Abstract
Frailty and type 2 diabetes mellitus (T2DM) can occur concurrently and are increasingly prevalent in older populations. There is a marked variability in frailty progression between men and women. This study aimed to investigate sex differences in the prevalence and factors associated with frailty in older outpatients with T2DM. This multicentre cross-sectional study included 638 outpatients (aged ≥ 60 years; median age 71 years [interquartile range, 66–77]; male, 55.5%) and was conducted from January 2019 to July 2020. Frailty was assessed using the Fried frailty phenotype. Factors associated with frailty were assessed using a logistic regression analysis. The overall frailty prevalence was 28.2% (men, 26.8%; women, 29.9%; P = 0.388). In the adjusted model, the factors associated with greater odds of being frail were older age (odds ratio [OR], 1.08; 95% confidence interval [CI], 1.05–1.11; P < 0.001) and body mass index (BMI) less than 20 kg/m2 (OR, 1.96; 95% CI, 1.16–3.32; P = 0.012). Higher education (OR, 0.64; 95% CI, 0.42–0.98; P = 0.041) and productive work (OR, 0.11; 95% CI, 0.03–0.36; P < 0.001) were protective factors against frailty. Frailty was associated with all four factors in women, but only with older age and productive work in men. Our study found that the prevalence of frailty in older outpatients with T2DM was 28.2%, though not significantly different between men and women. While older age and BMI less than 20 kg/m2 can increase the odds of frailty, and higher education and productive work can decrease the odds of frailty in women, only age and productive work were associated with frailty in men with T2DM.
Similar content being viewed by others
Introduction
Frailty is an important geriatric syndrome characterised by increased vulnerability and decreased ability of older adults to regain homeostasis following stressor events1. The prevalence of frailty increases with age, and the presence and severity of this clinical state can influence the manifestation and clinical outcomes of multiple comorbidities, including endocrine and cardiovascular diseases2,3. Frailty is also a risk factor for the development of major adverse cardiovascular events4. Fortunately, the progression of frailty can be delayed by early diagnosis and appropriate intervention5.
Type 2 diabetes mellitus (T2DM) is a major global health and economic burden due to an ageing population6. Management of T2DM in older adults is difficult owing to the coexistence of geriatric syndromes, such as polypharmacy, multimorbidity, falls, delirium, and frailty7. Frailty is not only an independent predictor of incident T2DM in older adults8, but is also associated with mortality, complications, and lower quality of life in people with diabetes2. Although T2DM was confirmed not to be significantly correlate with frailty in the geriatric patients9, the identification and assessment of frailty is increasingly recognised in recent clinical guidelines for diabetes to determine targets and therapeutic approaches for older patients with T2DM10,11.
Accumulating evidence has shown that there is a male–female health-survival paradox in the older population. Although women have a longer life expectancy than men12, systematic reviews of community-dwelling populations have found greater levels of disability, more comorbidities, and a higher prevalence of frailty in older women than in older men13,14. The discrepancy between health and survival may suggest that women tolerate frailty better than men. It may also be related to the differences in social, behavioural, and biological factors between the two groups15,16.
Vietnam, a lower middle-income country, entered a growth phase in their ageing population in 2011. In 2019, people aged ≥ 60 years accounted for 13.2% (men, 6.1%; women, 7.2%) of the total Vietnamese population17. The country has also undergone an epidemiological transition, with health alterations from infectious diseases to non-communicable diseases18. In Vietnam, 6% of the total population had diabetes19, and among older individuals, the diabetes rate was approximately 29%20. However, little clinical information is available to understand whether the characteristics of frailty in older adults with T2DM differ between the sexes. Therefore, the aim of this study was to investigate sex differences in the prevalence and factors associated with frailty in older outpatients with T2DM.
Material and methods
Study design and participants
This cross-sectional study was conducted in outpatients aged ≥ 60 years with T2DM at three geriatric clinics from January 2019 to July 2020. Participants met the inclusion criteria if they were diagnosed with T2DM for one year or more before enrolment based on a fasting plasma glucose level of ≥ 7.0 mmol/L after no caloric intake for at least 8 h and/or haemoglobin A1c (HbA1c) level of ≥ 6.5%. To ensure consistent management, trained geriatricians treated all patients for T2DM with any medication and with individualised HbA1c targets based on the recent guidelines of the European Society of Cardiology and European Association for the Study of Diabetes11. Exclusion criteria were hospital admission, active malignancy, serious mental condition, or heart failure categorised as New York Heart Association class III-IV. All participants provided written informed consent and underwent a comprehensive geriatric assessment, including demographic characteristics, Fried frailty phenotype, and comorbidities. Our study follows The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement21.
Sample size calculation
The sample size was calculated for the first aim of this study using a single population proportion formula: n = Z21−α/2*[p*(1 − p)/d2], where n = the required sample size, Z1−α/2 = 1.96 (with α = 0.05, and 95% confidence interval), p = prevalence of frailty in older outpatients with T2DM in Vietnam, and d = precision (assumed as 0.04). Because the prevalence is unknown, we set p as 0.5 to obtain the maximum possible value of p*(1 − p) as 0.25. This study required a minimum of 600 participants.
Variables and definitions
Geriatricians managing the patients were responsible for collecting demographic data, clinical characteristics, and measuring body weight and height. Self-reported information was obtained on age, sex, marital status, level of education, living status (alone or with anyone), and productive work. The patients’ educational level was classified as lower education (no school, elementary, and junior high school) or higher education (senior high school, university, and above). Productive work was defined as participants having any form of paid or unpaid job. Comorbidities were obtained from interviews and electronic medical records. Polypharmacy and multimorbidity data were collected based on the prescriptions of the patients. Polypharmacy was defined as five or more medications22. Multimorbidity was defined as the presence of two or more chronic diseases23.
Body mass index (BMI) was calculated as the quotient of body weight (kg) and height (m2). Body weight and height were measured following a standardised protocol using identical equipment at all study sites. Because of the differences in body mass index classifications between the World Health Organization and Asia–Pacific guidelines, the BMI of our patients was categorised into five groups (< 20, 20–24.9, 25–29.9, 30–34.9, and ≥ 35 kg/m2) according to a previous study investigating the relationship between frailty and BMI in older people24.
Assessment of frailty
Patients were physically examined and placed into one of three categories using the Fried frailty phenotype: frail (≥ 3 criteria present), pre-frail (1–2 criteria present), or non-frail (0 criteria present)25. A Vietnamese version of Fried criteria was carefully explained to each patient, and caregivers were asked to ensure that the reporting context was correct (Table S1). The five components are as follows.
-
1.
Unintentional weight loss of ≥ 4.5 kg or ≥ 5% body weight in the past year.
-
2.
Weakness: Grip strength of the dominant hand was measured once in the sitting position using a Jamar 5030-J1 hydraulic hand dynamometer (JLW Instruments, Chicago, IL 60,607, United States) with relaxed shoulders and encouragement. Weakness was defined as the lowest quintile of grip strength, stratified according to sex and body mass index (BMI). The BMI cut-off points were ≤ 29.0, ≤ 30.0, and ≤ 32.0 kg for BMI ≤ 24.0, 24.1–28.0, and > 28.0, respectively, in men and ≤ 17.0, ≤ 17.3, ≤ 18.0, and ≤ 21.0 kg for BMI ≤ 23.0; 23.1–26.0; ≤ 26.1–29.0, and > 29.0, respectively, in women.
-
3.
Exhaustion: Two questions from the Centre for Epidemiologic Studies Depression Scale were used: ‘I felt that everything I did was an effort last week’ and ‘I could not get going last week’25. Participants answering ‘frequently’ or ‘always’ to at least one of these two questions were categorised as having met the criterion for exhaustion.
-
4.
Slowness: The walking time of participants over a 4.57 m distance was adjusted for gender and height. The cut-off points for slow walking speed were established as height ≤ 173 cm and time ≥ 7 s (equivalent to 0.65 m/s) or height > 1.73 cm and time ≥ 6 s (equivalent to 0.76 m/s) for men, and height ≤ 1.59 cm and time ≥ 7 s (0.65 m/s) or height > 1.59 cm and time ≥ 6 s (0.76 m/s) for women.
-
5.
Low physical activity: We used the short version of the Minnesota Leisure Time Activity questionnaire, which included questions on 18 activities: walking, chores, mowing the lawn, raking, gardening, hiking, jogging, biking, exercise cycling, dancing, aerobics, bowling, golf, singles tennis, doubles tennis, racquetball, callisthenics, and swimming25. The total weekly kilocalories of physical activity expenditure were calculated using a standardised algorithm. Low activity levels were defined as < 383 kcal in men and < 270 kcal in women.
Statistical analysis
All collected data were analysed using the IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA). Categorical variables were described as frequencies and percentages (%). The Kolmogorov–Smirnov test was conducted to assess the distribution of continuous variables. Continuous variables were described using means and standard deviations for normal distribution and median and interquartile range (IQR) (25–75th percentile) for non-normal distribution. Chi-square test or Fisher’s exact test was used to compare categorical variables. Student’s t-test or one-way ANOVA was used to determine the statistical significance of the difference between two or more study group means. The Mann–Whitney and Kruskal–Wallis tests were used to compare two or more groups with non-normal distribution. To determine factors associated with frailty, the non-frail and pre-frail groups were pooled together in a non-frail group. Univariate logistic regression analysis was performed to identify potential factors associated with frailty. Variables with P values < 0.2 in the univariate analysis, were selected for multivariate logistic regression. All variables were examined for their interaction and multicollinearity. Multicollinearity was assessed using variance inflation factor (VIF)26. Multicollinearity is present when the VIF is higher than 5. All tests were two-sided, and the significance level was set at P < 0.05.
Ethical approval
The present study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam (approval number, 01/QĐ-ĐHYD [approved on January 16, 2019]).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Prevalence of frailty in older outpatients with T2DM
Of the 2215 patients admitted to our geriatric clinics during the study period, 1577 were excluded because they did not have diabetes (1544 patients), required hospital admission (17 patients), had serious mental conditions (6 patients), or had missing responses (10 patients). Figure 1 shows the flow diagram for the sample selection. The 638 older patients with T2DM enrolled in this study had a median age of 71 years (IQR: 66–77; range, 60–92) and male predominance (55.5%). The overall prevalence of non-frail, pre-frail, and frail categories was 17.9% (n = 114), 53.9% (n = 344), and 28.2% (n = 180), respectively.
Differences in main characteristics between men and women
Table 1 summarises the characteristics of the patients according to sex. The men and women did not differ in terms of living status, productive work, polypharmacy, multimorbidity, and some medical disorders (i.e. hypertension, heart failure, stroke, and chronic pulmonary diseases). However, there were significant differences in age, marital status, level of education, BMI group, coronary artery disease, osteoarthritis, and chronic kidney disease between the two groups. The women’s group was significantly older than the men’s group, and there were more women than men aged 75 years and over (46.1% vs. 31.1%, P < 0.001). Importantly, the women’s group had a significantly lower rate of higher education than the men’s group (37.7% vs. 76.3%, P < 0.001). The two most common conditions in both groups were hypertension and osteoarthritis, followed by chronic kidney disease in the men’s group and coronary heart disease in the women’s group.
Differences in main characteristics between the Fried frailty phenotype groups
To further understand the characteristics of frailty in older outpatients with T2DM, we compared the participants according to the frailty phenotype (Table 2). There was a trend for increasing age between the three groups with the median age of non-frail, pre-frail, and frail individuals being 68, 70, and 76 years, respectively (P < 0.001). Furthermore, there were significant differences between the three frailty phenotype groups in terms of sex, BMI, and productive work. Women (47.2%) and BMI < 20 kg/m2 (22.2%) were the most prevalent, whereas those with any form of job (1.7%) were the lowest in the frail group. Although there were no statistically significant differences among the three groups in marital status, level of education, living alone, polypharmacy, multimorbidity, and medical disorders, the rate of divorced/widowed was higher in the frail group than in the pre-frail and non-frail groups (31.1%, 23.0%, and 16.7%, respectively), and there were fewer highly educated older adults in the frail group than in the pre-frail and non-frail groups (52.8%, 60.2%, and 65.8%, respectively). Hypertension, osteoarthritis, and chronic kidney disease were the three most prevalent medical disorders, and were more frequently reported in the frail group.
Differences in Fried frailty phenotype components between men and women
In this study, frailty status varied according to sex. Frailty was more prevalent in women than in men (29.9% and 26.8%, respectively), but the difference was not statistically significant for the category of two states of the Fried frailty phenotype (P = 0.388) (Table 3). Of the five Fried frailty phenotype components, the proportion of participants with low grip strength was the highest (68.0%). This criterion was also the most prevalent among the men and women’s groups. Compared with the men’s group, the women’s group had higher rates of lower grip strength (75.4% vs. 62.1%, P = 0.001) and low walking speed (44.7% vs. 26.8%, P < 0.001), but a lower rate of less physical activity (31.3% vs. 43.8%, P = 0.002).
Associated factors of frailty in older outpatients with T2DM
Univariate and multivariate logistic regression analyses were performed to identify the potential factors associated with frailty (Table 4). In the adjusted model, two factors that increased the odds of frailty were older age and BMI < 20 mg/m2. In contrast, higher education and productive work were inversely associated with frailty. While all four factors were associated with frailty in women, only age and productive work were associated with frailty in men (Table 5).
Discussion
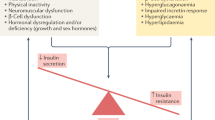
Frailty is an expression of physiological impairments and decreased functional reserve in multiple organ systems as a part of the ageing process, whereas T2DM is a pathological dysfunction characterised by hyperglycaemia and insulin resistance. These two clinical states can appear concurrently or consecutively in older adults2. They share several common pathophysiological mechanisms, such as metabolic impairment, increased oxidative stress, inflammatory dysregulation, and sarcopenia27. The presence of frailty in older patients with T2DM can increase the likelihood of adverse events and mortality28,29, whereas pathological metabolic changes in T2DM can increase the likelihood of frailty in older adults27. Nevertheless, sex differences in the patterns of frailty among older patients with T2DM are not fully understood. The results of this study provide three key observations. First, the prevalence of frailty in older outpatients with T2DM in Vietnam was 28.2%. There was no significant difference in prevalence of frailty between men and women. Second, there were differences in some characteristics between the men’s and women’s groups. Third, older age, productive work, higher education, and BMI < 20 kg/m2 were associated with frailty in the total study population and women, but only the first two factors were associated with frailty in men.
In a recent meta-analysis, the median community frailty prevalence using the frailty phenotype in individuals with diabetes was 13%, whereas the prevalence of frailty in outpatient populations varied widely due to heterogeneity in study settings, demographics, and especially in frailty assessment methods and differences in how frailty components were specified2. Our multicentre study is the first in Vietnam to find that the prevalence of frailty in older outpatients with T2DM was 28.2% when the Fried phenotype was used. Despite the differences in frailty assessment methods, our results are in accordance with the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial using the frailty index and a recent study in Taiwan using the Tilburg frailty indicator showed that the prevalence of frailty in older outpatients with T2DM were 25.6% and 26.6%, respectively30,31. Since the benefits from intensive glucose-lowering and glycaemic goals were altered in patients with frailty10,30, the high overlap between the two states found in the previous studies and ours highlights the importance of identifying frailty in older patients with diabetes.
The physiological variability in the ageing process between men and women and sex differences in biological factors (e.g. inflammatory cytokines, abdominal adiposity, and cognitive impairment) and psychosocial factors (e.g. healthcare utilisation and self-reported behaviours) may interactively contribute to sex differences in frailty32. A meta-analysis showed that older women had higher frailty index scores than older men12. However, the female sex as an associated factor of frailty was found in some studies, but no significant association was found in other studies33,34. Among older adults with T2DM, a recent study revealed a higher rate of frailty in women than in men, but sex was not a significant factor associated with frailty31. Based on the positive association between age and frailty33, the greater prevalence of frailty in our women’s group may be related to a higher mean age in the women’s group than in the men’s group. Further research in a larger population and long-term follow-up is needed to elucidate the presence or absence of an association between the female sex and frailty.
Previous evidence from the Study of Osteoporotic Fractures showed that older women with diabetes had a greater decline in walking speed, but not in handgrip strength, than older women without diabetes35. This finding can be explained by the results from the Health, Aging, and Body Composition Study showing a decline in leg muscle strength and quality, but no differences in arm muscle strength and quality between older adults with and without T2DM36. However, interestingly, our study revealed that low grip strength was the most frequent component of the frailty phenotype in older adults with T2DM (68.0%), but not low walking speed. Our contradictory findings can be explained by variations within the Fried frailty criteria used, especially in handgrip strength protocols. Although handgrip strength is a reliable assessment of muscle weakness, the values of this method could be influenced by many factors, such as different dynamometer, posture and arm position, dominant and non-dominant hands, and the differences in normative reference values stratified by the BMI cut-off points between world regions37,38. Our study used the original Fried frailty criteria with grip strength stratified by the BMI cut-off points for developed regions25, but not for developing regions such as Vietnam. This may explain the high rate of weakness assessed by grip strength in our study population. While Tamura et al.39 set the cut-off points for weakness as handgrip strength < 26 kg in men and < 18 kg in women regardless of the BMI, they found 49.5% of older outpatients with cardiometabolic disease having low grip strength. It was also the most frequent component of the Fried phenotype.
Consistent with two previous studies including older adults with diabetes39,40, our study found significantly lower handgrip strength and slower walking speed in women than in men. These findings can be explained by several mechanisms. First, muscle mass and strength are determined by metabolic characteristics based on the regulation of sex hormones in nutrient sensing and the metabolism of organic compounds. In men, higher basal insulin levels promote more glycogen synthesis in muscle cells, and higher testosterone levels coupled with upregulated insulin-like growth factor signalling, results in greater muscle mass and strength41. A lower skeletal muscle mass than men, and oestrogen deficiency upon menopause negatively affects skeletal muscle protein turnover in women42,43. Second, in the older population, insulin resistance which can result in protein degradation, is associated with decreased quadriceps muscle strength and is a major risk factor for sarcopenia44,45. A recent study revealed that only older women with diabetes showed a higher prevalence of sarcopenia than those without diabetes, but these were not different in older men40. Third, women have a higher percentage of body fat than men, and there are sex differences in fat distribution. While men tend to have a central fat distribution with more abdominal visceral fat, women have a peripheral fat distribution with greater adipose tissue in the hips and thighs46. The data from the Framingham Heart Study revealed the impact of fat distribution on physical strength when intramuscular fat was associated with increased odds of low walking speed47. Taken together, the mechanisms may explain why men are faster and stronger than women, but other factors still contribute to the differences in physical performance between older men and women with T2DM (Table S1).
Frailty is a geriatric syndrome that is affected simultaneously by many sociodemographic, physical, biological, and psychological factors. Identifying these factors may help geriatricians recognise those with a high likelihood of frailty. Importantly, understanding the disparities in the factors associated with frailty between men and women, may be necessary to develop an individualised approach for frailty prevention and management12. Our study found that age, BMI, working status, and levels of education were factors associated with frailty. First, the findings are consistent with previous studies showing a positive association between frailty and increased chronological age in the overall older population, based on epidemiological evidence and biological mechanisms33,48. The putative mechanisms of increased susceptibility to frailty with ageing include loss of proteostasis, genomic instability, inflammation, epigenetic alterations, loss of stem cell regeneration, telomere shortening, deregulated nutrient sensing, and mitochondrial dysfunction49. Second, BMI is an important physical factor with a U-shaped association with frailty, in that BMI ≥ 25 kg/m2 and BMI < 20 kg/m2 were associated with a higher prevalence of frailty in older adults50. However, little is known about the relationship between BMI and frailty in the older population with diabetes. Although excess body weight is a major risk factor for T2DM, there was a significantly decreased risk of mortality in overweight patients as compared with normal weight patients, and the survival benefits of obesity were only detected in older patients51. Our study found that only underweight, defined as BMI < 20 kg/m2, was associated with frailty in older patients with T2DM. Importantly, this association was only present in the women’s group. This may be related to the fact that older men have a greater percentage of skeletal muscle mass than older women52, and BMI is inadequate to reflect older adults' strengths. Further studies are needed to understand the impact of BMI on frailty among older adults with T2DM.
The two protective factors of frailty found in our study were productive work and higher education. Previous studies have shown that older adults who continue to work or engage in any productive work beyond the retirement age are less likely to become frail. Prolonged work participation may offer a sense of independence and social connections for older individuals53. However, until now, the impact of the type and density of work on frailty remains elusive. In addition to productive work, many studies have revealed that higher education levels can place older adults at a lower risk of being frail54,55. Although education is not directly related to the pathophysiology of frailty, a better education may impact an individual’s lifestyle, such as increased awareness of healthy behaviours or increased ability to access social support and health services that may influence the progression of frailty55. Interestingly, higher education was a protective factor only in women, but not in men in the current study. This finding may require more insight into the sex-related preventive effects of higher education in the older population.
Our study has several limitations. First, the prevalence of frailty and burden of medical disorders in our study may not be generalisable to the general older adult population since the sample only included patients visiting geriatric clinics. Second, the study was performed at only three geriatric clinics in Vietnam. The results of our study may not be transferable to the general population or to other countries. Third, the impact of comorbidities and marital status on frailty was not fully evaluated because of the low rates of some diseases and the low rate of single participants in our study. Fourth, we were unable to evaluate the impact of specific antidiabetic agents and glycaemic goals on frailty because there were often switches of treatment regimens for diabetes and different individualised HbA1c goals in every patient. Fifth, due to the cross-sectional nature of the study design, we could not evaluate causal relationships between frailty and the associated factors. Further longitudinal studies are warranted to clarify these relationships.
Conclusions
This is the first study to determine the prevalence of frailty in older patients with T2DM visiting geriatric clinics in Vietnam. Our findings add to the literature by demonstrating that older age and BMI < 20 kg/m2 were associated with increased odds of frailty, whereas higher education and productive work were associated with decreased odds of frailty. The sex differences in frailty in our geriatric outpatients with T2DM may suggest appropriate sex-related approaches to the management of frailty in these patients.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Hoogendijk, E. O. et al. Frailty: Implications for clinical practice and public health. Lancet 394, 1365–1375. https://doi.org/10.1016/S0140-6736(19)31786-6 (2019).
Hanlon, P. et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: A systematic review and study-level meta-analysis. Lancet Healthy Longev. 1, e106–e116. https://doi.org/10.1016/S2666-7568(20)30014-3 (2020).
Damluji, A. A. et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur. Heart J. 42, 3856–3865. https://doi.org/10.1093/eurheartj/ehab468 (2021).
Orkaby, A. R. Moving beyond chronological age: Frailty as an important risk factor for cardiovascular disease. Eur. Heart J. 42, 3866–3868. https://doi.org/10.1093/eurheartj/ehab481 (2021).
Travers, J., Romero-Ortuno, R., Bailey, J. & Cooney, M.-T. Delaying and reversing frailty: A systematic review of primary care interventions. Br. J. Gen. Pract. 69, e61–e69. https://doi.org/10.3399/bjgp18X700241 (2019).
Sinclair, A. et al. Diabetes and global ageing among 65–99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 162, 108078. https://doi.org/10.1016/j.diabres.2020.108078 (2020).
Yang, Y. C. et al. Geriatric syndromes and quality of life in older adults with diabetes. Geriatr. Gerontol. Int. 19, 518–524. https://doi.org/10.1111/ggi.13654 (2019).
Veronese, N. et al. Frailty is associated with an increased risk of incident type 2 diabetes in the elderly. J. Am. Med. Dir. Assoc. 17, 902–907. https://doi.org/10.1016/j.jamda.2016.04.021 (2016).
Wojszel, Z. B. & Magnuszewski, L. Type 2 diabetes correlates with comorbidity and nutritional status but not with functional health in geriatric ward patients: A cross-sectional study in Poland. Diabetes Metab. Syndr. Obes. 13, 4599–4607. https://doi.org/10.2147/DMSO.S279388 (2020).
American Diabetes, A. 12. Older adults: Standards of medical care in diabetes-2021. Diabetes Care 44, S168–S179. https://doi.org/10.2337/dc21-S012 (2021).
Cosentino, F. et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 41, 255–323. https://doi.org/10.1093/eurheartj/ehz486 (2020).
Gordon, E. H. et al. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 89, 30–40. https://doi.org/10.1016/j.exger.2016.12.021 (2017).
Collard, R. M., Boter, H., Schoevers, R. A. & Voshaar, R. C. O. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 60, 1487–1492. https://doi.org/10.1111/j.1532-5415.2012.04054.x (2012).
Shamliyan, T., Talley, K. M. C., Ramakrishnan, R. & Kane, R. L. Association of frailty with survival: A systematic literature review. Ageing Res. Rev. 12, 719–736. https://doi.org/10.1016/j.arr.2012.03.001 (2013).
Oksuzyan, A., Juel, K., Vaupel, J. W. & Christensen, K. Men: Good health and high mortality. Sex differences in health and aging. Aging Clin. Exp. Res. 20, 91–102. https://doi.org/10.1007/BF03324754 (2008).
Hubbard, R. E. & Rockwood, K. Frailty in older women. Maturitas 69, 203–207. https://doi.org/10.1016/j.maturitas.2011.04.006 (2011).
WHO. World Report on Aging and Health. Available online: https://www.worldometers.info/world-population/vietnam-population/. Accessed June 17, 2021). (2017).
Hinh, N. D. & Minh, H. V. Public health in Vietnam: Scientific evidence for policy changes and interventions. Glob. Health Action 6, 20443. https://doi.org/10.3402/gha.v6i0.20443 (2013).
Ngoc, N. B., Lin, Z. L. & Ahmed, W. Diabetes: What challenges lie ahead for Vietnam?. Ann. Glob. Health 86, 1. https://doi.org/10.5334/aogh.2526 (2020).
Nguyen, H. T., Nguyen, A. H. & Nguyen, G. T. X. Prevalence and associated factors of frailty in patients attending rural and urban geriatric clinics. Australas. J. Ageing https://doi.org/10.1111/ajag.13016 (2021).
von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 61, 344–349. https://doi.org/10.1016/j.jclinepi.2007.11.008 (2008).
Palmer, K. et al. Association of polypharmacy and hyperpolypharmacy with frailty states: A systematic review and meta-analysis. Eur. Geriatr. Med. 10, 9–36. https://doi.org/10.1007/s41999-018-0124-5 (2019).
Vetrano, D. L. et al. Frailty and multimorbidity: A systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 74, 659–666. https://doi.org/10.1093/gerona/gly110 (2019).
Hubbard, R. E., Lang, I. A., Llewellyn, D. J. & Rockwood, K. Frailty, body mass index, and abdominal obesity in older people. J. Gerontol. A Biol. Sci. Med. Sci. 65, 377–381. https://doi.org/10.1093/gerona/glp186 (2010).
Fried, L. P. et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156. https://doi.org/10.1093/gerona/56.3.m146 (2001).
Vatcheva, K. P., Lee, M., McCormick, J. B. & Rahbar, M. H. Multicollinearity in regression analyses conducted in epidemiologic studies. Epidemiology (Sunnyvale) 6, 227. https://doi.org/10.4172/2161-1165.1000227 (2016).
Morley, J. E., Malmstrom, T. K., Rodriguez-Mañas, L. & Sinclair, A. J. Frailty, sarcopenia and diabetes. J. Am. Med. Dir. Assoc. 15, 853–859. https://doi.org/10.1016/j.jamda.2014.10.001 (2014).
Wong, E. et al. Diabetes and risk of physical disability in adults: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 1, 106–114. https://doi.org/10.1016/S2213-8587(13)70046-9 (2013).
Cacciatore, F. et al. Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta Diabetol. 50, 251–260. https://doi.org/10.1007/s00592-012-0413-2 (2013).
Nguyen, T. N. et al. The impact of frailty on the effectiveness and safety of intensive glucose control and blood pressure-lowering therapy for people with type 2 diabetes: Results from the ADVANCE trial. Diabetes Care 44, 1622–1629. https://doi.org/10.2337/dc20-2664 (2021).
Lin, C. L., Yu, N. C., Wu, H. C. & Liu, Y. C. Risk factors associated with frailty in older adults with type 2 diabetes: A cross-sectional study. J. Clin. Nurs. https://doi.org/10.1111/jocn.15953 (2021).
Hagg, S. & Jylhava, J. Sex differences in biological aging with a focus on human studies. Elife https://doi.org/10.7554/eLife.63425 (2021).
Feng, Z. et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS ONE 12, e0178383. https://doi.org/10.1371/journal.pone.0178383 (2017).
Llibre Rodriguez, J. J. et al. The prevalence and correlates of frailty in urban and rural populations in Latin America, China, and India: A 10/66 population-based survey. J. Am. Med. Dir. Assoc. 19, 287-295 e284. https://doi.org/10.1016/j.jamda.2017.09.026 (2018).
Lee, C. G. et al. Changes in physical performance in older women according to presence and treatment of diabetes mellitus. J. Am. Geriatr. Soc. 61, 1872–1878. https://doi.org/10.1111/jgs.12502 (2013).
Park, S. W. et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 30, 1507–1512. https://doi.org/10.2337/dc06-2537 (2007).
Sousa-Santos, A. R. & Amaral, T. F. Differences in handgrip strength protocols to identify sarcopenia and frailty: A systematic review. BMC Geriatr. 17, 238. https://doi.org/10.1186/s12877-017-0625-y (2017).
Dodds, R. M. et al. Global variation in grip strength: A systematic review and meta-analysis of normative data. Age Ageing 45, 209–216. https://doi.org/10.1093/ageing/afv192 (2016).
Tamura, Y. et al. Prevalence of frailty, cognitive impairment, and sarcopenia in outpatients with cardiometabolic disease in a frailty clinic. BMC Geriatr. 18, 264. https://doi.org/10.1186/s12877-018-0955-4 (2018).
Kang, S. et al. Sex differences in sarcopenia and frailty among community-dwelling Korean older adults with diabetes: The Korean frailty and aging cohort study. J. Diabetes Investig. 12, 155–164. https://doi.org/10.1111/jdi.13348 (2021).
Comitato, R., Saba, A., Turrini, A., Arganini, C. & Virgili, F. Sex hormones and macronutrient metabolism. Crit. Rev. Food Sci. Nutr. 55, 227–241. https://doi.org/10.1080/10408398.2011.651177 (2015).
Doherty, T. J. Invited review: Aging and sarcopenia. J. Appl. Physiol. (1985) 95, 1717–1727. https://doi.org/10.1152/japplphysiol.00347.2003 (2003).
Collins, B. C., Laakkonen, E. K. & Lowe, D. A. Aging of the musculoskeletal system: How the loss of estrogen impacts muscle strength. Bone 123, 137–144. https://doi.org/10.1016/j.bone.2019.03.033 (2019).
Barzilay, J. I. et al. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged ≥ 70 years. Diabetes Care 32, 736–738. https://doi.org/10.2337/dc08-1781 (2009).
Wang, X., Hu, Z., Hu, J., Du, J. & Mitch, W. E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147, 4160–4168. https://doi.org/10.1210/en.2006-0251 (2006).
Karastergiou, K., Smith, S. R., Greenberg, A. S. & Fried, S. K. Sex differences in human adipose tissues: The biology of pear shape. Biol. Sex Differ. 3, 13. https://doi.org/10.1186/2042-6410-3-13 (2012).
Therkelsen, K. E., Pedley, A., Hoffmann, U., Fox, C. S. & Murabito, J. M. Intramuscular fat and physical performance at the Framingham Heart Study. Age (Dordr) 38, 31. https://doi.org/10.1007/s11357-016-9893-2 (2016).
Ferrucci, L., Levine, M. E., Kuo, P. L. & Simonsick, E. M. Time and the metrics of aging. Circ. Res. 123, 740–744. https://doi.org/10.1161/CIRCRESAHA.118.312816 (2018).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039 (2013).
Rietman, M. L. et al. The association between BMI and different frailty domains: A U-shaped curve?. J. Nutr. Health Aging 22, 8–15. https://doi.org/10.1007/s12603-016-0854-3 (2018).
Gao, F. et al. Impact of obesity on mortality in patients with diabetes: Meta-analysis of 20 studies including 250,016 patients. J. Diabetes Investig. 9, 44–54. https://doi.org/10.1111/jdi.12677 (2018).
Janssen, I., Heymsfield, S. B., Wang, Z. M. & Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. (1985) 89, 81–88. https://doi.org/10.1152/jappl.2000.89.1.81 (2000).
Sewdas, R. et al. Why older workers work beyond the retirement age: A qualitative study. BMC Public Health 17, 672. https://doi.org/10.1186/s12889-017-4675-z (2017).
Hoogendijk, E. O. et al. Explaining the association between educational level and frailty in older adults: Results from a 13-year longitudinal study in the Netherlands. Ann. Epidemiol. 24, 538-544 e532. https://doi.org/10.1016/j.annepidem.2014.05.002 (2014).
Kendhapedi, K. K. & Devasenapathy, N. Prevalence and factors associated with frailty among community-dwelling older people in rural Thanjavur district of South India: A cross-sectional study. BMJ Open 9, e032904. https://doi.org/10.1136/bmjopen-2019-032904 (2019).
Acknowledgements
We thank the patients for their participation in our study.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
H.T.N. conceived and designed the research. All authors were involved in data collection. H.T.N. and A.H.N. performed the statistical analysis. H.T.N. wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, H.T., Nguyen, A.H. & Le, P.T.M. Sex differences in frailty of geriatric outpatients with type 2 diabetes mellitus: a multicentre cross-sectional study. Sci Rep 12, 16122 (2022). https://doi.org/10.1038/s41598-022-20678-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20678-7
This article is cited by
-
Potential confounders of the obesity paradox in older patients following transcatheter aortic valve replacement
European Geriatric Medicine (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.