Abstract
Acute kidney injury (AKI) often occurs in patients in the intensive care unit (ICU). AKI duration is closely related to the prognosis of critically ill patients. Identifying the disease course length in AKI is critical for developing effective individualised treatment. To predict persistent AKI at an early stage based on a machine learning algorithm and integrated models. Overall, 955 patients admitted to the ICU after surgery complicated by AKI were retrospectively evaluated. The occurrence of persistent AKI was predicted using three machine learning methods: a support vector machine (SVM), decision tree, and extreme gradient boosting and with an integrated model. External validation was also performed. The incidence of persistent AKI was 39.4–45.1%. In the internal validation, SVM exhibited the highest area under the receiver operating characteristic curve (AUC) value, followed by the integrated model. In the external validation, the AUC values of the SVM and integrated models were 0.69 and 0.68, respectively, and the model calibration chart revealed that all models had good performance. Critically ill patients with AKI after surgery had high incidence of persistent AKI. Our machine learning model could effectively predict the occurrence of persistent AKI at an early stage.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) refers to the sudden loss of excretory renal function and is a common and complex clinical syndrome that occurs in patients in the intensive care unit (ICU)1. The clinical manifestations vary based on the duration and severity, and the required methods of treatment also differ. The duration of AKI is an important factor in predicting the mortality of critically ill patients2. A systematic review reported that AKI duration was independently correlated with patient mortality and the occurrence of cardiovascular events3. In 2017, the Acute Dialysis Quality Initiative (ADQI) Group classified AKI into transient and persistent AKI based on whether the AKI persists for more than 48 h. Predicting the course of AKI can help identify phenotypes that require different treatment regimens, thereby facilitating individualised management. Guidelines recommend that when persistent AKI is diagnosed, clinicians should carefully reassess the patient, re-evaluate the aetiology of AKI, and re-examine the treatment regimen4. For patients with transient AKI, unnecessary examinations and treatments, such as renal replacement therapy (RRT), can be avoided, as their renal function will recover in a short time5. Therefore, the duration of AKI holds clinical significance.
However, early identification of persistent AKI is difficult, and traditional urine biochemistry6 and the renal resistive index7 exhibit poor prediction results. The latest research revealed that prediction models based on big data from modern electronic case systems combined with machine learning algorithms were more accurate than traditional methods8. In particular, the latest integrated machine learning algorithms combine the best-performing algorithms to create an integrated model to achieve a better modelling effect, which has begun to make important contributions in various fields, such as industry, agriculture, education, and medicine9,10,11,12.
Therefore, this study aimed to predict the occurrence of persistent AKI in critically ill surgical patients, based on machine learning algorithms and integrated models, for accurate early identification of high-risk patients.
Methods
Study design and research participants
Our study was reported according to the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement13. A retrospective study was conducted including 955 patients with AKI who were first admitted to the ICU of Dongyang People’s Hospital between January 1, 2010, and September 30, 2020. External validation was performed using the MIMIC III dataset14, a freely accessible online critical care database. The inclusion criteria were patients who were first admitted to the ICU and underwent surgical treatment. The exclusion criteria were chronic kidney disease diagnosis, age < 18 years, ICU stay < 48 h, AKI diagnosis before ICU admission, or missing values of variables > 20%.
This study followed all related local guidelines and regulations, including the human genetic-related research regulations. This study was approved by the Ethics Committee of Dongyang People’s Hospital (Dong Ren Yi 2022-YX-056). The need for obtaining informed consent was waived by the local Ethics Committee (Ethical Committee of Dongyang People’s Hospital) because of the retrospective and observational study design. The data were anonymised before analysis. One author (X.J.) obtained permission to access the Medical Information Mart for Intensive Care (MIMIC) database after the completion of “Protecting Human Research Participants,” an online training course launched by the National Institutes of Health (Certification Number: 7632299).
Data collection
We collected data using the medical record information mining software provided by Shanghai Le9 Healthcare Technology Co., Ltd. The retrieved data included the following: (1) basic clinicodemographic information (age, sex, disease severity [Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sepsis-related Organ Failure Assessment (SOFA) score], duration of operation, RRT, urine volume, and complications); (2) AKI-related drugs such as antibiotics, diuretics, and contrast agents; (3) laboratory measurements on the first day of ICU admission; and (4) vital signs on the first day of ICU admission, including maxima, minima, and averages.
Diagnostic criteria
AKI was defined and staged according to the 2012 Kidney Disease: Improving Global Outcomes criteria15 as follows: increase in serum creatinine (Scr) by 50%, increase in Scr by 26.5 mol/L within 2 days, or urine volume < 0.5 mL/kg/h for 6 h. Baseline Scr was defined as the lowest Scr level within 6 months before ICU admission16. For patients without previous Scr data, it was estimated using the following formula17: Scr = 0.74–0.2 (if female) + 0.08 (if black) + 0.0039* age (in years). Based on the 2017 expert consensus4, persistent AKI was defined as a duration of AKI > 48 h; otherwise, it was designated as transient AKI.
The primary endpoint was the incidence of persistent AKI. The secondary outcomes included hospitalisation mortality, length of ICU stay, length of hospital stay, mechanical ventilation duration, and hospitalisation cost.
Data processing
A total of 83 potentially related variables were preliminarily screened. After excluding three variables with > 20% of missing values, the remaining 80 variables were subjected to data pre-processing using the Classification And REgression Training (CARET) package in the R language. Thirteen variables showing a strong correlation (correlation coefficient > 0.9) with other independent variables were eliminated. The remaining 67 variables were then subjected to feature selection using the backward selection method, random forest sampling, and 10% cross-checking. Thereafter, the variables were ranked according to their importance. The 12 most important variables were retained.
Outliers were detected using the interquartile range (IQR), i.e., the difference between the upper and lower quartiles of the boxplot. The outliers were excluded and handled as missing values. Variables with > 20% missing values were deleted. The missing values of variables were replaced using multiple imputations.
Model establishment
First, support vector machines (SVMs), decision trees C5.0, and extreme gradient boosting (XGBoost) were trained for three machine learning (ML) models using the following R packages: CARET, XGBoost, C50, e1071, and gbm. The hyperparameters were adjusted using a grid search. Thereafter, the CaretEnsemble18 was used to create ensemble models from these three models. Samples were randomly divided into training and test sets in a 7:3 ratio. All ML models were evaluated using 10 × cross-validation.
Model validation and evaluation
The model performance was evaluated in the area under the receiver operating characteristic curve (AUC). The calibration performance of the model was evaluated using calibration curves. The confusion matrix was evaluated using accuracy, precision, specificity, and recall as parameters; the cut-off point was 0.5.
Model interpretation
Model interpretation was implemented using variable importance, which was sorted using the function “varImp” within the CARET package in R. Moreover, local interpretable model-agnostic explanations (LIME) and iBreakdown algorithms provided individual interpretation19,20.
Statistical analysis
Descriptive statistics were analysed conventionally using the CBCgrps package in R21. Normally distributed measurement data were expressed as x ± s and compared between groups using the two-independent-samples t-test. Meanwhile, non-normally distributed data were expressed as median (P25, P75) and compared using the Mann–Whitney U test. Enumeration data were expressed in terms of the rate and percentage and compared between the groups using the χ2 test. All statistical analyses were performed using R (software version 4.1.2). A P-value of 0.05 was considered significant.
Results
Study population and baseline characteristics
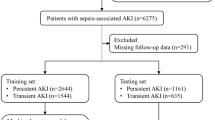
Our study included a total of 955 patients complicated by AKI admitted to the ICU after surgery, including 376 patients with persistent AKI with an incidence of 39.4%; in the MIMIC III data set, the incidence was 45.1% (1429/3170 cases). The 955 patients with AKI were randomly divided into the training and internal verification sets at a ratio of 7:3. MIMIC III was used as the external verification. Figure 1 shows a flow chart of the study design. Table 1 presents a comparison of baseline data among the training, internal verification, and external verification sets.
Table 2 shows a comparison of baseline data and clinical prognoses of patients in the persistent and transient AKI groups. There was no significant difference in age or sex. Comparison of SOFA and APACHE II scores showed that the severity of disease in the persistent AKI group was higher, and the clinical outcomes included time on ventilator, length of ICU stay, in-hospital mortality, and hospitalisation expenses. There were significant differences between the two groups. In addition, the use of contrast agents and diuretics was greater in the persistent AKI group. We also compared the baseline data and clinical outcomes of patients in the persistent and transient AKI groups in the MIMIC III data set, as shown in Supplementary Table S1.
Model establishment and evaluation
We used ML algorithms C5.0, SVM, and XGBoost to establish models. Supplementary figure S1−3 illustrate the hyperparameter tuning. We created an integrated model using these three models. The relative influence of each ML model in the integrated model is shown in Fig. 2, which shows that SVM and XGBoost greatly influenced the integrated model. Figure 3 presents the comparison of the prediction performance of machine learning C5.0, SVM, XGBoost, and integrated models in the internal verification set. The model calibration chart shows that all models had good predictive performance, with the integrated model showing the highest AUC (0.86, 95% CI: 0.814–0.906), and the SVM showing the second highest AUC (0.856, 95% CI: 0.812–0.901). The ROC comparison and model calibration chart in the external verification set are shown in Supplementary figure S4. The AUC value of the SVM was 0.693 (95% CI: 0.676–0.711). Supplementary Table S2 lists other evaluation indexes of prediction performance of the ML models.
Evaluation of model performance in the internal validation dataset. (A) The calibration plot shows the consistency between observed and predicted risks for persistent acute kidney injury. (B) Discrimination of the machine-learning models in the internal validation dataset. SVM, support vector machine; XGBoost, extreme gradient boosting; AUC, area under the curve. The number in parentheses indicates the 95% confidence interval.
Model interpretation
Figure 4 and Supplementary Figures S5 and S6 show the order of importance of variables in the models. The Cr level, RRT, and lac level on ICU admission exhibited the greatest influence on the models. LIME provided interpretation for individual patients, and we estimated the contribution of the variables of two patients (Patients 2 and 3), as shown in Figs. 5 and 6. We used the iBreakdown method (Supplementary Figure S7) to estimate the contribution of each variable to the probability of persistent AKI for Patient 1, which showed that the initial Cr = 56 was closely correlated with a reduced risk of persistent AKI.
Heatmap plot showing the contribution of each variable to the classification of the sample patients. The relative contribution of each variable was calculated using the LIME algorithm. Data of patients 2 and 3 are shown as examples. The red colour indicates that the relevant variable contradicts a given label, while the blue colour indicates support. AKI, acute kidney injury; SOFA, Sepsis-related Organ Failure Assessment; Uo_24h, Urine volume for 24 h on intensive care unit admission; LIME, Local Interpretable Model-Agnostic Explanations.
The LIME feature plot shows the contribution of each variable to the classification of the sample patients. The red colour indicates that the relevant variable contradicts a given label, while the blue colour indicates support. AKI, acute kidney injury; SOFA, Sepsis-related Organ Failure Assessment; Uo_24h, Urine volume for 24 h on intensive care unit admission; LIME, Local Interpretable Model-Agnostic Explanations.
Discussion
Principal results
Our research utilised vital signs and related information in the 24 h after admission to the ICU, which could distinguish between persistent and transient AKI in advance, based on SVM or ML integration algorithms. The AUCs in the internal and external verification sets were approximately 0.86 and 0.69, respectively, showing that the model detection performance was good. The prediction effect of the integrated ML model was not as good as that of the SVM in the external verification sets, which might be related to the model we chose. We combined only three common ML algorithms: C5.0, SVM, and XGBoost. In a previous study, Rahman et al. integrated ML elastic network regression, random forest, and XGBoost to predict the early recurrence of oesophageal cancer after surgery, and the best AUC of the integrated ML algorithm was 0.805, whereas the AUC of XGBoost was 0.804, with a slight difference between them22. Similarly, super learner, an algorithm specifically integrating ML, has great potential in improving prediction quality in applied health science23. It contains a large number of ML algorithms, and the number of applications is increasing. Therefore, our models require further improvement. By integrating more ML algorithms, the performance of the model may be further improved in the future.
Comparison with prior work
Previous research has shown that the incidence of persistent AKI varies with the severity of the disease. For example, in a large retrospective cohort study, involving over 54,000 patients with AKI from 2012 to 2019, the incidence of persistent AKI was 42%24. In a prospective study among ICU patients, the proportion of patients with persistent AKI among ICU patients at six hospitals was as high as 61.8% (175/283)25. Our research showed that the incidence of persistent AKI in ICU patients with AKI after surgery was 39.4–45.1% and was relatively low among ICU patients; this was because AKI was correlated with reversible factors such as hypovolaemia and ischaemia, which were common in postoperative patients. Early identification of these patients can help to identify the phenotypes that require different treatment schemes, thereby reducing excessive intervention treatment.
In the past few years, there has been an increasing number of prediction models for AKI based on ML, but few studies have focused on ICU patients. A study by Ding et al., which used free databases to screen important variables that affect persistent AKI based on ML algorithms, and the nomogram produced showed good prediction performance26. Another study by Luo et al. used five ML algorithms to identify persistent AKI in ICU patients with sepsis at an early stage, and the AUC of the model with the optimal algorithm reached 0.76. However, most of these studies only used creatinine as the standard for AKI diagnosis, and generally lacked external verification27. Our research included creatinine and urine volume data, and used MIMIC data for verification, which made the model suitable for more general condition.
Limitations
First, this study was retrospective in nature, and urine volume was not recorded every hour. Therefore, when we calculated urine volume for 6 h, the data were inaccurate, and for patients transferred to other departments or discharged from the hospital within 48 h following admission to the ICU, urine volume data was not collected. However, our data are more consistent with actual practice than when only a creatinine standard is used to diagnose AKI. Second, there is a black box effect in ML. We explained the models by three methods, namely rank of variable importance in the models, LIME, and iBreakdown, which allows individualised application to patient. Finally, 12 variables were included in the models, including the maximum and average vital signs on first day after ICU admission. The method of calculation was somewhat complicated. It is better to automatically identify persistent AKI by adding data to the current electronic medical record system.
Conclusions
Critically ill patients with AKI after surgery displayed a high incidence of persistent AKI and poor prognoses. We could predict the occurrence of persistent AKI in advance using the machine learning models and the integrated model; external verification by the MIMIC III database, and the model calibration was good. In the future, the electronic medical record system can be integrated to automatically identify persistent AKI to facilitate individualised treatment and improve patient outcomes.
Data availability
The datasets of Dongyang People’s Hospital are available from the corresponding author on reasonable request. The MIMIC III dataset is a freely accessible online critical care database (https://mimic.physionet.org/).
References
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Primers. 7, 52 (2021).
Han, S. S. et al. Duration of acute kidney injury and mortality in critically ill patients: A retrospective observational study. BMC Nephrol. 14, 133 (2013).
Mehta, S. et al. The prognostic importance of duration of AKI: A systematic review and meta-analysis. BMC Nephrol. 19, 91 (2018).
Chawla, L. S. et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat. Rev. Nephrol. 13, 241–257 (2017).
Hoste, E. et al. Identification and validation of biomarkers of persistent acute kidney injury: The RUBY study. Intensive Care Med. 46, 943–953 (2020).
Pons, B. et al. Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: Multicenter cohort study. Crit. Care. 17, R56 (2013).
Darmon, M. et al. Performance of Doppler-based resistive index and semi-quantitative renal perfusion in predicting persistent AKI: Results of a prospective multicenter study. Intensive Care Med. 44, 1904–1913 (2018).
Lee, T. H., Chen, J. J., Cheng, C. T. & Chang, C. H. Does artificial intelligence make clinical decision better? A review of artificial intelligence and machine learning in acute kidney injury prediction. Healthcare 9, 162 (2021).
Bhagat, S. K., Tung, T. M. & Yaseen, Z. M. Heavy metal contamination prediction using ensemble model: Case study of Bay sedimentation, Australia. J. Hazard. Mater. 403, 123492 (2021).
Peppes, N., Daskalakis, E., Alexakis, T., Adamopoulou, E. & Demestichas, K. Performance of machine learning-based multi-model voting ensemble methods for network threat detection in Agriculture 4.0. Sensors (Basel). 21, 7475 (2021).
Karalar, H., Kapucu, C. & Gürüler, H. Predicting students at risk of academic failure using ensemble model during pandemic in a distance learning system. Int. J. Educ. Technol. High Educ. 18, 63 (2021).
Bannick, M. S., McGaughey, M. & Flaxman, A. D. Ensemble modelling in descriptive epidemiology: Burden of disease estimation. Int. J. Epidemiol. 49, 2065–2073 (2021).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 350, g7594 (2015).
Johnson, A. E. et al. MIMIC-III, a freely accessible critical care database. Sci. Data 3, 160035 (2016).
Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 120, c179–c184 (2012).
Shen, Y., Zhang, W., Cheng, X. & Ying, M. Association between postoperative fluid balance and acute kidney injury in patients after cardiac surgery: A retrospective cohort study. J. Crit. Care 44, 273–277 (2018).
Závada, J. et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol. Dial. Transplant. 25, 3911–3918 (2010).
Zhang, Z., Chen, L., Xu, P. & Hong, Y. Predictive analytics with ensemble modeling in laparoscopic surgery: A technical note. Laparosc. Endosc. Robot. Surg. 5, 25–34 (2022).
Staniak, M. & Biecek, P. Explanations of model predictions with live and breakDown packages. R J. 10, 395 (2019).
Ribeiro, M. T., Singh, S. & Guestrin, C. “Why Should I Trust You?”: Explaining the Predictions of Any Classifier 11351144 (Assoc. for Computing Machinery, 2016).
Zhang, Z., Gayle, A. A., Wang, J., Zhang, H. & Cardinal-Fernández, P. Comparing baseline characteristics between groups: An introduction to the CBCgrps package. Ann. Transl. Med. 5, 484 (2017).
Rahman, S. A. et al. Machine learning to predict early recurrence after oesophageal cancer surgery. Br. J. Surg. 107, 1042–1052 (2020).
Naimi, A. I. & Balzer, L. B. Stacked generalization: An introduction to super learning. Eur. J. Epidemiol. 33, 459–464 (2018).
Ozrazgat-Baslanti, T. et al. Association of persistent acute kidney injury and renal recovery with mortality in hospitalised patients. BMJ Health Care Inform. 28, e100458 (2021).
Perinel, S. et al. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: Results of a multicenter cohort study. Crit. Care Med. 43, e269–e275 (2015).
Ding, C. & Hu, T. Development and external verification of a nomogram for patients with persistent acute kidney injury in the Intensive Care Unit. Int. J. Gen. Med. 14, 5005–5015 (2021).
Pickkers, P. et al. Acute kidney injury in the critically ill: An updated review on pathophysiology and management. Intensive Care Med. 47, 835–850 (2021).
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was funded by Zhejiang Medical Association (2018ZYC-A134, 2019ZYC-A172), Jinhua Science and Technology Bureau (2020-4-127) and Dongyang Science and Technology Bureau (21-337).
Author information
Authors and Affiliations
Contributions
X.D.J. and S.G. designed the study, performed the data extraction, and analysed and drafted the manuscript. C.J.D. and Y.X.H. critically revised the manuscript. X.P.C. supervised the study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, X., Hu, Y., Guo, S. et al. Prediction of persistent acute kidney injury in postoperative intensive care unit patients using integrated machine learning: a retrospective cohort study. Sci Rep 12, 17134 (2022). https://doi.org/10.1038/s41598-022-21428-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21428-5
This article is cited by
-
Künstliche Intelligenz und akute Nierenschädigung
Medizinische Klinik - Intensivmedizin und Notfallmedizin (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.