Abstract
Arthropod declines have been linked to agricultural intensification. However, information about the impacts of intensification is still limited for many crops, as is our understanding of the responses of different arthropod taxa and trophic groups, thus hindering the development of effective mitigation measures. We investigated the impacts of olive farming intensification on canopy-dwelling arthropods in the Mediterranean region. Intensification involves the increased use of agrochemicals, mechanisation and irrigation, but also structural changes from traditional orchards with low densities of large and old trees, to intensive and superintensive orchards with high to very high densities of smaller and younger trees, respectively. Canopy arthropods were vacuum-sampled at 53 sites representing the three orchard intensification levels, in spring, summer and autumn 2017. We evaluated how the arthropod community varied across intensification levels, and in response to orchard structure, management and landscape context. We found no changes in the diversity of arthropod taxa across intensification levels after correcting for sample coverage, but arthropod abundance declined markedly along the intensification gradient. Decreased abundance was associated with changes in orchard structure, lower herbaceous cover, and higher herbicide and insecticide use. The abundance of a specialized olive pest was lower in landscapes with higher woodland cover. The negative effects of intensification were stronger in spring and summer than in autumn, and parasitoids and predators were particularly affected. Overall, results suggest that retaining herbaceous cover, reducing agrochemical inputs and preserving natural woody elements in the landscape, may contribute to mitigate impacts of olive farming intensification on canopy arthropods, particularly on beneficial species.
Similar content being viewed by others
Introduction
Arthropods are declining in many terrestrial ecosystems1,2,3, posing a serious threat to ecosystem functioning and human wellbeing, given their key role in ecosystem processes and services4,5,6. Agricultural intensification is considered one of the main drivers of arthropod declines, both at local and landscape scales3,7. However, information about the impacts of intensification is still lacking for many crops. Moreover, little is known about whether different arthropod taxa and trophic groups are equally vulnerable to intensification3. Addressing these issues is thus critical to understand if and how intensified agriculture can be managed to mitigate negative impacts on arthropods.
Olive (Olea europaea L. 1753) is one of the crops that has rapidly expanded and intensified in recent decades, mainly due to a combination of agricultural policies and market mechanisms8,9. Cultivation of this crop is largely concentrated in the Mediterranean biodiversity hotspot10,11, where olive orchards have been part of rural landscapes for millennia12 and harbour considerable arthropod diversity13,14. Traditional orchards are rain fed, typically composed of large and old trees (> 50 years) planted at low densities (ca. 100 trees ha−1), and are managed with reduced or no use of agrochemicals and mechanisation15,16. The soil is often tilled to remove vegetative cover between trees17,18, but a herbaceous layer may be retained under organic production models19,20. Over the past decades, these orchards have been replaced by larger and more intensively managed ones, that are irrigated, spray insecticides to control pests and herbicides to reduce the herbaceous layer, and have mechanical harvesting15,16. Intensification has also involved major changes in orchard structure, with intensive orchards characterized by high densities of relatively small and young trees (200–450 trees ha−1) planted at regular intervals, and superintensive orchards characterized by very high densities (1500–2000 trees ha−1) of dwarf tree varieties planted as hedgerows21,22. Despite ongoing intensification, these three levels of structural intensity still coexist at regional and even landscape levels9,23.
These changes, together with landscape simplification resulting from the ongoing expansion of intensified olive farming, are likely to have considerable impacts on arthropod diversity. Until now, however, most studies about the intensification of olive farming have focused on vertebrates, showing major declines in bat and breeding bird diversity and abundance from traditional, through intensive, to superintensive orchards24,25,26, and in response to increased landscape simplification25. Regarding arthropods, various studies have examined the effects of intensifying some management practices in olive orchards, showing that communities tend to be more diverse and/or abundant in orchards with reduced or no insecticide spraying, and where ground cover is present13,14,27,28,29,30. Some of these studies also explored the effects of landscape simplification on arthropods, reporting mostly negative, but also neutral or even positive effects14,29,30. However, detailed investigation of the effects of orchard structure on arthropods is still lacking, as previous studies were mostly carried out in orchards structurally similar to the traditional archetype, with none assessing superintensive orchards. This is an important aspect to consider as, for instance, large and old trees in traditional orchards might provide cavities and other refuges for a range of arthropod species31,32, which are generally absent in the younger and smaller trees of intensive and superintensive orchards. Therefore, examining the entire intensification gradient, while also considering changes in the landscape context, is essential to fully understand the impacts of olive farming intensification on arthropods.
Here, we examine how orchard- and landscape-level changes along an intensification gradient influence olive tree canopy-dwelling arthropod communities. We focused on canopy-dwelling arthropods, because they represent a complex community with a high diversity of taxonomic and functional groups and appear to be particularly sensitive to management practices in olive orchards13,27. We considered the overall community as well as functional trophic groups, because arthropod responses to disturbance might be strongly influenced by ecological traits33,34, in particular those related to feeding strategy35,36. The study was carried out from spring to autumn as arthropod responses are likely to vary due to seasonal changes in orchard management and weather conditions (e.g., rain and temperature). For instance, there might be stronger negative responses in periods with more concentrated insecticide use to control pests (e.g., olive moth on flowers in spring and olive fruit fly on olives in autumn)24. Likewise, greater resource availability in some seasons (e.g., more food and shelter associated with the herbaceous understory in spring) might differentially affect arthropod responses. Moreover, harsher weather in some seasons (e.g., hot and dry conditions in summer) could lead to more pronounced negative effects of intensification. Specifically, the goals of our study were to understand how the arthropod community varies across orchard intensification levels (traditional, intensive and superintensive), and in response to orchard structure, management and landscape context. Community variation was assessed in terms of (1) overall diversity and abundance, (2) diversity and abundance of trophic groups, and (3) abundance of individual taxa. We further examined (4) whether these responses varied across seasons.
Material and methods
Study area and sampling design
The study was conducted in the Alentejo region, southern Portugal (Fig. 1). The climate is Mediterranean, with mean daily temperatures ranging from 5.8 to 12.8 °C in winter and from 16.3 to 30.2 °C in summer37. Annual rainfall (500–600 mm) is largely limited to October–March. The landscape is mildly undulating (100–400 m a.s.l.) and dominated by open agricultural land, cork (Quercus suber) and holm oak (Q. rotundifolia) woodlands, olive orchards (Olea europaea) and vineyards (Vitis vinifera). In Alentejo, widespread intensification of olive farming has taken place in recent decades9, with the region currently accounting for ca. 75% of national olive production and containing approximately half of the Portuguese olive growing area (ca. 185,000 ha)38, including most of the recently established intensive and superintensive orchards (ca. 40,000 ha)39. Expansion of intensive olive farming in this area has been promoted by subsidies under the European Union Common Agricultural Policy (CAP), the increased market demand for olive oil, and the availability of new irrigation infrastructures9,40.
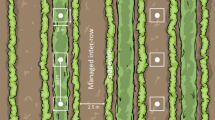
Location of the 53 points sampled for canopy-dwelling arthropods in traditional, intensive and superintensive orchards across the Alentejo region, southern Portugal. The two inset maps show the northern and southern sections of the study area in detail. Along the top are photographs representing traditional (left), intensive (middle) and superintensive (right) orchards. The maps were generated using the free and open source Geographic Information System QGIS v3.10 (https://qgis.org/en/site/).
Sampling of canopy arthropods was made at points previously selected by Costa et al.25, following a stratified random procedure designed to represent the greatest possible range of variation in orchard structure. Initially, we selected 60 sampling points within 38 olive orchards across the study region, but excluded seven points located in three orchards that were largely unmanaged, and in two orchards for which no agrochemical information was obtained. A total of 53 points in 33 orchards was thus retained (Fig. 1). We established one point per orchard, except in the largest orchards where we established a maximum of three points at least 500 m apart. All points were placed as far as possible from orchard edges. Consent to carry out the study at the selected orchards was obtained from the respective land-owners and/or managers.
Arthropod sampling
Canopy arthropods were vacuum sampled using a Modified CDC backpack aspirator model 1412 (John W. Hock Company, Gainsville, FL, USA). This method was chosen because it enables a rapid and efficient collection of canopy arthropods41, it provides data representative of arthropod community structure42, and has been extensively used for sampling olive tree canopies43,44,45. It should be noted, however, that vacuum sampling is less efficient for more mobile insects, including two of the major olive pests: olive moth (Prays oleae; Praydidae) and olive fruit fly (Bactrocera oleae; Tephritidae). Due to logistical constraints it was impractical to carry out the targeted sampling using traps or lures that are necessary to efficiently sample these insects46, and so results for families Praydidae and Tephritidae need to be interpreted with care. Sampling was carried out in spring (19 April–21 May), summer (14–29 June) and autumn (19 September–10 October) of 2017. This spans the periods of highest arthropod activity47 and coincides with the olive tree’s flowering, fruit development and ripening stages48, respectively, when the main agrochemical treatments are undertaken24,49. In common with several other studies45,49,50,51, sampling could only be carried out in one year, which limits the power of the conclusions. However, despite potential interannual fluctuations in arthropod populations1,52, this timeframe was deemed reasonable as it is less likely that responses across orchard intensification levels also vary widely between years53,54. At each sampling point, five trees were marked, one located at the centre of the point and the other four at 20 m from the centre, approximately in the four cardinal directions. Each tree at each sampling point was aspirated once per season for one minute, yielding a total of 795 samples (53 sampling points × 5 trees × 3 seasons = 795 aspirations).
Specimens were stored in ethanol (70%) and subsequently identified to family level, because more detailed identification of every specimen was too time-consuming and/or impractical due to lack of taxonomic expertise. However, family-level identification can detect the effects of human-induced disturbance on terrestrial arthropod communities with little loss of information55,56,57. Nomenclature and taxonomy followed widely used entomological references58,59,60,61,62,63,64. Specimens from orders Ephemeroptera, Psocoptera, Thysanoptera and sub-order Nematocera could not be identified to family level, corresponding to 35.2% of 4665 collected arthropods. All arthropods successfully identified to family level (n = 3024) were assigned to one of four trophic groups based on their diets and feeding strategy: herbivores, predators, parasitoids and scavengers. Families reported to feed predominantly on arthropods, plant matter and dead matter and/or fungi, were classified as predators, herbivores and scavengers, respectively. Parasitoids have different feeding habits as larvae and adults, but were classified in a single separate group because of their parasitic nature. Chrysopidae also change their feeding habits during their life cycle but were classified as predators due to their importance as predators of olive tree pests65. Although the family Formicidae is composed of predator, scavenger and indirect herbivore species66, ants in olive tree canopies are mostly predatory67 and were thus also classified as predators. An additional “mixed” category was created for arthropod families composed of species with a mixture of feeding strategies.
Environmental variables
At each sampling point (n = 53) we characterized orchard structure (Table 1) and management (Table 2). Six variables characterizing orchard structure were measured in the field following the procedures described in Costa et al.25, and included: tree age, trunk diameter at breast height (dbh), trunk height, canopy volume, intra-row and inter-row tree distances (Table 1). We then performed a clustering analysis on these variables, which confirmed that the selected orchards represented three well-defined structural intensification levels: traditional, intensive and superintensive (for details see Supplementary methods). Two of the variables characterising orchard management were also estimated in the field, including presence/absence of irrigation (assessed based on the presence of a drip irrigation system), and herbaceous cover (assessed visually as the percentage of inter-row herbaceous cover). Two other management variables were obtained from enquiries to the land-owners and/or managers, including: presence/absence of herbicide application to control herbaceous ground cover, and presence/absence of insecticide spraying on canopies to control insect pests. Only binary variables could be used to characterise agrochemical applications, because the timing and quantities applied at each orchard were often not disclosed during enquiries. Likewise, information about the types of herbicides and insecticides used could only be obtained for part of the orchards. Herbaceous cover and herbicide and insecticide application were estimated for each sampling season, because they were expected to vary over time at each point. Enquiries were carried out in accordance with Portuguese legislation relative to statistical confidentiality (Law No. 22/2008 of 13 May—article 6), as well as the General Data Protection Regulation (EU) 2016/679 (GDPR).
The potential effects of landscape context on local arthropod communities were also accounted for68,69, considering the percentage cover by dominant land uses in the region within a 500-m radius around each sampling point, namely: cork and holm oak woodlands, olive orchards, and open agricultural land26. This radius was selected because many arthropod taxa in olive orchards29,70 and in other crops71 are known to respond to landscape composition at this spatial scale. Due to intercorrelations between the cover by the dominant land use types, only cork and holm oak woodland cover was used in analyses (Table 2). These woodlands are highly biodiverse72 and are the most widespread semi-natural habitat around olive orchards in southern Portugal73. Semi-natural habitats can act as sources of food, shelter, as well as breeding sites for numerous arthropods, including those that provide key services such as natural biocontrol68,69. Landscape composition was obtained in a Geographic Information System environment, from Portugal’s digital land cover map of 201574, which was the date most closely matching the sampling year (2017).
Data analysis
We estimated arthropod community diversity for each intensification level and season, using the Hill diversity framework75 and the package iNEXT76, following Penado et al.77. To account for the effects of rarity on diversity estimates, we computed taxa richness (Hill number q = 0), and Hill-Shannon (q = 1) and Hill-Simpson (q = 2) diversities, because increasing the Hill number reduces the leverage of rare taxa75. Diversity estimates for a given intensification level were made by pooling all individuals collected at all sampling points across seasons, and then separately for each season. Estimates were made considering identifications to the highest possible taxonomic resolution, that is, family level except for orders Ephemeroptera, Psocoptera, Thysanoptera and sub-order Nematocera. Because diversity metrics are sensitive to sampling effort and relative abundance, we standardised diversity estimates to equal-coverage75,77, with coverage estimating the proportion of individuals in the (whole) community that belong to the taxa present in the sample75. To standardise diversity estimates, we first assessed variation in sample coverage with increasing number of individuals identified, and then set a fixed coverage value, corresponding to the minimum coverage obtained when each sample (traditional, intensive, superintensive) is extrapolated to double the number of individuals identified75,77. Diversity estimates (± 95% Confidence Intervals) were obtained at the fixed coverage value, either by extrapolation for samples with coverage lower than the standard value, or through rarefaction otherwise75,77.
We then examined the effects of intensification on community- and taxon-level abundance, and how such effects depend on ecological traits, using the Hierarchical Modelling of Species Communities (HMSC) framework78. Although HMSC also produces estimates of community richness, these were not used because they are uncorrected for sample coverage, and can thus be highly influenced by the number of individuals collected at each sampling point. HMSC is a class of hierarchical Bayesian joint species distribution models that relate community- and taxon-level responses to environmental covariates, while determining whether responses are influenced by taxon traits and also accounting for random effects related to study design and spatial dependencies78. For the analyses, we built a community matrix (sites/orchard/season × taxaall) pooling data for each arthropod taxon (orders/sub-orders and families) from the five replicate olive trees per sampling point per season. We then built a second community matrix (sites/orchard/season × taxafamilies) where only data at family level were used, thereby excluding Ephemeroptera, Psocoptera, Thysanoptera, and sub-order Nematocera. The environmental matrix (sites/season × covariates) was built considering as covariates the orchard intensification levels, their structural, management and landscape variables (Tables 1, 2), and season (spring, summer, autumn) depending on the particular analysis performed (see below). The trait matrix (taxa × traits) was built considering the assignment of each arthropod family to one of the five trophic groups. We also considered a matrix of site coordinates to account for spatial dependencies78.
In model building, we used abundance (count) community matrices as response variables, assuming a lognormal Poisson distribution and log-link function to allow for more variation around the expectation78. We used the first community matrix (sites/orchard/season × taxaall) and the environmental matrix to build an initial model considering only season and orchard intensification levels, as well as their interaction terms, to evaluate broad changes in community patterns along the intensity gradient over the seasons. We then built a second model using structural, management and landscape covariates, and their interactions with season, thereby evaluating community responses to different aspects of intensification and whether responses varied seasonally. The structural variables were all correlated, therefore, we only retained trunk diameter at breast height (dbh) to avoid multicollinearity79. This variable was selected because it was the most strongly correlated with the first dimension of the PCA (Supplementary Table S1), which accounted for 76.4% of the variation in orchard structure (Supplementary Fig. S2). Irrigation was dropped from the analysis because it was strongly correlated with dbh (r = − 0.916, P < 0.001). Although the correlation between dbh and insecticide use was also high (r =− 0.648, P < 0.001), they were both retained because the value was below the threshold of r = 0.779 (Supplementary Table S2). In all the models we included an orchard-level random effect to account for similar management practices in sampling points belonging to the same orchard, a site-level random effect to control for repeated visits to the same sampling point, and the matrix of site coordinates for spatial dependencies. We subsequently repeated the entire process, using the second community matrix (sites/orchard/season × taxafamilies) together with the trait matrix, to evaluate changes in trophic group responses along the intensification gradient across seasons. The fitted models were used to predict abundance for the overall community and for each trophic group, by summing the corresponding predicted abundance of individual taxa. Statistical support for differences in arthropod abundance between orchard intensification levels, and in response to structural, management and landscape variables, was assessed from the overlap between 84% credible intervals (CI). These intervals more adequately approximate an α = 0.05 error rate, while comparisons using 95% intervals are overly conservative80. Non-overlapping CIs thus provide statistical support for differences at α ~ 0.0580.
Models were fitted to the data with the HMSC-R package81 for R82. Each model was run with five Markov chains for 250,000 iterations, from which the first 150,000 were discarded as transient. We assessed model convergence by inspecting the trace plots of β estimates and the width of credible intervals of model estimates, discarding rare taxa (< 10 individuals) as this improved convergence. The models’ explanatory power was evaluated using R22,81.
Results
Overall community composition
Twelve arthropod orders were identified from the 4665 collected specimens: Araneae, Coleoptera, Diptera, Ephemeroptera, Hemiptera, Hymenoptera, Lepidoptera, Neuroptera, Orthoptera, Psocoptera, Raphidioptera and Thysanoptera (Supplementary Table S3). Ninety families were identified from nine of these orders (3024 specimens), of which four families accounted for half of the identified arthropods: Psyllidae, composed entirely of the secondary olive pest Euphyllura olivina (Hemiptera) 29.6%, Scelionidae (Hymenoptera) 9%, Oxyopidae (Araneae) 6.3%, and Chrysopidae (Neuroptera) 5.4%.
Community diversity across orchard intensification levels
A higher number of taxa was collected in traditional orchards than in either intensive and superintensive orchards, while sample coverage was very high and similar across orchard types (97–99%), though slightly decreasing across the intensification gradient (Table 3). However, after standardising for sample coverage, the estimates of taxa richness were lower in traditional than in both intensive and superintensive orchards, while Hill-Shannon and Hill-Simpson diversities increased along the intensification gradient, albeit with large and partly overlapping confidence intervals. In spring, the number of taxa collected and sample coverage declined across intensification levels, while taxa richness estimates corrected for coverage increased from traditional to intensive orchards, and were lowest in superintensive orchards; Hill-Shannon and Hill-Simpson diversities increased with intensification. In summer the patterns were mostly similar for taxa collected, coverage and Hill-Simpson estimates, while taxa richness and Hill-Shannon diversity tended to decrease from traditional through intensive to superintensive orchards. In autumn the patterns of variation in relation to intensification level were broadly similar to spring (Table 3).
Arthropod responses to orchard intensification levels
The proportion of explained variation varied widely between taxa in the HMSC model (R2: 0.04–0.79; mean ± SD: 0.19 ± 0.15). Orchard intensification level accounted for over a third of the explained variation in taxon abundance (35.1 ± 15.8%; 9.5–89.7%), while almost half was accounted for by season (46.4 ± 21.0%; 0.8–88.4%) (Fig. 2). Overall, trophic group was responsible for 15.3% of the variation in arthropod responses to covariates. The effects of trophic group were strongest in superintensive orchards in summer, when they were responsible for 35% of the variation in taxon responses (Supplementary Table S4).
Proportion of variance in total arthropod abundance explained by orchard intensification level, season, sampling point-level, orchard-level and spatial random effects. The proportions of variance explained by fixed and random effects are shaded in tones of green and grey, respectively. Arthropod drawings by Juan Pablo Cancela.
Models revealed a strong seasonal change in total arthropod abundance, which declined from spring to autumn (Fig. 3a). These declines occurred mainly within traditional orchards, while less seasonal variation was observed in intensive and superintensive orchards (Fig. 3a). Models also revealed general arthropod declines with orchard intensification, although responses varied across seasons, trophic groups and individual taxa (Figs. 3, 5a). Total abundance was highest in traditional orchards and similar in intensive and superintensive orchards in spring, while tending to decline across intensification levels in summer, and showing little variation in autumn (Fig. 3a). There was statistical support for higher predator abundance in traditional orchards than in intensive and superintensive orchards in spring, while in summer and autumn there was little variation between intensification levels (Fig. 3d). Herbivore abundance was marginally lower in superintensive than in traditional orchards in summer, but no statistically supported differences were found in other periods (Fig. 3b). Parasitoid abundance was highest in traditional orchards in spring, lowest in superintensive orchards in summer, and declined across intensification levels in autumn (Fig. 3c). Scavengers and arthropods from the mixed category were much less abundant than the other groups, but still followed the general patterns of stronger negative responses to intensification in spring and summer than in autumn (Fig. 3e,f). Regarding individual taxa, most statistically supported effects of intensification were negative, and more so in spring (Fig. 5a).
Seasonal variation in total arthropod abundance and abundance per trophic group predicted from Hierarchical Modelling of Species Communities (posterior mean and 84% credible intervals), across orchard intensification levels: traditional (squares), intensive (circles) and superintensive (triangles). Arthropod drawings by Juan Pablo Cancela.
Arthropod responses to orchard structure, management and landscape context
Models using structural, management and landscape variables performed similarly to those based only on orchard intensification level, with the proportion of explained variation also varying widely between taxa (R2: 0.03–0.81; mean ± SD: 0.20 ± 0.16). The structural covariate (dbh) accounted on average for the largest proportion of explained variation (21.2 ± 8.1%; 4.4–38.6%), followed by season (19.0 ± 14.8%; 1.0–56.3%), herbaceous cover (15.4 ± 5.0%; 0.6–29.6%) and woodland cover (9.7 ± 4.9%; 2.2–24.1%). Herbicide (8.4 ± 3.7%; 0.9–16.6%) and insecticide (8.2 ± 3.6%; 1.1–13.6%) applications accounted for the lowest proportions of explained variation (Supplementary Fig. S4). Overall, trophic group was responsible for 21.4% of variation in arthropod responses to covariates. The effects of trophic group were particularly strong regarding responses to the use of herbicides in summer (45%) and of insecticides in spring (57%) and summer (59%) (Supplementary Table S5).
Arthropod abundance showed generally positive responses to the structural variable included in the models (dbh), although responses varied across seasons, trophic groups and individual taxa (Figs. 4, 5b). There was statistical support for total arthropod abundance increasing with dbh in spring, and for parasitoid abundance increasing with dbh in summer (Fig. 4). Parasitoid abundance also increased marginally with dbh in spring, while herbivore abundance increased marginally with dbh in summer. Weak responses were detected for the remaining trophic groups (Fig. 4). Statistically supported effects of dbh on individual taxa were mostly positive and observed in spring and summer (Fig. 5b). Regarding management variables, there was no statistical support for total and trophic group abundance varying with herbicide use (Fig. 4), although many individual taxa were negatively affected in summer (Fig. 5b). There was also no variation in total and trophic group abundance with insecticide use, except for marginal declines in scavenger abundance in summer and autumn (Fig. 4). Although several individual taxa were negatively affected by insecticide use, a few were positively affected in summer (Fig. 5b). Predator abundance increased marginally with herbaceous cover in spring, while total abundance and that of the other trophic groups showed weak responses to this covariate (Fig. 4). There were also positive effects of herbaceous cover on the abundance of some taxa, particularly in spring (Fig. 5b). Arthropod responses to woodland cover were generally weak (Fig. 4), although there was statistical support for a negative effect on two herbivorous taxa in spring and summer, one of which was family Psyllidae (Fig. 5b), composed entirely of the pest E. olivina.
Variation in total abundance and abundance per trophic group predicted from Hierarchical Modelling of Species Communities (posterior mean and 84% credible intervals) in relation to trunk diameter at breast height (dbh), herbaceous cover, herbicide use, insecticide use and woodland cover in spring (green), summer (red) and autumn (blue). The average percentage of variance in arthropod abundance explained by all environmental variables together, for all arthropods and per trophic group, is provided in brackets at the end of each row of panels. Within each panel, the average percentage of variance relative to the total amount, and accounted for by each variable, is presented. Arthropod drawings by Juan Pablo Cancela.
Heatmaps of effects (posterior estimates of β obtained from Hierarchical Modelling of Species Communities) of orchard intensification level, season, and their interaction (a), and of structural, management and landscape variables, and their interactions with season (b) on individual taxon abundance. The represented responses received high statistical support (posterior probability > 95%) of being positive (blue) or negative (red). Arthropod drawings by Juan Pablo Cancela.
Discussion
Our study revealed strong effects of olive farming intensification on canopy arthropods, though the patterns observed varied depending on the community parameters analysed (diversity versus abundance), trophic group and season. We found no consistently negative effects of intensification on arthropod diversity, but there were generally marked declines in abundance from traditional, through intensive, to superintensive orchards. These declines were mainly related to changes in orchard structure across the intensification gradient, together with the removal of herbaceous cover and use of agrochemicals, while landscape context showed minimal effects. The negative effects of intensification were more pronounced in spring and summer than in autumn, and were strongest for parasitoid and predator abundance. Taken together, our results suggest that the ongoing intensification of olive production will likely lead to declines in the abundance of canopy arthropods, including beneficial taxa, though there may be management options to at least partly mitigate such effects.
The lack of negative effects of intensification on diversity is surprising as other studies have reported reduced arthropod diversity in more intensive agricultural fields83,84, including in olive orchards51,85. It is unlikely that this was due to the relatively coarse taxonomic resolution used in our study, as others have found intensification effects using identifications only up to family or even order levels28,29,86. However, most previous studies did not standardise diversity estimates to equal sample coverage, and so responses could be confounded with that of sampling effort or community abundance75,77. Our results illustrate this potential shortcoming, as we found that numbers of both taxa and individuals collected tended to decline as expected along the intensification gradient, but the negative effects on diversity either disappeared or even reversed after standardising diversity estimates. This occurred despite very high coverages (92–99%), which suggests that our sampling captured the abundant taxa dominating local communities, but missed a large number of rarer taxa, particularly in more intensive orchards75. Traditional orchards might thus support higher diversity of abundant taxa (i.e., the raw richness estimates) than more intensive orchards, but the latter appear to have a larger proportion of rarer taxa that increased total diversity. It can be hypothesized that less abundant and probably less stable arthropod communities in more intensive orchards (see below), are more susceptible to the arrival of a large number of external rare taxa, thereby artificially inflating total diversity of the system.
In contrast to diversity, arthropod abundance declined along the intensification gradient, with effects found at the community, trophic group and individual taxa levels. These declines were at least partly related to changes in orchard structure, as suggested by lower total arthropod and parasitoid abundance in orchards with smaller tree dbh, which was used as a proxy for structural intensification. It is uncertain, however, whether dbh was indeed the main driver of arthropod responses, because structural variables were highly intercorrelated, and so their effects were hard to disentangle. For instance, superintensive orchards have small and young (low dbh) trees with low canopy volume, but planted at high densities, which may be detrimental for thermophilous Mediterranean insects that appear to favour open canopy plantations87. Seemingly, smaller and younger trees in more intensive orchards do not have the large and complex trunks with many cavities and crevices characteristic of trees in traditional orchards, which may be more favourable to support populations of many arthropod taxa31,32. Therefore, it is likely that arthropod responses to orchard structure resulted from the joint changes in tree density, age and size along the intensification gradient.
Besides orchard structure, arthropod abundance also seemed to respond to orchard management in line with the results of previous studies30,43, though some effects (e.g., of herbicide and insecticide use) were weaker than expected. This problem might be associated to some extent with using relatively coarse variables, particularly in the case of agrochemicals. In fact, we could only use presence/absence variables to characterise herbicide and insecticide use, because more detailed information was not disclosed by most farmers, thereby reducing the power to detect their effects. However, it is likely that the paucity of management effects was also associated with collinearity problems, making it more difficult to disentangle the unique effects of management versus structural variables. For instance, irrigation was very highly correlated with dbh (r = − 0.916), and thus had to be removed altogether from the analysis. Herbaceous cover, herbicide and insecticide use were retained in the analysis, but negative effects were mostly observed on individual taxa and only on two trophic groups (predators and scavengers). These results suggest that variations in arthropod communities observed in our study were mainly a consequence of the drastic transitions in structure and management practices that occur from traditional, through intensive, to superintensive orchards, rather than reflecting responses to individual variables.
Landscape cover by oak woodlands (semi-natural habitat) influenced arthropod abundance in olive orchards, though responses were weak compared to previous research29,47. This could be related to the small amount of oak woodlands in the landscapes studied, which might be too small to significantly affect arthropod abundance at orchard level88. For instance, 70% of the sampling points were surrounded by less than 10% woodland cover within the 500-m radius buffer. It is also plausible that higher intensity of agricultural practices in more intensive orchards (60% of sampling sites) diluted or counteracted the beneficial effects of available semi-natural habitat88. Moreover, the intensive and superintensive orchards studied were often embedded in landscapes dominated by intensified olive farming, further limiting the potential effects of semi-natural habitats on local arthropod abundances. It is noteworthy, however, that there were negative effects of woodland cover on the specialized olive tree pest, E. olivina. This provides some support for the idea that semi-natural habitats can limit the movement and spread of pests89, and thus contribute to the reduction of olive pest abundance25,90,91.
The effects of intensification observed in our study were most pronounced for predators and, particularly, for parasitoids, which is consistent with previous research showing strong intensification effects on arthropods at higher trophic levels92. Parasitoid abundance was lower in structurally more intensive orchards, as indicated by its increase with dbh, though it is uncertain whether responses were mainly driven by orchard structure itself, or by management practices related to it (see above). For instance, dbh was negatively correlated with the use of insecticides, consisting mainly of broad-spectrum dimethoate, which can play a role in parasitoid declines13,93. Herbivore abundance was also lower in structurally more intensive orchards, which might also be a consequence of insecticide use, and can affect parasitoids through reduced host availability. Predators increased with herbaceous cover, which is in line with studies suggesting that the herbaceous layer is an important source of predatory arthropods in olive orchards45,94. Finally, both parasitoids and predators responded negatively to herbicides, which may reflect direct lethal effects of their application, but also indirect effects mediated by reduced abundance of herbivorous hosts and prey, and reduced cover and habitat quality of the herbaceous layer. Overall, these results suggest that structural and management changes along the intensification gradient can have far-reaching negative effects on predators and parasitoids, potentially compromising the provision of natural biocontrol95,96.
Arthropod responses to intensification varied across seasons, with generally stronger effects in spring than in summer and, particularly, than in autumn. Spring had the largest differences in total arthropod abundance, and in parasitoid and predator abundance, between traditional and more intensive orchards. This might be because there were better conditions in traditional orchards for arthropods to overwinter and then rapidly build their populations up in spring, due for instance to a more developed herbaceous layer and lower usage of agrochemicals94,97. In summer, declines in abundance along the intensification gradient were less pronounced, though there were still strongly negative effects of superintensive orchards on parasitoids and the mixed group, as well as on several individual taxa. Summer was also the period when we found the strongest negative effects of herbicides on the abundance of several arthropod taxa. The reasons for this are uncertain, but one possibility is that more herbicides were applied in summer than in other periods, though data to test this idea was not collected. Another possibility is that the pattern observed was related to the harsh summer conditions, as high temperatures and reduced moisture can decrease the ability of arthropods to cope with very intensive management regimes98. For instance, herbicides could have had more lethal effects when there were additional stresses associated with the harsh summer conditions, while chemical removal or degradation of the herbaceous layer might have reduced the availability of refuges to endure such conditions. Irrespective of the causes, these results suggest that disregarding seasonal effects may hinder a complete understanding of the effects of olive farming intensification on canopy arthropods.
Conclusions
Our study adds to previous research showing strong negative effects of olive farming intensification on biodiversity25,26. In our case, these negative effects primarily involved marked declines in canopy arthropod abundance, particularly that of parasitoid and predator trophic groups, which were most evident in the superintensive orchards. Such declines appeared to be driven by the drastic changes in orchard structure and management occurring across the three main intensification levels, probably reflecting responses to overall shifts in olive farming systems rather than to any specific structural or management variable99,100. Still, ours and previous studies suggest that impacts of intensification could be less pronounced in orchards with reduced use of agrochemicals and with a more developed herbaceous layer13,94, though the feasibility and consequences of implementing such practices in intensive and superintensive orchards is still uncertain. Likewise, there is some support for the importance of maintaining semi-natural habitats at the landscape scale to reduce biodiversity impacts and pest abundance within orchards25,101, but quantification of the amount and types of habitats required to achieve such goals is still lacking. Overall, our results suggest that the rapid expansion of intensive and superintensive orchards is negatively impacting arthropod communities that inhabit Mediterranean farmland. This can reduce the ability of beneficial groups such as parasitoids and predators to deliver valuable biocontrol services, and it can propagate up the food chain to negatively affect birds and bats24,102. In particular, longer (i.e., multi-year) sampling would be desirable to fully understand how intensification influences arthropod communities in the long term. This could also enable us to determine how impacts can be mitigated in more intensive production systems, and how the implementation of mitigation measures can be supported through public incentives and market mechanisms103.
Data availability
All supplementary information can be downloaded from the journal’s website. Data and code are provided in the Supplementary Information files.
References
Seibold, S. et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574, 671–674 (2019).
van Klink, R. et al. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368, 417–420 (2020).
Wagner, D. L. Insect declines in the anthropocene. Annu. Rev. Entomol. 65, 457–480 (2020).
Wilson, E. O. The little things that run the world (the importance and conservation of invertebrates). Conserv. Biol. 1, 344–346 (1987).
Isaacs, R., Tuell, J., Fiedler, A., Gardiner, M. & Landis, D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 7, 196–203 (2009).
Hallmann, C. A. et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12, e0185809 (2017).
Raven, P. H. & Wagner, D. L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. U.S.A. 118, e2002548117 (2021).
Neves, B. & Pires, I. M. The Mediterranean diet and the increasing demand of the olive oil sector: Shifts and environmental consequences. Region. 5, 101–112 (2018).
Silveira, A. et al. The sustainability of agricultural intensification in the early 21st century: Insights from the olive oil production in Alentejo (Southern Portugal). In Changing Societies: Legacies and Challenges. The Diverse Worlds of Sustainability, 247–275 (2018).
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Salomone, R. & Ioppolo, G. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily). J. Clean. Prod. 28, 88–100 (2012).
Rallo, L. et al. High-density olive plantations. Hortic. Rev. Am. Soc. Hortic. Sci. 41, 303–383 (2013).
Santos, S. A. P., Pereira, J. A., Torres, L. M. & Nogueira, A. J. A. Evaluation of the effects, on canopy arthropods, of two agricultural management systems to control pests in olive groves from north-east of Portugal. Chemosphere 67, 131–139 (2007).
Gkisakis, V., Volakakis, N., Kollaros, D., Bàrberi, P. & Kabourakis, E. M. Soil arthropod community in the olive agroecosystem: Determined by environment and farming practices in different management systems and agroecological zones. Agric. Ecosyst. Environ. 218, 178–189 (2016).
Beaufoy, G. EU Policies for Olive Farming. Unsustainable on all counts (WWF and Birdlife International, Brussels, 2001).
EFNCP. The environmental impact of olive oil production in the EU: Practical options for improving the environmental impact. European Forum on Nature Conservation and Pastoralism & Asociación para el Análisis y Reforma de la Política Agro-rural, Brussels. https://ec.europa.eu/environment/agriculture/pdf/oliveoil.pdf (2000).
Vanwalleghem, T. Quantifying the effect of historical soil management on soil erosion rates in Mediterranean olive orchards. Agric. Ecosyst. Environ. 142, 341–351 (2011).
Simões, M. P., Belo, A. F., Pinto-Cruz, C. & Pinheiro, A. C. Natural vegetation management to conserve biodiversity and soil water in olive orchards. Span. J. Agric. Res. 12, 633–643 (2014).
Milgroom, J., Soriano, M. A., Garrido, J. M., Gómez, J. A. & Fereres, E. The influence of a shift from conventional to organic olive farming on soil management and erosion risk in southern Spain. Renew. Agric. Food Syst. 22, 1–10 (2007).
Lodolini, E. M. & Neri, D. Organic olive farming. African J. Agric. Res. 8, 6426–6434 (2013).
Rallo, L. Iberian olive growing in a time of change. Chron. Horticult. 49, 27–30 (2010).
Diez, C. M. et al. Cultivar and tree density as key factors in the long-term performance of super high-density olive orchards. Front. Plant Sci. 7, 1–13 (2016).
Allen, H. D., Randall, R. E., Amable, G. S. & Devereux, B. J. The impact of changing olive cultivation practices on the ground flora of olive groves in the Messara and Psiloritis regions, Crete, Greece. L. Degrad. Dev. 17, 249–327 (2006).
Herrera, J. M., Costa, P., Medinas, D., Marques, J. T. & Mira, A. Community composition and activity of insectivorous bats in Mediterranean olive farms. Anim. Conserv. 18, 557–566 (2015).
Costa, A. et al. Structural simplification compromises the potential of common insectivorous bats to provide biocontrol services against the major olive pest Prays oleae. Agric. Ecosyst. Environ. 287, 106708 (2020).
Morgado, R. et al. A Mediterranean silent spring? The effects of olive farming intensification on breeding bird communities. Agric. Ecosyst. Environ. 288, 106694 (2020).
Ruano, F. et al. Use of arthropods for the evaluation of the olive-orchard management regimes. Agric. For. Entomol. 6, 111–120 (2004).
Jerez-Valle, C., García, P. A., Campos, M. & Pascual, F. A simple bioindication method to discriminate olive orchard management types using the soil arthropod fauna. Appl. Soil Ecol. 76, 42–51 (2014).
Carpio, A. J., Castro, J. & Tortosa, F. S. Arthropod biodiversity in olive groves under two soil management systems: Presence versus absence of herbaceous cover crop. Agric. For. Entomol. 21, 58–68 (2018).
Rey, P. J. et al. Landscape-moderated biodiversity effects of ground herb cover in olive groves: Implications for regional biodiversity conservation. Agric. Ecosyst. Environ. 277, 61–73 (2019).
Mccomb, W. C. & Noble, R. E. Invertebrate use of natural tree cavities and vertebrate nest boxes. Am. Midl. Nat. 107, 163–172 (1982).
Bovyn, R. A., Lordon, M. C., Grecco, A. E., Leeper, A. C. & LaMontagne, J. M. Tree cavity availability in urban cemeteries and city parks. J. Urban Ecol. 5, 1–9 (2019).
Ribera, I., Dolédec, S., Downie, I. & Foster, G. Effect of land disturbance and stress on species traits of ground beetle assemblages. Ecology 82, 1112–1129 (2001).
Barbaro, L. & van Halder, I. Linking bird, carabid beetle and butterfly life-history traits to habitat fragmentation in mosaic landscapes. Ecography 32, 321–333 (2009).
Steffan-Dewenter, I. & Tscharntke, T. Butterfly community structure in fragmented habitats. Ecol. Lett. 3, 449–456 (2000).
Gámez-Virués, S. et al. Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 6, 8568 (2015).
Medinas, D. et al. Road effects on bat activity depend on surrounding habitat type. Sci. Total Environ. 660, 340–347 (2019).
INE. Estatísticas Agrícolas – 2018. Lisboa. Instituto Nacional de Estatística. https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_publicacoes&PUBLICACOESpub_boui=358629204&PUBLICACOESmodo=2 (2019).
Rodríguez-Cohard, J. C., Sánchez-Martínez, J. D. & Garrido-Almonacid, A. Strategic responses of the European olive-growing territories to the challenge of globalization. Eur. Plan. Stud. 28, 2261–2283 (2020).
Reis, P. O olival em Portugal. Dinâmicas, tecnologias e relação com o desenvolvimento rural. Instituto Nacional de Investigação Agrária e Veterinária. http://www.iniav.pt/fotos/editor2/caderno_olivalemportugal.pdf (2014).
Yi, Z., Jinchao, F., Dayuan, X., Weiguo, S. & Axmacher, J. C. A comparison of terrestrial arthropod sampling methods. J. Resour. Ecol. 3, 174–182 (2012).
Leather, S. R. Insect Sampling in Forest Ecosystems (Wiley-Blackwell, New Jersey, 2008).
Paredes, D., Cayuela, L. & Campos, M. Synergistic effects of ground cover and adjacent vegetation on natural enemies of olive insect pests. Agric. Ecosyst. Environ. 173, 72–80 (2013).
Porcel, M., Cotes, B., Castro, J. & Campos, M. The effect of resident vegetation cover on abundance and diversity of green lacewings (Neuroptera: Chrysopidae) on olive trees. J. Pest Sci. 90, 195–206 (2017).
Álvarez, H. A. et al. Semi-natural habitat complexity affects abundance and movement of natural enemies in organic olive orchards. Agric. Ecosyst. Environ. 285, 106618 (2019).
Paredes, D., Cayuela, L., Gurr, G. M. & Campos, M. Is ground cover vegetation an effective biological control enhancement strategy against olive pests?. PLoS ONE 10, e0117265 (2015).
Gkisakis, V. D. et al. Olive canopy arthropods under organic, integrated, and conventional management. The effect of farming practices, climate and landscape. Agroecol. Sustain. Food Syst. 42, 843–858 (2018).
Sanz-Cortés, F. et al. Phenological growth stages of olive trees (Olea europaea). Ann. Appl. Biol. 140, 151–157 (2002).
Rodríguez, E., González, B. & Campos, M. Natural enemies associated with cereal cover crops in olive groves. Bullet. Insectol. 65, 43–49 (2012).
Morente, M., Campos, M. & Ruano, F. Evaluation of two different methods to measure the effects of the management regime on the olive-canopy arthropod community. Agric. Ecosyst. Environ. 259, 111–118 (2018).
Cardenas, M., Pascual, F., Campos, M. & Pekar, S. The spider assemblage of olive groves under three management systems. Environ. Entomol. 44, 509–518 (2015).
Hegazi, E. M. et al. Seasonality in the occurrence of two lepidopterous olive pests in Egypt. Insect Sci. 18, 565–574 (2011).
Markó, V., Keresztes, B., Fountain, M. T. & Cross, J. V. Prey availability, pesticides and the abundance of orchard spider communities. Biol. Control 48, 115–124 (2009).
Picchi, M. S., Marchi, S., Albertini, A. & Petacchi, R. Organic management of olive orchards increases the predation rate of overwintering pupae of Bactrocera oleae (Diptera: Tephritidae). Biol. Control 108, 9–15 (2017).
Caruso, T. & Migliorini, M. Micro-arthropod communities under human disturbance: Is taxonomic aggregation a valuable tool for detecting multivariate change? Evidence from Mediterranean soil oribatid coenoses. Acta Oecol. 30, 46–53 (2006).
Schipper, A. M., Lotterman, K., Geertsma, M., Leuven, R. S. E. W. & Hendriks, A. J. Using datasets of different taxonomic detail to assess the influence of floodplain characteristics on terrestrial arthropod assemblages. Biodivers. Conserv. 19, 2087–2110 (2010).
Timms, L. L., Bowden, J. J., Summerville, K. S. & Buddle, C. M. Does species-level resolution matter? Taxonomic sufficiency in terrestrial arthropod biodiversity studies. Insect Conserv. Divers. 6, 453–462 (2013).
Unwin, D. M. A Key to the Families of British Beetles (Field Studies Council, 1984).
Goulet, H. & Huber, J. Hymenoptera of the World: An identification Guide to Families. (Agriculture Canada publication, 1993).
Johnson, N. F. & Triplehorn, C. A. Borror and DeLong’s Introduction to the Study of Insects 7th edn. (Thomson Brooks/Cole, Belmont, 2005).
Fletcher, M. J., and updates. Identification keys and checklists for the leafhoppers, planthoppers and their relatives occurring in Australia and neighbouring areas (Hemiptera: Auchenorrhyncha). https://idtools.dpi.nsw.gov.au/keys/auch/index.html (2009).
Mata, L. & Goula, M. Clave de familias de Heterópteros de la Península Ibérica (Insecta, Hemiptera, Heteroptera). Versión 1. Publicaciones del Centre de Recursos de Biodiversitat Animal, Universitat de Barcelona. http://www.ub.edu/crba/publicacions/Clau%20heteropters/Volum4_Clave_de_Familias_de_Heteropteros_de_la_P.Iberica.pdf (2011).
Oosterbroek, P. The European families of the Diptera. Identification, diagnosis, biology. (Royal Dutch Society for Natural History (KNNV) Publishing, Utrecht, 2015).
World Spider Catalog. Version 19. Natural History Museum Bern. http://wsc.nmbe.ch (2018).
Campos, M. Lacewing in Andalusian olive orchards. In Lacewing in the Crop Environment (eds McEwen, P. et al.) 492–497 (Cambridge University Press, Cambridge, 2001).
Wilson, E. O. & Hölldobler, B. The rise of the ants: A phylogenetic and ecological explanation. Proc. Natl. Acad. Sci. U. S. A. 102, 7411–7414 (2005).
Martínez-Núñez, C. et al. Ant community potential for pest control in olive groves: Management and landscape effects. Agric. Ecosyst. Environ 305, 107185 (2021).
Bianchi, F. J. J. A., Booij, C. J. H. & Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B. 273, 1715–1727 (2006).
Holland, J. M. et al. Semi-natural habitats support biological control, pollination and soil conservation in Europe. A review. Agron. Sustain. Dev. 37, 31 (2017).
Paredes, D. et al. Landscape simplification increases Bactrocera oleae abundance in olive groves: Adult population dynamics in different land uses. J. Pest Sci. https://doi.org/10.1007/s10340-022-01489-1 (2022).
Thies, C., Roschewitz, I. & Tscharntke, T. The landscape context of cereal aphid–parasitoid interactions. Proc. R. Soc. B. 285, 203–210 (2005).
Pinto-Correia, T., Ribeiro, N. & Sá-Sousa, P. Introducing the montado, the cork and holm oak agroforestry system of Southern Portugal. Agrofor. Syst. 82, 99–104 (2011).
Morgado, R. et al. Drivers of irrigated olive grove expansion in Mediterranean landscapes and associated biodiversity impacts. Landsc. Urban Plan. 225, 104429 (2022).
Direção-Geral do Território. Carta de Uso e Ocupação do Solo de Portugal Continental para 2015 (COS2015). http://www.dgterritorio.pt/dados_abertos/cos/ (2015).
Roswell, M., Dushoff, J. & Winfree, R. A conceptual guide to measuring species diversity. Oikos 130, 321–338 (2021).
Hsieh, T. C., Ma, K. H. & Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 7, 1451–1456 (2016).
Penado, A. et al. From pastures to forests: Changes in Mediterranean wild bee communities after rural land abandonment. Insect Conserv. Divers. 15, 325–336 (2022).
Ovaskainen, O. & Abrego, N. Joint Species Distribution Modelling. With Applications in R. (Cambridge University Press, 2020).
Dormann, C. F. et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46 (2013).
Macgregor-Fors, I. & Payton, M. E. Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. PLoS ONE 8, e56794 (2013).
Tikhonov, G. et al. Joint species distribution modelling with the r-package Hmsc. Methods Ecol. Evol. 11, 442–447 (2019).
R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2021).
Wickramasinghe, L. P., Harris, S. H., Jones, G. & Jennings, N. V. Abundance and species richness of nocturnal insects on organic and conventional farms: Effects of agricultural intensification on bat foraging. Conserv. Biol. 8, 1283–1292 (2004).
Galloway, A. D., Seymour, C. L., Gaigher, R. & Pryke, J. S. Organic farming promotes arthropod predators, but this depends on neighbouring patches of natural vegetation. Agric. Ecosyst. Environ. 310, 107295 (2021).
Hevia, V., Ortega, J., Azcárate, F. M., López, C. A. & González, J. A. Exploring the effect of soil management intensity on taxonomic and functional diversity of ants in Mediterranean olive groves. Agric. For. Entomol. 21, 109–118 (2019).
Vitanović, E. et al. Arthropod communities within the olive canopy as bioindicators of different management systems. Span. J. Agric. Res. 16, e0301 (2018).
Vasconcelos, S. et al. Long-term consequences of agricultural policy decisions: How are forests planted under EEC regulation 2080/92 affecting biodiversity 20 years later?. Biol. Conserv. 236, 393–403 (2019).
Tscharntke, T. et al. When natural habitat fails to enhance biological pest control—five hypotheses. Biol. Conserv. 204, 449–458 (2016).
Ortega, M., Pascual, S. & Rescia, A. J. Spatial structure of olive groves and scrublands affects Bactrocera oleae abundance: A multi-scale analysis. Basic Appl. Ecol. 17, 696–705 (2016).
Martínez-Núñez, C. et al. Direct and indirect effects of agricultural practices, landscape complexity and climate on insectivorous birds, pest abundance and damage in olive groves. Agric. Ecosyst. Environ. 304, 107145 (2020).
Paredes, D., Karp, D. S., Chaplin-Kramer, R., Benítez, E. & Campos, M. Natural habitat increases natural pest control in olive groves: Economic implications. J. Pest Sci. 92, 1111–1121 (2019).
Attwood, S. J., Maron, M., House, P. N. & Zammit, C. Do arthropod assemblages display globally consistent responses to intensified agricultural land use and management?. Glob. Ecol. Biogeogr. 17, 585–599 (2008).
Miranda, M. A., Miquel, M., Terrassa, J., Melis, N. & Monerris, M. Parasitism of Bactrocera oleae (Diptera; Tephritidae) by Psyttalia concolor (Hymenoptera; Braconidae) in the Balearic Islands (Spain). J. Appl. Entomol. 132, 798–805 (2008).
Álvarez, H. A., Morente, M., Campos, M. & Ruano, F. L. madurez de las cubiertas vegetales aumenta la presencia de enemigos naturales y la resiliencia de la red trófica de la copa del olivo. Ecosistemas 28, 92–106 (2019).
Rusch, A., Valantin-Morison, M., Sarthou, J. P. & Roger-Estrade, J. Biological control of insect pests in agroecosystems. Effects of crop management, farming systems, and seminatural habitats at the landscape scale: A review. Adv. Agron. 109, 219–259 (2010).
Greenop, A., Cook, S. M., Wilby, A., Pywell, R. F. & Woodcock, B. A. Invertebrate community structure predicts natural pest control resilience to insecticide exposure. J. Appl. Ecol. 57, 2441–2453 (2020).
Porcel, M., Ruano, F., Cotes, B., Peña, A. & Campos, M. Agricultural management systems affect the green lacewing community (Neuroptera: Chrysopidae) in olive orchards in southern Spain. Environ. Entomol. 42, 97–106 (2013).
Stamou, G. P. Arthropods of Mediterranean-Type Ecosystems (Springer, 2012).
Santos, J. L. et al. A farming systems approach to linking agricultural policies with biodiversity and ecosystem services. Front. Ecol. Environ. 19, 168–175 (2021).
Ribeiro, P. F. et al. An applied farming systems approach to infer conservation-relevant agricultural practices for agri-environment policy design. Land Use Policy 58, 165–172 (2016).
Herrera, J. M. et al. A food web approach reveals the vulnerability of biocontrol services by birds and bats to landscape modification at regional scale. Sci. Rep. 11, 1–10 (2021).
Solomou, A. D. & Sfougaris, A. I. Bird community characteristics as indicators of sustainable management in olive grove ecosystems of Central Greece. J. Nat. Hist. 49, 301–325 (2015).
Piñeiro, V. et al. A scoping review on incentives for adoption of sustainable agricultural practices and their outcomes. Nat Sustain. 3, 809–820 (2020).
Acknowledgements
We thank the property owners and managers for granting us access to the surveyed orchards, and Amália Oliveira and Sílvia Barreiro for assistance during fieldwork. This research was funded by the Portuguese Foundation for Science and Technology (FCT) under PTDC/AAG-REC/6480/2014. SV, JMH, BS, MP, GJ-N, FM and RH were supported by FCT grants/contracts SFRH/BD/121388/2016, IF/00001/2015, SFRH/BD/137803/2018, SFRH/BPD/97025/2013, SFRH/BD/133017/2017, IF/01053/2015 and UID/BIA/04004/2021, respectively. PS was supported by a grant from project PORBIOTA (POCI-01-0145-FEDER-022127), supported by POCI under the PORTUGAL 2020 Partnership Agreement through FEDER. PB was supported by EDP Biodiversity Chair and MJ by SLU Centre for Biological Control. Work was co-funded by the project NORTE-01-0246-FEDER-000063, supported by Norte Portugal Regional Operational Programme (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Contributions
S.V., P.B., J.M.H. and F.M. conceived the ideas, and S.V., P.B., J.M.H., M.J., R.H. and F.M. designed the study. JMH and BS selected the sampling sites. S.V. collected the arthropods, and N.M.-R., G.J.-N., B.S., S.V. and J.M.H. collected the environmental variables. S.P., P.S. and S.V. identified the arthropods. S.V. and M.P. analysed the data. S.V. led the writing with substantial contributions from P.B., R.H., M.J. and F.M.. All authors critically reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vasconcelos, S., Pina, S., Herrera, J.M. et al. Canopy arthropod declines along a gradient of olive farming intensification. Sci Rep 12, 17273 (2022). https://doi.org/10.1038/s41598-022-21480-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21480-1
This article is cited by
-
Mixed effects of oak woodlands on biocontrol potential and pest pressure in olive orchards under management intensification
Journal of Pest Science (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.