Abstract
Malaria remains a major health problem and vector control is an essential approach to decrease its burden, although it is threatened by insecticide resistance. New approaches for vector control are needed. The females of Anopheles gambiae s.l. mate once in their life and in the swarms formed by males. Trapping swarms of Anopheles gambiae s.l. males is a potential new intervention for vector control, alternative to the use of insecticides, as it would disrupt mating . The proof-of-concept pilot study aiming at investigating swarm trapping as a potential vector control intervention, was carried out in 6 villages as in eastern Gambia. Swarms of Anopheles gambiae s.l. were identified and their size, height, and duration determined during the baseline year. Swarm trapping by local volunteers was implemented the following transmission season in 4 villages while the other 2 villages were taken as controls. Entomological outcomes were monitored by Human Landing Catches and Pyrethrum Spray Catches. A cross-sectional survey to determine malaria prevalence was carried out at the peak of the malaria transmission season for two consecutive years. At baseline, 23 swarming sites of Anopheles gambiae s.l. were identified. Before the intervention, mean indoor resting density per house and malaria prevalence were similar between control and intervention villages. Following the intervention, Anopheles gambiae s.l. indoor resting density was 44% lower in intervention than in control villages (adj IRR: 0.0.56; 95% CI 0.47–0.68); the odds of malaria infections were 68% lower in intervention than in control villages (OR: 0.32; 95% CI 0.11–0.97). Swarm trapping seems to be a promising, community-based vector control intervention that could reduce malaria prevalence by reducing vector density. Such results should be further investigated and confirmed by larger cluster-randomized trials.
Similar content being viewed by others
Introduction
Vector-borne diseases represent a considerable health burden in tropical and subtropical regions. Malaria alone, transmitted exclusively by Anopheles mosquitoes (Diptera: Culicidae) infected with Plasmodium protozoan parasites, caused in 2019 about 229 million cases and 409,000 deaths, most of them in sub-Saharan Africa1. Between 2000 and 2015, thanks to the scale up of standard control interventions, the malaria burden decreased substantially in several sub-Saharan countries1,2. However, transmission is still ongoing and resistance against antimalarial drugs and insecticides has emerged3,4. Moreover, where coverage of standard control interventions is high, residual transmission is maintained by outdoor transmission5,6,7,8. Therefore, new tools are needed as standard control interventions are unable to further reduce and then interrupt malaria transmission1.
Vector control based on the use of insecticides is a key component of malaria control2. Besides insecticide resistance, vector control is challenged by vectors feeding and resting outdoor, allowing them to escape standard control interventions6. There is growing interest in the use of genetically modified mosquitoes or laboratory-reared male mosquitoes that, by mating with wild female mosquitoes, would reduce or suppress vector populations through several mechanisms, including sterile insect technique and incompatible insect technique9,10. One major challenge for their large-scale implementation is the capacity of such laboratory-reared males to successfully compete with wild ones as basic life history traits and mating preference remain poorly understood 11. Moreover, mass rearing and negative effects on off-target species should be considered12. In Sub-Saharan Africa, the logistics of implementing such novel tools would be particularly challenging given the lack of biomedical industries, ethic community/government acceptance and expertise to monitor its implementation.
Trapping swarms of male mosquitoes may be an alternative approach for vector control. An. gambiae s.l. males form swarms daily, at sunset, for 10–30 min and in the same locations for several years13; females mate in flight once in their life, stock the sperm in their spermatheca and then lay eggs every two-days after blood feeding14. Mass trapping of males during swarming could significantly reduce indoor resting density by reducing mating and thus malaria transmission. However, for its successful deployment, swarms should be easily identifiable and consistently in the same locations as in Burkina Faso, Mali and Benin, where local residents were trained to identify and collect swarms14,15. Such an intervention, despite its apparent difficulties, has the advantage of targeting malaria vectors that may be resistant to insecticides. In Burkina Faso, swarms trapping decreased vector density by 80%16, although the impact of such intervention on malaria prevalence was not measured. As female mating increases vector’s susceptibility to human malaria parasites17, trapping male mosquitoes may reduce malaria transmission also by this mechanism.
Results
Characteristics of Anopheles gambiae s.l. reproductive swarms in Eastern Gambia
Twenty-three swarming sites of Anopheles gambiae s.l., between 3 and 5 swarms per village, were identified (Table 1), most of them near households (Fig. 1). These swarms usually appeared at the same location a few minutes after sunset, around 18:50. All swarms were species-specific, mostly An. coluzzii and An. arabiensis (Table 1). Swarm size was 61.34 (standard deviation SD: 25.41), 57.64 (SD: 27.72), and 68 (SD: 11.11) for An. coluzzii, An. arabiensis and An. gambiae s.s., respectively, with no difference between species (Fig. 2A, p = 0.44). Mean swarm duration in seconds was 609.94 (SD: 121.88), 481.72 (SD: 70.96) and 635.8 (SD: 100.62) for An. coluzzii, An. arabiensis and An. gambiae s.s., respectively, with An. arabiensis having the shorter duration (Fig. 2B, p < 0.0001) while there was no difference between An. coluzzii and An. gambiae s.s.. Conversely, swarm height (in centimeter) was significantly higher for An. arabiensis (171.8 SD: 10.49) than for An. coluzzii (119.05 SD: 10.27) and An. gambiae s.s. (112 SD: 13.50) (Fig. 2C, p < 0.0001).
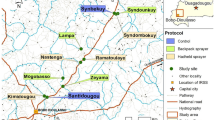
Distribution of Anopheles gambiae s.l. reproductive swarms in six villages of eastern Gambia. A The map of The Gambia shows the five administrative regions: WCR (West Coast Region); NBR (North Bank Region), LRR (Lower River Region), CRR (Central River Region) and URR (Upper River Region). The study area is in URR; Blue dots: control villages (Mamasutu and Bakadagy); Red dots: intervention villages (Chamoi, Dampha kunda, Tambasansang and Madina Yoro) the red dots. The control and intervention villages were ~ 23 km apart showing on the satellite image obtained from Google Earth Pro 7.3.4.8642. The green circles correspond to the positive Anopheles gambiae s.l. swarming positions and red circles correspond to the negative ones.
Swarm trapping
Forty-eight swarm collections (twice a week) during the intervention period captured 36,327 male mosquitoes, 30,131 from An. coluzzii swarms and 6196 from An. arabiensis swarms. The highest number of mosquitoes was collected in September (Supplementary Fig. 1).
Indoor resting density and other entomological measurements before and after intervention
Before the intervention, mean indoor resting density per house was similar between control and intervention villages (adjusted IRR: 1.04; 95% CI 0.80–1.35) (p = 0.964) (Fig. 3A,B, Table 2 and Table S1), and between villages (Fig. 3B). In 2018, mean indoor resting density was significantly lower in intervention than in control villages (adjusted IRR: 0.56; 95% CI 0.47–0.68) (p < 0.001). Such a difference was particularly marked between August and October (Fig. 3C,D and Table 2).
In 2017, a total of 153 An. gambiae s.l. were collected by HLC, all of them negative for P. falciparum sporozoites. Biting rates ranged between 1 and 1.91 per human/night and were similar between villages.
In 2018, a total of 2560 An. gambiae s.l. were collected by HLC, most of them either An. coluzzii (52.61%) or An. arabiensis (36.95%) (Supplementary Fig. S2A). The biting rate was 10.80 per human/night (95% CI 10.15–11.48) in intervention villages and 15.87 per human/night (95% CI 15.09–16.68) in control villages (p = 0.89, Fig. S2B and Table S2). The sporozoite rate was 0.1% (95% CI 0.02–0.54) in intervention villages and 0.2% (95% CI 0.07–0.56) in control villages (p = 0.9, Supplementary Fig. S2C and Table S3). The EIR was 0.01 (95% CI 0.00–0.06) in the intervention and 0.06 (95% CI 0.01, 0.2) in the control arm (p-value = 0.89, Supplementary Fig. S2D).
Malaria prevalence
A total of 921 and 892 individuals were included into the cross-sectional survey in 2017 and 2018, respectively (Supplementary Table S4).
In 2017, malaria prevalence ranged between 1.24 and 2.33%, and was similar between intervention (1.88%) and control villages (2.07%) (OR: 0.91; 95% CI 0.35–2.37, p = 0.849), (Fig. 4 and Table 3). In 2018, malaria prevalence ranged between 0 and 3.87% and was significantly lower in intervention (0.93%) than in control villages (2.91%) (OR: 0.32, 95% CI 0.11–0.97, p = 0.044, Fig. 4, Table 3 and Table S5).
Malaria prevalence in the target and control villages of Anopheles reproductive swarm trapping. The target villages are: Chamoi, Dampha kunda, Tambasansang and Madina Yoro; and control ones are: Bakadagy and Mamasutu. The bars corresponds to the malaria prevalence ± 95% Confident Interval in 2017 and 2018. The significance of the difference is indicated (ns, p > 0.05; *p < 0.05).
Discussion
Mass trapping of Anopheles gambiae males during swarming decreased significantly both indoor resting vector density and prevalence of malaria infection in intervention villages, although it did not have any effect on other entomological parameters, i.e., sporozoite rate and EIR. This is not surprising as indoor resting density should decrease by at least 80% for a significant effect on EIR18,19. An effect on indoor resting density of similar magnitude was also observed for other mosquito control tools such as solar mosquito trap20 and attractive toxic sugar baits (ATSB)21. Our primary goal was to investigate non-chemical strategies that could be effective for vector control, namely trapping swarming males with insect sweep nets rather than treating them with insecticides as previously described16. Despite no available data comparing both approaches, their effectiveness is probably similar as far targeted Anopheles gambiae populations are sensitive to the insecticide used.
Trapping Anopheles swarms has never been implemented as a vector control intervention although the contribution of male mosquitoes to higher indoor resting density and transmission intensity has been recently reported17,22, suggesting this approach has the potential of becoming a vector control tool15,16. In addition, this is an intervention that could be implemented with limited resources. Local volunteers were easily trained to collect swarm after sunset, at a time when they would have finished their usual daily activities such as farming or schooling. They carried out their task enthusiastically and reliably. A similar approach was taken for the control of larval habitats in Tanzania23. Despite a limitation to knowing the exhaustivity of swarming event numbers happening in the village, we can ensure that most swarms were identified in this pilot study accordingly to our experience in Anopheles’ reproductive biology study.
Swarms characteristics were investigated prior to swarms trapping. Indeed, knowing the vectors’ biological characteristics is essential to determine the place and the timing for the implementation of new vector control tools. Swarming of Anopheles gambiae s.l. began a few minutes after sunset, as also observed in Benin, Burkina Faso, Mali and Tanzania15. Despite reports of Anopheles gambiae s.l. indoor swarming in Tanzania24, all swarms in this study were outdoor, in open areas and close to human habitations. Moreover, swarm’s height and duration were similar to those described in other studies, although they were of smaller size than in Burkina Faso and Mali15,22,25,26. The association between indoor resting density and swarm size has already been reported in other countries, e.g., Benin14; small swarm size may be related with low indoor resting density oberved in the study sites.
Although only 5 out of the 23 swarms identified were An. arabiensis, this species represented about a third of all vectors collected by HLC, probably reflecting the increasing abundance of this species in eastern Gambia27. This may suggest that An. arabiensis swarms were missed, possibly because they are usually at a higher height from the ground25. The efficiency of swarm trapping may vary by species, with higher yield for An. coluzzii and An. gambiae s.s.. than for An. arabiensis.
Study villages were not randomized to either intervention or control arm, and this is a major limitation given that the two study groups, control and intervention, were more than 20 km apart and thus not necessarily comparable. Although the year prior to the intervention, malaria prevalence and the entomological parameters were similar between intervention and control villages, other factors could have been responsible of the intervention’s observed effect. For example, awareness on malaria may have increased because of the research team activity, resulting in prompt treatment seeking and/or increased use of ITN.
Malaria prevalence was determined by microscopy and thus a substantial proportion of low-density infections may have been missed. Nevertheless, microscopy diagnosed infections of higher parasite density and thus more transmissible to the vector28.
The low anopheline biting rates observed in 2017, at baseline, is probably due to the timing of the entomological collections as these started just after the peak of indoor resting density. In the intervention year, entomological collections started at the beginning of the transmission season, in July, explaining the higher number of mosquitoes collected by HLC. Nevertheless, the trend of indoor resting density in control villages was similar to that observed in 2017.
In conclusion, swarm trapping seems to be a promising, community-based vector control intervention that could reduce malaria prevalence by reducing vector density. Such results should be further investigated and confirmed by larger cluster-randomized trials.
Materials and methods
The main goal of this study was to pilot mass swarm trapping as a potential vector control intervention. There were two objectives, first to describe swarming and mating behavior of malaria vectors and thus identify the swarm positions, and then determine the effect of mass swarm trapping on malaria transmission. In addition, all methods described below to achieve these objectives, were carried out in accordance with relevant guidelines and regulations.
Study sites and design
The study was carried out in eastern Gambia, in Upper River Region (URR). Malaria transmission in The Gambia is seasonal and heterogeneous across the country, with relatively high prevalence in eastern Gambia5, despite high coverage of control interventions6. Six villages were identified and baseline data (swarming behaviour, Anopheles gambiae s.l. density and malaria prevalence) were collected in 2017. Mass swarm trapping was implemented in 2018 in four villages (Chamoi, Dampha Kunda, Tambasansang, and Madina Yoro) while the other two villages (Mamasutu and Bakadagy), about 20 km from the intervention villages to limit contamination29, were taken as controls (Fig. 1).
Anopheles gambiae s.l. reproductive swarm characterization
Each study village was divided into several areas. Possible An. gambiae s.l. swarm markers (locations with high chances to find a swarm) were identified at daytime by trained volunteers (see Table 1) who went back in the evening to actively search for swarming events. They looked towards the lightest part of the sky, from ground level to about 4 m above the swarm markers. Swarms were confirmed by field supervisors and their duration and height above the ground was recorded. Swarms were sampled once, 5–10 min after their formation, using a standardized insect sweep net (120 cm diameter attached to a 1–1.5 m long stick, depending on the swarming height)14. The swarm location (latitude and longitude) was mapped within 2 m accuracy on background data using the Global Positioning System (GPS-Gamin®). Swarms were collected in the same locations for six evenings. Mosquitoes were transferred into cups, knocked down in a freezer, identified morphologically30 as belonging to Anopheles gambiae s.l., counted and kept in silica gel in 1.5 ml Eppendorf tubes for further molecular identification.
Swarms trapping as an intervention
The intervention (collection of swarms) was implemented between July and December 2018, covering the whole malaria transmission season. Trained local volunteers in intervention villages collected with a large insect net Anopheles gambiae s.l. males attending the swarming event for two consecutive days per week, at the same positions previously identified (Fig. 1). Collected mosquitoes were starved to death, morphologically confirmed as An. gambiae s.l.30, counted, and stored individually in 1.5 ml Eppendorf tube with silica gel at − 20 °C until DNA extraction.
Entomological catches
Human landing catches (HLC)
HLC (indoors and outdoors) were carried out in all villages, from 8.00 p.m. to 7.00 a.m, in three locations per village, and for one night per survey (i.e., six person-night per village per survey). Surveys were carried out 6 weeks apart. There were 2 surveys in 2017 (September–December), and 4 surveys (July–December) in 2018. The collection teams were rotated between collection points on different nights to minimize sampling bias.
Pyrethrum spray catches for indoor resting collection (PSC)
PSC were carried out monthly, from September to December in 2017 and from July to December in 2018, in ten randomly selected houses per village, and two rooms per house14. Indoor resting density per house was estimated as the average number of malaria vectors per house, by month and village.
Identification of Anopheles gambiae species complex
DNA was extracted from head/thoraces with Qiagen QIAxtractor robot according to the manufacturer’s protocol. Species-specific genotyping was performed31 and form-specific restriction enzyme digestion used to distinguish between An. arabiensis (292 bp), An. gambiae s.s (110 and 257 bp), An. coluzzii (367 bp), An. melas (435 bp) and hybrid coluzzii/gambiae (110, 257 and 367 bp)32.
Screening for Plasmodium sporozoites
DNAs extracted from mosquitoes’ head/thoraces (collected by HLC) was analysed using TaqMan SNP genotyping protocol33 to detect sporozoites of Plasmodium falciparum, P. ovale, P. malariae, and P. vivax.
Malariometric survey
A cross-sectional survey was carried out at peak transmission season (November) for 2 consecutive years (2017 and 2018). In each villages, 150 individuals at least 6 months old and no history of travel within the previous month were randomly selected. After obtaining a written informed consent, a blood sample for microscopy was collected by finger-prick. Thick blood films were stained with 2% Giemsa for 30 min and examined by two independent microscopists. A third reader resolved any discrepancy. Blood smears were considered negative after reading 100 high power fields. Patients with clinical malaria (history of fever in the previous 24 h and/or body temperature ≥ 37.5 °C with a positive Rapid Diagnostic Test) were treated with artemether-lumefantrine, the first line treatment in The Gambia. The sample size was estimated on the assumption malaria prevalence would be 10%6; with 150 individuals per village, prevalence would be estimated with a precision of ± 5%.
Ethical approval and consent
The study was approved by the Scientific Coordinating Committee of the Medical Research Council Unit The Gambia (SCC 1548) and the Gambia Government/MRC Joint Ethics Committee. The study team obtained verbal consent from the study communities at village meetings before field activities. Written informed consent was obtained from all individuals aged ≥ 18 years; parents/guardians provided written consent for children (< 18 years of age); assent was obtained from children aged 12–17 years. All household selected for entomological collection (using HLC and PSC) and volunteers for HLCs and swarm collection provided additional written informed consent.
Data collection and statistical analyses
Data were collected using five different case report forms: swarm characterization, mass swarm trapping intervention, indoor resting densities assessment, mosquito sampling by HLC, and malariometric survey. Data were double entered into a Microsoft Excel datasheet, and the data supervisor reconciled the discrepancies via the verification process. Anopheles species morphological and molecular identification, sporozoites in salivary glands, and microscopy reading of thick blood films were extracted from the malaria diagnosis platforms into an Excel datasheet after double-checking and discrepancy reconciliation.
The indoor resting density was estimated as the mean of indoor resting An. gambiae s.l. females collected in ten houses per village. The biting rate was estimated by dividing the number of collected An. gambiae s.l. females by the number of volunteers and collection nights. The sporozoite rate was the proportion of P. falciparum positive mosquitoes divided by the total number of mosquitoes screened by PCR assay. The entomological inoculation rate (EIR) was estimated by multiplying the sporozoite rate by the biting rate34.
Swarm characteristics (swarm size, swarming duration and swarming height) were compared between Anopheles species (An. gambiae s.s., An. coluzzii and An. arabiensis) using non-parametric Kruskal–Wallis test after confirming non-gaussian distribution with Shapiro–Wilk test. Indoor resting density, biting rate and EIR were compared between control and intervention villages using unadjusted negative binomial regression. The Z-test for the difference in two proportions was used to compare the sporozoite rate between control and intervention arms. Unadjusted logistic regression was used to compare malaria prevalence between control and intervention villages. Furthermore, the epidemiological and entomological endpoints of this study were estimated using village-level analysis and permutation tests to compare them between the two groups All statistical analyses were done with R35 (version 3.5.0), and the figures with Prism 9.
Data availability
Data supporting the conclusions of this article are included within the article. Raw data will be made available upon request to the corresponding author.
References
World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges (World Health Organization, 2020).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
Assogba, B. S. et al. Dynamic of resistance alleles of two major insecticide targets in Anopheles gambiae (s.l.) populations from Benin, West Africa. Parasites Vectors 13, 134 (2020).
Amambua-Ngwa, A. et al. Major subpopulations of Plasmodium falciparum in sub-Saharan Africa. Science 365, 813–816 (2019).
Mwesigwa, J. et al. On-going malaria transmission in The Gambia despite high coverage of control interventions: a nationwide cross-sectional survey. Malar. J. 14, 314 (2015).
Mwesigwa, J. et al. Residual malaria transmission dynamics varies across The Gambia despite high coverage of control interventions. PLoS ONE 12, e0187059 (2017).
Killeen, G. F., Govella, N. J., Lwetoijera, D. W. & Okumu, F. O. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar. J. 15, 225 (2016).
Sherrard-Smith, E. et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl. Acad. Sci. USA 116, 15086–15095 (2019).
McGraw, E. A. & O’Neill, S. L. Beyond insecticides: New thinking on an ancient problem. Nat. Rev. Microbiol. 11, 181–193 (2013).
Flores, H. A. & O’Neill, S. L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 16, 508–518 (2018).
Howell, P. I. & Knols, B. G. Male mating biology. Malar. J. 8, S8 (2009).
Alphey, L. Genetic control of mosquitoes. Annu. Rev. Entomol. 59, 205–224 (2014).
Diabaté, A. et al. Spatial distribution and male mating success of Anopheles gambiaes warms. BMC Evol. Biol. 11, 184 (2011).
Assogba, B. S. et al. Characterization of swarming and mating behaviour between Anopheles coluzzii and Anopheles melas in a sympatry area of Benin. Acta Trop. 132, S53–S63 (2014).
Diabate, A. & Tripet, F. Targeting male mosquito mating behaviour for malaria control. Parasites Vectors 8, 347 (2015).
Sawadogo, S. P. et al. Targeting male mosquito swarms to control malaria vector density. PLoS ONE 12, e0173273 (2017).
Dahalan, F. A., Churcher, T. S., Windbichler, N. & Lawniczak, M. K. N. The male mosquito contribution towards malaria transmission: Mating influences the Anopheles female midgut transcriptome and increases female susceptibility to human malaria parasites. PLoS Pathog. 15, e1008063 (2019).
Brady, O. J. et al. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 110, 107–117 (2016).
Catano-Lopez, A. et al. An alternative model to explain the vectorial capacity using as example Aedes aegypti case in dengue transmission. Heliyon 5, e02577 (2019).
Homan, T. et al. The effect of mass mosquito trapping on malaria transmission and disease burden (SolarMal): A stepped-wedge cluster-randomised trial. Lancet 388, 1193–1201 (2016).
Traore, M. M. et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 19, 72 (2020).
Diabaté, A. et al. Spatial swarm segregation and reproductive isolation between the molecular forms of Anopheles gambiae. Proc. R. Soc. B. 276, 4215–4222 (2009).
Vanek, M. J. et al. Community-based surveillance of malaria vector larval habitats: A baseline study in urban Dar es Salaam, Tanzania. BMC Public Health 6, 154 (2006).
Nambunga, I. H. et al. Wild populations of malaria vectors can mate both inside and outside human dwellings. Parasites Vectors 14, 514 (2021).
Dabiré, K. R. et al. Occurrence of natural Anopheles arabiensis swarms in an urban area of Bobo-Dioulasso city, Burkina Faso, West Africa. Acta Trop. 132, S35–S41 (2014).
Kaindoa, E. W. et al. New evidence of mating swarms of the malaria vector, Anopheles arabiensis in Tanzania. Wellcome Open Res 2, 88 (2017).
Opondo, K. O. et al. Does insecticide resistance contribute to heterogeneities in malaria transmission in The Gambia?. Malar. J. 15, 166 (2016).
Slater, H. C. et al. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat. Commun. 10, 1433 (2019).
Guerra, C. A. et al. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasites Vectors 7, 276 (2014).
Gillies, M. T. A Supplement to the Anophelinae of Africa South of the Sahara. 146.
Scott, J. A., Brogdon, W. G. & Collins, F. H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 49, 520–529 (1993).
Fanello, C., Santolamazza, F. & Della Torre, A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP: Identification of An. gambiae species and forms. Med. Vet. Entomol. 16, 461–464 (2002).
Bass, C. et al. PCR-based detection of Plasmodium in Anopheles mosquitoes: A comparison of a new high-throughput assay with existing methods. Malar. J. 7, 177 (2008).
Gnanguenon, V. et al. Transmission patterns of Plasmodium falciparum by Anopheles gambiae in Benin. Malar. J. 13, 444 (2014).
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. (Accessed 20 October 2021)
Acknowledgements
We thank all the study participants for their participation. We also thank microscopists Bekai Njie, Yusupha Bayo and Alhagie Jabbi at Malaria Microscopy Lab of MRCG-LSHTM for their assistance on the slide reading. This work was supported by Malaria Research Capacity Development in West and Central Africa (MARCAD) through DELTAS Africa Initiative Grant DEL-15-010 of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from Wellcome Trust (WT: 107741/A/15/Z) and the UK Government. B.S.A. was supported by MARCAD programme as a postdoc fellow. The funders do not have any responsibility about the conclusion drew by the authors in this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: B.S.A., U.D.A.; data curation: B.S.A., L.C., M.W.; formal analysis: B.S.A., M.W., U.D.A.; funding acquisition: B.S.A., U.D.A.; investigation: B.S.A., S.S., J.A.; methodology: B.S.A., A.N, S.S., S.T.C., M.M.C., L.J.; supervision: B.S.A., U.D.A.; validation: B.S.A., U.D.A.; writing—original draft: B.S.A., U.D.A.; writing-review and editing: B.S.A., K.O.O, M.J., A.D., J.A, U.D.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assogba, B.S., Sillah, S., Opondo, K.O. et al. Anopheles gambiae s.l. swarms trapping as a complementary tool against residual malaria transmission in eastern Gambia. Sci Rep 12, 17057 (2022). https://doi.org/10.1038/s41598-022-21577-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21577-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.