Abstract
This prospective, randomized controlled trial evaluated the effect of neostigmine for intraoperative neuromonitoring (IONM) during thyroid surgery. Forty subjects undergoing thyroidectomy with IONM, randomized into neostigmine administration after tracheal intubation (Group N, n = 20) or control treatment with normal saline (Group C, n = 20), completed the trial. Electromyography amplitudes of the vagus nerve (V1) were recorded before thyroid dissection. The time from the initial V1 signal check to successful V1 stimulation was recorded. In Group N, all the patients had a successful V1 signal at the first check, whereas ten (50%) patients in Group C had a time delay between the initial V1 check and successful V1 (p < 0.001). The mean delay time among the delayed patients in Group C was 11.2 ± 1.4 min. The mean time from skin incision to successful V1 stimulation was significantly shorter in Group N than in Group C (15.4 ± 2.4 min vs. 19.9 ± 5.7 min, p = 0.003). In Groups N and C, the mean V1 amplitudes were 962.2 ± 434.5 μV vs. 802.3 ± 382.7 μV (p = 0.225), respectively, and the mean R1 amplitudes were 1240.0 ± 836.5 μV vs. 1023.4 ± 455.8 μV (p = 0.316), respectively. There was one bucking event in Group N. In conclusion, neostigmine administration immediately after tracheal intubation can be useful to reverse neuromuscular blockade for successful IONM in thyroid surgeries.
Similar content being viewed by others
Introduction
The use of intraoperative neuromonitoring (IONM) has increased in thyroid surgery because such monitoring has been shown to reduce recurrent laryngeal nerve (RLN) injury with its use1. Accordingly, interest in improving the quality of IONM is growing. One of the key factors for successful IONM is the adequate reversal of neuromuscular blockade. Although neuromuscular blocking agents (NMBAs) are needed to optimize tracheal intubation conditions and operating conditions, excessive neuromuscular blockade may interfere with electromyography (EMG) signals during IONM2.
Sugammadex, a rapid-acting NMBA, is considered to be the most effective reversal agent for IONM to date3, but its high price and poor accessibility in many countries limit its use in many health care systems4. Furthermore, its fast reversal kinetics and complete reversal of neuromuscular blockade may result in unwanted patient movement during surgery5,6. Neostigmine, a cholinesterase inhibitor, is a safe, effective, and comparatively inexpensive alternative reversal agent7,8. The feasibility of neostigmine as a reversal agent for IONM during thyroid surgery was demonstrated in one previous observational study9. However, there is no strong evidence for using neostigmine to create a good condition for IONM in thyroid surgery.
The purpose of the present study was to investigate the effects of neostigmine on IONM during thyroid surgery compared with no use of reversal agents for IONM. We hypothesized that neostigmine would shorten the time to obtain good conditions for successful IONM, without increasing intraoperative bucking events.
Methods
This prospective randomized controlled trial was approved by the Institutional Review Board of the Seoul Metropolitan Government Seoul National University Boramae Medical Center (no. 10-2021-12) and was registered on ClinicalTrials.gov (NCT04873531; https://register.clinicaltrials.gov/prs/app/action/ViewOrUnrelease?uid=U00026JX&ts=16&sid=S000AXJ8&cx=tqaqzt, 26 April 2021) before enrolment of subjects. Adult patients (≥ 19 years old) with American Society of Anesthesiology physical status I-III who were scheduled to undergo thyroid surgery with IONM were enrolled between May 2021 and August 2021. Patients who required neuromuscular blocking agents other than rocuronium or were scheduled to undergo endoscopic or robotic thyroid surgery were excluded from the study. Written informed consent was obtained from all patients.
Randomization
Patients were randomly allocated in a 1:1 ratio to the neostigmine group (Group N) or the control group (Group C). Patients in Group N (n = 22) were administered 0.03 mg/kg neostigmine with 0.006 mg/kg glycopyrrolate after tracheal intubation, and those in Group C (n = 22) were administered the same volume of normal saline after tracheal intubation. Patients in Group C were administered neostigmine and glycopyrrolate after skin closure. An investigator who did not have a clinical role in this study generated the random allocation sequence using computer-generated block randomization (size: 6 blocks). The patient, operating surgeon, and surgical assistants were blinded to the allocation.
Anesthesia
Upon patient arrival to the operating theater without premedication, standard monitoring was applied, including pulse oximetry, electrocardiography, and noninvasive blood pressure. Bispectral index monitoring was applied to obtain an adequate depth of general anesthesia with maintenance of a value of 40–60. Acceleromyography was applied at the adductor pollicis muscle and ulnar nerve of the left hand for evaluation of neuromuscular blockade. After administration of 30 mg lidocaine, induction and maintenance of anesthesia were performed with a continuous infusion of propofol and remifentanil using a target-controlled infusion system with Orchestra™ infusion pumps (Fresenius Vial, Brézins, France). After loss of consciousness, 0.6 mg/kg rocuronium was administered to relax the muscles for tracheal intubation.
The patient was positioned for thyroid surgery with a standard pillow beneath their neck and back prior to tracheal intubation to prevent tracheal tube displacement during positioning of the patient for surgery10. A nerve integrity monitor (NIM) standard reinforced EMG endotracheal tube (Medtronic, Jacksonville, FL, USA) with a 7.0 mm internal diameter for men and a 6.0 mm internal diameter for women was used for tracheal intubation. A single anesthesiologist (J-M.L.) performed tracheal intubation by direct laryngoscopy in all patients. The anesthesiologist aimed to place the midpoint of the EMG tube electrodes between both vocal cords while evaluating the modified Cormack-Lehane grade. After tube fixation, the anesthesiologist administered a mixture of 0.03 mg/kg neostigmine with 0.006 mg/kg glycopyrrolate to the patients in Group N or normal saline of the same volume to the patients in Group C. This process ensured the surgeon remained blinded to group allocation.
All setup and monitoring processes were performed in compliance with the standards outlined in the International Neural Monitoring Study Group guidelines11. The NIM response 3.0 system (Medtronic, Jacksonville, FL, USA) was used for nerve monitoring. The stimulation duration was set at 100 ms, the event threshold was set at 100 μV, and the stimulus current was set at 1 mA with a frequency of 4 Hz.
Operative techniques
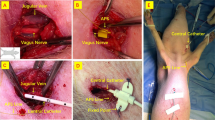
Operations were performed by a single surgeon (Y.J.C) who was blinded to the patient’s group allocation. A conventional low collar skin incision was made along the skin crease at the lower neck. After the isthmus was divided, the avascular plane between the cricothyroid muscle and the upper pole of the thyroid gland was exposed. After mobilizing the upper pole, EMG signals were recorded according to standardized IONM procedures11. During the operation, the surgeon checked whether the signal from the vagus nerve stimulation (V1) was successful. We defined the time of the initial V1 signal check as the time when the surgeon first tested the V1 signal after exposure of the carotid sheath, and the time of successful V1 stimulation as the time when a V1 signal > 100 μV was detected four or more times in succession under electrical stimulation currents of 1 mA at a frequency of 4 Hz. If successful V1 stimulation was not detected, the surgeon halted the operation and rechecked the V1 signal at 1-min intervals until successful V1 stimulation was detected. The time of the initial V1 check and successful V1 stimulation were recorded. After successful V1 stimulation, the surgeon continued the operation, which included confirmation of the RLN (R1) signal. The time and amplitudes of successful V1 and R1 stimulations were recorded. The train-of-four (TOF) ratio was recorded at the points of administration of rocuronium, tracheal intubation, administration of neostigmine or normal saline, and signal checks for V1 and R1. The surgeon suspended the operation if there was significant patient movement or bucking during surgery, and resumed procedures when the event was resolved. After the mass was completely removed, the surgeon checked the signals of the recurrent laryngeal nerve (R2) and the vagus nerve (V2) to recheck whether nerve impairment by surgery occurred. In patients who underwent total thyroidectomy, only the results of the lobe that was approached first were recorded.
Outcomes
The primary outcome was the time from skin incision to successful V1. We defined a case with ‘time delay for V1’ as the case when V1 was not detected at the initial V1 check, and recorded the time between the initial V1 check to successful V1. The secondary outcomes included the occurrence of intraoperative bucking events and the mean EMG amplitudes of the V1, R1, R2, and V2 signals. The time when the anesthesia induction began was recorded as the time at which the observation started.
Statistics
Quantitative variables of patient characteristics and outcome measures are presented as the mean ± standard deviation (SD). Categorical variables are presented as numbers with percentages. Depending on the distribution of variables according to the Kolmogorov–Smirnov test, continuous variables were compared using Student’s t test. Binary outcomes were compared using χ2 tests or Fisher’s exact tests. For the primary outcomes, the incidence of time delay was compared by Fisher’s exact test, and the time from skin incision to successful V1 stimulation was compared by Student’s t test between the two groups. For the secondary outcomes, the amplitudes of V1, R1, R2, and V2 were compared by Student’s t test and the incidence of the intraoperative bucking events was compared by Fisher’s exact test between the two groups. A p value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics v. 26 software (IBM Corp., Armonk, NY, USA).
Sample size calculation
One study reported that satisfactory qualitative nerve monitoring was achieved in 95% of patients after the administration of a combination of 2.5 mg neostigmine and 0.4 mg glycopyrrolate. In patients who received a placebo, satisfactory nerve monitoring was attained in only half of the cases12. We therefore assumed that the use of neostigmine and glycopyrrolate for reversal of neuromuscular blockade could prevent a delay in 95% or more patients and that no administration could cause a delay in approximately 50% of patients. To test this hypothesis, 19 patients per group were needed, with a type I error of 0.05, and 90% power. Considering a dropout rate of 15%, the sample size was calculated to be 44 patients.
Results
Patient characteristics
A total of 44 patients were enrolled in this study. Patient screening, enrolment, randomization, and analysis are shown in the CONSORT flow diagram in Fig. 1. The modified Cormack-Lehane grade was 1/2A/2B/3/4 in 2/18/16/6/0 among 42 patients in whom tracheal intubation was performed with a direct laryngoscope. Four patients who had no V1 signal after a significant amount of time were excluded from the analysis. Among them, two patients in Group N and one patient in Group C had no V1 or R1 signal but had laryngeal twitches. Postoperative laryngoscopic examination revealed normal vocal cord movement, suggesting that the absence of a V1 signal was due to technical failure. One patient in Group C had no V1 or R1 signal and no laryngeal twitch. Postoperative laryngoscopic examination revealed transient vocal cord palsy suggesting vagus nerve or RLN injury during upper pole manipulation or thyroid traction before the initial V1 signal check. Finally, 40 patients (20 patients in each group) were eligible for analysis. The clinical characteristics of the patients are summarized in Table 1. There were no significant differences between groups in terms of sex, age, body mass index, tumor size, operative extent, or pathological diagnosis. There were no adverse events, such as bradycardia or tachycardia, due to neostigmine-glycopyrrolate administration or any tissue injury by the involuntary intraoperative bucking movement of patients during the study. Additionally, no RLN injury occurred in any of the patients included in the analysis (Table 1).
Time measurements of anesthetic and operative procedures
Table 2 shows the duration of the anesthetic and operative procedures. The mean time from rocuronium injection to tracheal intubation was 3.5 ± 0.9 min for Group N and 3.8 ± 0.8 min for Group C (p = 0.265). The mean time from skin incision to initial V1 signal check was comparable between the two groups (15.4 ± 2.4 vs. 15.0 ± 2.5 min, p = 0.606). For all patients in Group N, V1 stimulation was successful on the initial attempt, whereas half of the patients in Group C (10/20) required multiple attempts before successful stimulation of V1 (p < 0.001), with a mean delay time of 11.2 ± 1.4 min between the initial attempt and successful V1 stimulation. As the primary outcome in our study, the mean time from skin incision to successful V1 stimulation was significantly shorter in Group N than in Group C (15.4 ± 2.4 vs. 19.9 ± 5.7 min, p = 0.003). The mean time from skin incision to R1 stimulation was also shorter in Group N than in Group C (17.1 ± 2.5 vs. 23.4 ± 6.5 min, p < 0.001). The mean time from skin incision to successful R2/V2 stimulation and total operative time were comparable between Group N and Group C (31.26 ± 6.7 vs. 34.6 ± 7.7 min, p = 0.139 and 48.6 ± 10.8 vs. 54.3 ± 16.3 min, p = 0.196).
IONM quality and outcomes
IONM quality and outcomes are presented in Table 3. At the initial V1 signal check, the TOF counts were higher in Group N than in Group C (p < 0.001). The respective TOF counts were 0/4, 1/4, 2/4, 3/4, 4/4 in 1, 4, 4, 0, 11 patients in Group N; and 0/4, 1/4, 2/4, 3/4, 4/4 in 16, 3, 1, 0, 0 patients in Group C. At successful V1 stimulation, the TOF counts were significantly higher in Group N than in Group C (p < 0.001). The respective TOF counts were 0/4, 1/4, 2/4, 3/4, 4/4 in 1, 4, 4, 0, 11 patients in Group N and 0/4, 1/4, 2/4, 3/4, 4/4 in 10, 7, 3, 0, 0 patients in Group C. With R1 stimulation, the TOF counts were higher in Group N than in Group C (p = 0.019). For the secondary outcomes of our study, all patients in both groups except one in Group C had V1 amplitudes higher than 500 μV, and the mean V1 amplitudes were comparable between Groups N and C (962.2 ± 434.5 μV vs. 802.3 ± 382.7 μV, p = 0.225). All patients in both groups had R1 amplitudes higher than 500 μV, and the mean R1 amplitudes were comparable between Groups N and C (1240.0 ± 836.5 μV vs. 1023.4 ± 455.8 μV, p = 0.316). With R2 and V2 stimulation, TOF counts were not significantly different between groups. All patients in both groups had R2 and V2 amplitudes higher than 500 μV. Patients in Groups N and C had comparable mean R2 amplitudes (1186.8 ± 599.4 μV vs. 1098.5 ± 440.3 μV, p = 0.599) and V2 amplitudes (930.2 ± 433.3 μV vs. 893.3 ± 302.1 μV, p = 0.599). During the surgical procedure, bucking events were observed in one patient (5.0%) in Group N at the time of V1 stimulation and none in Group C, with no significant difference between the two groups (p = 0.311). The bucking movement in the patient in Group N subsided spontaneously within 1 min while the surgeon held the surgical procedures for a while.
Discussion
The results of the present study show that compared with placebo, the use of neostigmine for IONM provided a faster time to successful IONM. The mean time from skin incision to successful V1 stimulation was 15.4 ± 2.4 min for Group N and 19.9 ± 5.7 min for Group C (p = 0.003) in this study. In a previous study, Gunes et al.13 reported that in patients for whom no reversal agents were used the mean time from the point of initiation of surgery to the point of full recovery of laryngeal EMG was 50 min. Our center recently reported that the mean time from neostigmine administration to stimulation of the external branch of the superior laryngeal nerve was 21.0 ± 4.5 min9. Previous studies either reported results of IONM when no reversal agents were used or results of IONM when neostigmine was used as a reversal agent9,13. However, there has been a lack of comparison studies on this issue. To the best of our knowledge, this is the first randomized controlled trial to compare the IONM quality and the time to obtain adequate conditions for IONM between patients who were administered neostigmine as a reversal agent before the initiation of surgery and those who were not.
There are currently no clear guidelines on the management of neuromuscular blockade for IONM during thyroid surgery. Various strategies have been suggested for the optimal management of neuromuscular blockade. Some studies have focused on manipulating NMBAs, such as using relaxant-free anesthesia or lower doses of NMBAs14,15. However, it has been shown that avoiding NMBAs cannot improve tracheal intubation conditions and can increase the risk of difficult tracheal intubation16. Considering the potentially serious consequences of difficult tracheal intubation, good IONM conditions should never be prioritized over good tracheal intubation conditions. Therefore, we used rocuronium as an NMBA with a reversal agent when IONM was planned. Previous studies have reported sugammadex after rocuronium use is useful for IONM13,17,18. While some studies suggest a dose of 2 mg/kg sugammadex for reversal of neuromuscular blockade prior to IONM13,17, one study comparing 1 mg/kg and 2 mg/kg sugammadex showed that a dose of 1 mg/kg sugammadex induced fewer intraoperative bucking events than a 2 mg/kg dose, while still providing comparable IONM quality5. This demonstrates that the goal of administering NMBAs is not complete recovery from neuromuscular blockade, but just enough reversal so that IONM is possible while still providing good anesthetic and surgical conditions. In fact, when successful V1 stimulation was detected in Group C, the TOF counts were all below 3/4, but high-quality IONM was still possible. Because the threshold for the reversal of neuromuscular blockade is different for each muscle and nerve of the body, TOF monitoring applied at the adductor pollicis muscle may not have been able to accurately reflect reversal of muscles innervated with the vagus nerve or the RLN. This is in line with previous studies that have suggested that TOF monitoring may be of little value in IONM9,19,20. Therefore, we believed that reversal agents less potent than sugammadex, such as neostigmine, could be an alternative reversal agent for qualified IONM.
A previous observational study presented the use of neostigmine as a possible reversal agent for IONM9. Neostigmine is a commonly used reversal agent because it is safe, effective, inexpensive, and readily available21. The present study showed that the use of neostigmine after tracheal intubation did not cause a time delay until IONM was possible and provided adequate IONM quality without significantly increasing the incidence of intraoperative bucking movement during thyroid surgery. Now, it has been established that the use of neostigmine is beneficial for IONM during thyroid surgery in comparison with no use of reversal agents. Further studies comparing neostigmine and sugammadex are needed to determine the optimal strategy for IONM in thyroid surgery.
The optimal time to administer reversal agents is also unknown. Many studies have reported the administration of reversal agents directly before IONM13,17,18,22. One study noted that when sugammadex was administered directly after tracheal intubation, up to 35% of patients experienced intraoperative bucking events, more than 60% of which occurred during skin incision and flap dissection5. Therefore, delayed administration of sugammadex may be beneficial, especially after strong surgical stimuli, such as skin incision or flap dissection. However, delayed administration of neostigmine may not have the same effect as sugammadex because neostigmine results in slower and less complete reversal. We administered neostigmine after tracheal intubation because we assumed that the effect of neostigmine might be maximized when administered after tracheal intubation. Neostigmine works by blocking acetylcholinesterase activity, resulting in an increase of acetylcholine levels in the body. Its peak effect occurs in 10 min and duration of action is 20–30 min23. Considering the pharmacokinetics of neostigmine and the relatively short time from tracheal intubation to the initial V1 check (approximately 25 min) in our routine clinical practice, we planned to administer neostigmine after tracheal intubation even though it takes a longer time for the TOF ratio to recover if neostigmine is administered at the point of deep neuromuscular blockade24. In our experience, a high TOF ratio was not necessarily required for successful IONM during thyroid surgery5. One study in which neostigmine was given 30 min after NMBA administration for the induction of anesthesia reported that the IONM quality was significantly improved compared to that when no reversal agent was used12. In our study, the mean operative time was 48.6 ± 10.8 min and 54.3 ± 16.3 min in Groups N and C, respectively, and the mean time from rocuronium administration to neostigmine or placebo administration was approximately 7 min in all patients in both groups. The administration of reversal agents 30 min after anesthesia induction is not suitable for centers with short operative times. A previous IONM study, in which reversal agents were administered just after tracheal intubation, reported that only two out of 50 patients (4%) experienced intraoperative bucking events9. In the present study, the same timeline as the previous study was used for neostigmine administration in Group N, and one out of 20 patients (5%) in the group experienced intraoperative bucking events, which was lower than other reports in which sugammadex was administered5,6. Nevertheless, more research is needed to establish the optimal timing for the administration of reversal agents.
In this study, we defined four or more consecutive V1 signals > 100 μV responding to electrical stimulation currents as successful V1 stimulation. To the best of our knowledge, there is no clear reference on the definition of successful V1 stimulation. Therefore, we defined successful V1 stimulation by partially using the concept of TOF25. Four twitches to four stimulations means that approximately 25–30% recovery from neuromuscular blockade. Of course, this is not enough to recover self-respiration, but we believe that a nearly full recovery from neuromuscular blockade (TOF ratio > 0.7 or 0.9) is not required to obtain adequate conditions for IONM. In our protocol, we showed that the amplitude of the signal of V1 was sufficiently high (> 500 μV in 39 patients of both groups) when four or more consecutive twitches were detected upon V1 stimulation, even though the TOF count at the adductor pollicis muscle was 3 or less in 9/20 patients in Group N and 20/20 patients in Group C.
The question of whether sufficient neostigmine-induced reversal to create a clinically adequate condition for IONM happens as a sudden event or as a gradual process has not been previously addressed. In the present study, we defined four or more consecutive twitches responding to electrical stimulation as successful V1, and we rechecked the signal repeatedly in 1-min intervals until a successful V1 signal was produced for patients with unsuccessful initial V1 stimulation. Although we continuously checked the V1 signal to electrical stimulation at 1-min intervals in this study, the surgeon observed that successful V1 stimulation appeared rather abruptly. In other words, even if the recovery progress from neuromuscular blockade occurs gradually26, sudden successful V1 stimulation can be achieved in terms of the clinical condition for IONM, e.g., abrupt successful V1 stimulation 1 min after no or only one twitch. This might suggest that the neuromuscular blockade recovery needed for adequate IONM by reversal agents occurs in a narrow period of time. Even if reversal agents are not given, V1 should be checked at least every minute to minimize unnecessary loss of time in surgery.
There are some limitations to this study. First, factors other than the NMBAs and reversal agents may have affected the EMG results and were not controlled in the analysis. EMG amplitudes evoked by nerve stimulation vary from stimulation to stimulation and from patient to patient for multiple reasons including malposition of the endotracheal tube, manipulation of the gland or trachea during nerve stimulation, the volume of fluid in the surgical field, and nerve ensheathment within the fascia that may obstruct probe-to-nerve contact. Although we could not control for each of these points, much effort was put into creating a controlled environment for all surgical procedures. We also believe that this limitation was overcome to some extent by our randomized study design, despite the small sample size. Second, although we previously proposed using neostigmine as an alternative to sugammadex for IONM in thyroid surgeries, we did not compare neostigmine to sugammadex in the present study. This was because we aimed to first establish the feasibility of using neostigmine in comparison with the absence of reversal agents. We expect to see future studies that investigate the effects of neostigmine versus sugammadex as reversal agents for IONM during thyroid surgeries. Last, surgery was performed by a specialized and experienced endocrine surgeon, and thus, the operation times were relatively short. Therefore, the results of this study may not be generally applicable, and much caution is needed in the interpretation of the results. Nevertheless, the strength of this study was its randomization of patients, which prevented potential selection bias. We observed significant differences in the results between the two groups despite a small sample size.
In conclusion, the administration of neostigmine immediately after tracheal intubation successfully reversed the neuromuscular blockade induced by rocuronium without an increase in intraoperative bucking events. Therefore, neostigmine administration immediately after tracheal intubation may be useful to reverse neuromuscular blockade for successful IONM in thyroid surgeries.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Choi, S. Y. & Son, Y. I. Intraoperative neuromonitoring for thyroid surgery: The proven benefits and limitations. Clin. Exp. Otorhinolaryngol. 12, 335–336 (2019).
Lu, I. C., Wu, S. H. & Wu, C. W. Neuromuscular blockade management for intraoperative neural monitoring. Kaohsiung J. Med. Sci. 36, 230–235 (2020).
Venkatraghavan, L. et al. Effect of reversal of residual neuromuscular blockade on the amplitude of motor evoked potentials: A randomized controlled crossover study comparing sugammadex and placebo. Neurol. Sci. 43, 615–623 (2022).
O’Reilly-Shah, V. N. et al. Using a worldwide in-app survey to explore sugammadex usage patterns: A prospective observational study. Br. J. Anaesth. 119, 333–335 (2017).
Chai, Y. J. et al. Comparison of sugammadex dose for intraoperative neuromonitoring in thyroid surgery: A randomized controlled trial. Laryngoscope 131, 2154–2159 (2021).
Lu, I. C. et al. Reversal of rocuronium-induced neuromuscular blockade by sugammadex allows for optimization of neural monitoring of the recurrent laryngeal nerve. Laryngoscope 126, 1014–1019 (2016).
Luo, J. et al. Reevaluation and update on efficacy and safety of neostigmine for reversal of neuromuscular blockade. Ther. Clin. Risk Manag. 14, 2397–2406 (2018).
Orihara, M. et al. Comparison of incidence of anaphylaxis between sugammadex and neostigmine: A retrospective multicentre observational study. Br. J. Anaesth. 124, 154–163 (2020).
Oh, M. Y. et al. Investigation of potential neuropharmacological activity of neostigmine-glycopyrrolate for intraoperative neural monitoring in thyroid surgery. Kaohsiung J. Med. Sci. 38, 59–64 (2022).
Kim, J. et al. Feasibility of attachable ring stimulator for intraoperative neuromonitoring during thyroid surgery. Int. J. Endocrinol. 2020, 5280939 (2020).
Randolph, G. W. et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: International standards guideline statement. Laryngoscope 121(Suppl 1), S1-16 (2011).
Marshall, S. D., Boden, E. & Serpell, J. The effect of routine reversal of neuromuscular blockade on adequacy of recurrent laryngeal nerve stimulation during thyroid surgery. Anaesth. Intensive Care 43, 485–489 (2015).
Gunes, M. E. et al. Effect of intraoperative neuromonitoring on efficacy and safety using sugammadex in thyroid surgery: Randomized clinical trial. Ann. Surg. Treat. Res. 97, 282–290 (2019).
Han, Y. D., Liang, F. & Chen, P. Dosage effect of rocuronium on intraoperative neuromonitoring in patients undergoing thyroid surgery. Cell Biochem. Biophys. 71, 143–146 (2015).
Sneyd, J. R. & O’Sullivan, E. Tracheal intubation without neuromuscular blocking agents: is there any point?. Br. J. Anaesth. 104, 535–537 (2010).
Lundstrom, L. H. et al. Avoidance of neuromuscular blocking agents may increase the risk of difficult tracheal intubation: a cohort study of 103,812 consecutive adult patients recorded in the Danish Anaesthesia Database. Br. J. Anaesth. 103, 283–290 (2009).
Donmez, T. et al. Thyroid surgery, ionm and sugammadex sodium relationships: Benefits in sugammadex sodium use for ionm. Acta Endocrinol. (Buchar) 15, 454–459 (2019).
Empis de Vendin, O. et al. Recurrent laryngeal nerve monitoring and rocuronium: A selective sugammadex reversal protocol. World J. Surg. 41, 2298–2303 (2017).
Hemmerling, T. M. et al. Simultaneous determination of neuromuscular block at the larynx, diaphragm, adductor pollicis, orbicularis oculi and corrugator supercilii muscles. Br. J. Anaesth. 85, 856–860 (2000).
Lu, I. C. et al. Electromyographic study of differential sensitivity to succinylcholine of the diaphragm, laryngeal and somatic muscles: A swine model. Kaohsiung J. Med. Sci. 26, 640–646 (2010).
Nicolardot, J. et al. Neostigmine accelerates recovery from moderate mivacurium neuromuscular block independently of train-of-four count at injection: A randomised controlled trial. Br. J. Anaesth. 121, 497–499 (2018).
Namizato, D. et al. Anesthetic considerations of intraoperative neuromonitoring in thyroidectomy. J. Nippon Med. Sch. 86, 263–268 (2019).
Nair, V. P. & Hunter, J. M. Anticholinesterases and anticholinergic drugs. Contin. Educ. Anaesth. Crit. Care Pain 4, 164–168 (2004).
Bevan, J. C. et al. Early and late reversal of rocuronium and vecuronium with neostigmine in adults and children. Anesth. Analg. 89, 333–339 (1999).
McGrath, C. D. et al. Monitoring of neuromuscular block. Contin. Educ. Anaesth. Crit. Care Pain 6, 7–12 (2006).
Kim, K. S. et al. Relationshiop between first-twitch depression and -train-of-four ratio during sugammadex reversal of rocuronium-induced neuromuscular blockade. Korean J. Anesthesiol. 69, 239–243 (2016).
Funding
This research was supported by a grant of Patient-Centered Clinical Research Coordinating Center funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI19C0481, HC19C0103).
Author information
Authors and Affiliations
Contributions
Y.J.C. and J.M.L. designed and performed the study. M.Y.O. and J.M.L. analyzed the data. The main manuscript was written by M.Y.O. and Y.J.C., and revised by J.M.L. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oh, M.Y., Chai, Y.J., Huang, TY. et al. Administration of neostigmine after tracheal intubation shortens time to successful intraoperative neuromonitoring during thyroid surgery: a randomized controlled trial. Sci Rep 12, 16797 (2022). https://doi.org/10.1038/s41598-022-21282-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21282-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.