellitus was 11.9 ± 9.1 years, which included many subjects with a relatively long history of diabetes mellitus. The prevalence of diabetic retinopathy was 24.1% (10.3% for SDR and 6.9% for PPDR and PDR, respectively). As for diabetic nephropathy, 14 patients (24.1%) had Stage 2, 8 (3.4%) had Stage 3, and 10 (17.2%) had Stage 4. The percentages of patients who had ever been diagnosed with dyslipidemia or hypertension were 89.5% and 77.2%, respectively.
Pancreatic volume and diabetes mellitus
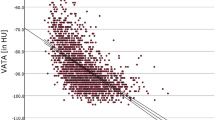
We evaluated how BSA-corrected pancreatic volume changes in subjects with diabetes mellitus (Fig. 1A). BSA-corrected pancreatic volume in subjects with normal glucose tolerance and diabetes mellitus were 60.9 ± 7.8 cm3 and 49.4 ± 15.3 cm3, respectively, although it did not reach a statistic significance. In the present study, pancreatic volume did not change when grouped by alcohol consumption (Fig. 1B). Next, we evaluated the correlation between BSA-corrected pancreatic volume and diabetes-related parameters. As shown in Figs. 1C–H, there was a negative correlation between BSA-corrected pancreatic volume and age, duration of diabetes, glycoalbumin, mean and max IMT, and there was a positive correlation between BSA-corrected pancreatic volume and HOMA2-β. The correlation between pancreatic volume and microvascular complications was evaluated (Fig. 1I,J). There was a positive correlation between albuminuria and pancreatic volume (p = 0.058). No significant correlation was found between progression of diabetic retinopathy and pancreatic volume (p > 0.05).
(A) Comparison of BSA-corrected pancreatic volume in subjects between with normal glucose tolerance and type 2 diabetes mellitus. (B–J) Correlation between BSA-corrected pancreatic volume and diabetes-related parameters. There was a negative correlation between BSA-corrected pancreatic volume and age, duration of diabetes, glycoalbumin, mean and max IMT, and there was a positive correlation between BSA-corrected pancreatic volume and HOMA2-β.
Multiple regression analysis was performed to evaluate factors affecting HOMA2-β and mean IMT. The results of the multiple regression analysis for HOMA2-β are shown in Table 2; BSA-corrected pancreatic volume was not an independent factor determining HOMA2-β (t = 1.03, p > 0.05). Table 3 shows the results of multiple regression analysis for mean IMT, which showed a significant correlation between BSA-corrected pancreatic volume and mean IMT (t = − 2.00, p < 0.05). Next, other atherosclerotic factors affecting mean IMT were included in the analysis. Model 2 included systolic blood pressure and LDL-cholesterol, which were independent factors affecting mean IMT (t = 2.52, p < 0.05). The t-value for BSA-corrected pancreatic volume in model 2 was − 1.74 (p = 0.061). In model 3, Brinkmann index was added to the analysis, and BSA-corrected pancreatic volume was an independent factor affecting mean IMT (t = − 2.06, p < 0.05).
Serrated changes of the pancreatic margins
The relationship between changes in the pancreatic limbus and various parameters was evaluated. The characteristic pancreatic limbus changes in diabetes mellitus is serrated changes (Fig. 2A). Serration of the pancreatic limbus was observed in 14.3% of subjects with normal glucose tolerance and 74.1% of those with diabetes mellitus (p < 0.005, Fig. 2B). Next, we evaluated the correlation between the presence of serrated pancreatic changes and various parameters (Figs. 2C–H). Subjects with serrated changes were older and had higher HbA1c. Visceral fat area was significantly larger in subjects with serrated changes (274.7 ± 79.2 cm2) compared to that in subjects without serrated changes (193.1 ± 73.8 cm2) (p < 0.0005). BSA-corrected pancreatic volume was 56.0 ± 11.8 cm3 in subjects without serrulation which was significantly larger compared to those with serrulation (47.9 ± 15.8 cm3) (p < 0.05), and mean IMT was significantly thicker in subjects with serrulation (p < 0.05). Next, we examined the possible association between the serrated pancreas and microvascular complications. As shown in Fig. 3 (upper panel). advanced diabetic retinopathy was significantly more common in subjects with serrated changes compared to those without serrated changes (p < 0.05). Similar results were obtained regarding diabetic nephropathy. As shown in Fig. 3 (lower panel), advanced diabetic nephropathy was significantly more common in subjects with serrated changes (p < 0.005).
(A) Representative serrated changes of the pancreatic margins in subjects with type 2 diabetes mellitus. (B) Percentage of serrated pancreatic changes in subjects with normal glucose tolerance and type 2 diabetes mellitus. Percentage of serrated pancreatic changes was significantly higher in subjects with type 2 diabetes mellitus compared to those with normal glucose tolerance (p < 0.005). (C–H) Correlation between the presence of serrated pancreatic changes and various parameters. Subjects with serrated changes were older and had higher HbA1c (p < 0.05). Visceral fat area was significantly larger in subjects with serrated changes compared to those without them (p < 0.0005). BSA-corrected pancreatic volume in subjects with serrated changes was significantly smaller compared to those without them (p < 0.05), and mean IMT was significantly thicker in subjects with serrulation (p < 0.05).
Discussion
Insulin resistance induced by increased visceral fat and fatty liver often contributes to the pathogenesis in type 2 diabetes mellitus8. In the previous study using abdominal MRI, the fat content of the pancreas was 9.7% in subjects with normal glucose tolerance and 20.4% in those with diabetes mellitus, and there was also a correlation between the fatty pancreas and HOMA-IR9. The increased incidence of type 2 diabetes in subjects with the fatty pancreas also suggests that the fatty pancreas may reflect insulin resistance10. In this study, we measured and analyzed pancreatic volume using T1-weighted fat-suppressed MRI, focusing on the decrease in pancreatic volume and serrated changes as findings reflecting an increase in pancreatic fat content. Furthermore, we examined the possible association between morphological changes in the pancreas and vascular complications in subjects with type 2 diabetes and found that morphological changes in the pancreas such as pancreatic volume or serrated changes in the pancreatic limbus were closely associated with vascular complications such as atherosclerosis and diabetic microangiopathy.
A similar study in the past reported a significant correlation between pancreatic volume reduction and HOMA-β5. In the same paper, HOMA-β may not have been accurately assessed because the mean blood glucose level at the time of evaluation was above 140 mg/dL11. The mean HbA1c of the current participants in this study was 8.9 ± 2.3%, and the analysis was performed using HOMA2-β, which can more accurately assess endogenous insulin secretory capacity even under hyperglycemic conditions12. Results showed a significant single correlation with BSA-corrected pancreatic volume, as in previous reports, but no correlation was found in multiple regression analysis. Fatty infiltration into the pancreas leads to decreased β-cell function and has been reported as one factor in the development of diabetes8. On the other hand, islets, which are responsible for the endocrine function of the pancreas, account for only about 1% of the volume of the pancreas, and further studies are needed to determine whether a decrease in the overall volume of the pancreas or serrated changes in the pancreatic limbus is directly correlated with the endogenous insulin secretory capacity.
We assume that possible mechanisms for atrophy of pancreatic volume due to fatty pancreas include fat deposition around the adenocellular cells and infiltration from deposited fat, and replacement by fat cells after destruction of adenocellular cells due to microischemia caused by inflammation or atherosclerosis13. A 25% increase in pancreatic fat content is associated with the development of type 2 diabetes and atherosclerosis14, and fat deposition in the pancreas in subjects with type 2 diabetes leads to insulin resistance and β-cell dysfunction15. In the present study, serrated changes were significantly correlated with increased visceral fat area, IMT thickening, and renal dysfunction, suggesting that pancreatic morphological changes in type 2 diabetes may more sensitively reflect ectopic fat deposition in the whole body. Progression of diabetic nephropathy and diabetic retinopathy were also more frequently observed in subjects with type 2 diabetes together with serrated changes. We assume that lipotoxicity, at least in part, contributes to the progression of diabetic microvascular complications in subjects with serrated changes by causing vascular endothelial damage and inducing oxidative stress and apoptosis in extravascular organs. Systemic lipotoxicity should be assumed in subjects with fat deposition in the pancreas, and serrated changes in the pancreas may be a useful finding in estimating the potential risk of microvascular and macrovascular complications of type 2 diabetes mellitus.
This study has several limitations. First, this was a single-center, retrospective study. Most of the patients in this study are Japanese, and their physiques and lifestyles may differ from those in other countries. Second, the number of cases in the control group, non-diabetic patients, was small because the study included patients who had undergone MRI. Larger case numbers are needed for objective validity about the evaluation of pancreatic morphology in non-diabetic patients. Next, it was not possible in this study to determine which factors, such as chronic inflammation, excessive alcohol, or fat deposition, caused the morphological changes in the pancreas. Lastly, it cannot be ruled out that most of the participants in this study had a history of dyslipidemia, potentially influencing the results.
Taken together, decreased BSA-corrected pancreatic volume and serrated changes in the pancreas are closely associated with diabetic microvascular and macrovascular complications, and these findings may be new indicators to reflect ectopic fat deposition in the whole body and to infer the progression of microvascular complications and atherosclerotic changes in subjects with type 2 diabetes mellitus.
Data availability
All data generated or analysed during this study are included in this published article.
References
Rudenski, A. S. et al. Natural history of pancreatic islet B-cell function in type 2 diabetes mellitus studied over six years by homeostasis model assessment. Diabet. Med. 5(1), 36–41 (1988).
Alzaid, A., Aideyan, O. & Nawaz, S. The size of the pancreas in diabetes mellitus. Diabet. Med. 10(8), 759–763 (1993).
Lim, S. et al. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol. 51(5), 739–748 (2014).
Butler, A. E. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52(1), 102–110 (2003).
Macauley, M. et al. Altered volume, morphology and composition of the pancreas in type 2 diabetes. PLoS One 10(5), e0126825 (2015).
Lu, J. et al. Association between pancreatic atrophy and loss of insulin secretory capacity in patients with type 2 diabetes mellitus. J. Diabetes Res. 2019, 6371231 (2019).
Du Bois, D. & Du Bois, E. F. A formula to estimate the approximate surface area if height and weight be known. Arch Intern. Med. 17, 863–871 (1916).
Ou, H. Y. et al. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS ONE 8(5), e62561 (2013).
Tushuizen, M. E. et al. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 30(11), 2916–2921 (2007).
Chai, J. et al. MRI chemical shift imaging of the fat content of the pancreas and liver of patients with type 2 diabetes mellitus. Exp. Ther. Med. 11(2), 476–480 (2016).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7), 412–419 (1985).
Song, Y. S. et al. Comparison of the usefulness of the updated homeostasis model assessment (HOMA2) with the original HOMA1 in the prediction of type 2 diabetes mellitus in Koreans. Diabetes Metab. J. 40(4), 318–325 (2016).
Takahashi, M. et al. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 109(10), 3013–3023 (2018).
Stamm, B. H. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: A systematic study of 112 autopsies in patients without known pancreatic disease. Hum. Pathol. 15(7), 677–683 (1984).
Guglielmi, V. & Sbraccia, P. Type 2 diabetes: Does pancreatic fat really matter?. Diabetes Metab Res Rev 34(2), e2955 (2018).
Funding
There is no funding associated with this manuscript.
Author information
Authors and Affiliations
Contributions
Y.I. researched data and wrote the manuscript. T.K., F.T., T.S., M.K., E.N., K.D., R.W., H.I., K.T., J.S., Y.F., Y.K., M.S., S.N., T.M., K.K. participated in discussion. H.K. participated in discussion and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
H.K. has received honoraria for lectures, received scholarship grants, and received research grant from Novo Nordisk Pharma, Sanofi, Eli Lilly, Boehringer Ingelheim, Taisho Pharma, Sumitomo Dainippon Pharma, Takeda Pharma, Ono Pharma, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Kissei Pharma, MSD, AstraZeneca, Astellas, Novartis, Kowa, Abbott. K.K. has been an advisor to, received honoraria for lectures from, and received scholarship grants from Novo Nordisk Pharma, Sanwa Kagaku, Takeda, Taisho Pharma, MSD, Kowa, Sumitomo Dainippon Pharma, Novartis, Mitsubishi Tanabe Pharma, AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Sanofi. Other authors (Yuichiro Iwamoto, Tomohiko Kimura, Fuminori Tatsumi, Toshitomo Sugisaki, Masato Kubo, Erina Nakao, Kazunori Dan, Ryo Wamata, Hideyuki Iwamoto, Kaio Takahashi, Junpei Sanada, Yoshiro Fushimi, Yukino Katakura, Masashi Shimoda, Shuhei Nakanishi, Tomoatsu Mune) do not have conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwamoto, Y., Kimura, T., Tatsumi, F. et al. Association between changes in pancreatic morphology and vascular complications in subjects with type 2 diabetes mellitus: a retrospective study. Sci Rep 12, 17166 (2022). https://doi.org/10.1038/s41598-022-21688-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21688-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.