Abstract
Circadian misalignments have been linked to adverse cardiometabolic outcomes. However, the association between irregular daily routine and the risk of cardiovascular disease (CVD) remains unknown. We examined this association in a prospective study in Japan. The study included 78,115 Japanese participants aged 45–74 years. The self-reported daily routine was evaluated using the question, ‘Is your daily routine or activity schedule regular?’ The response (yes/no) was obtained as a binary variable. Cox proportional hazard regression analysis was used to estimate the hazard ratios and 95% confidence intervals for the association between an irregular daily routine and CVD incidence risk. Among the participants, 23.7% reported an irregular daily routine. During the mean follow-up period of 13.3 years, we observed 4641 CVD events. An irregular daily routine was significantly associated with increased risks of CVD and total stroke in women, but not in men. This positive association between an irregular daily routine and the risk of CVD was weak in the high vegetable and fruit consuming population. An irregular daily routine is positively associated with the risk of incident CVD, especially in women. These associations may be weak in populations that consume a diet rich in vegetables and fruits.
Similar content being viewed by others
Introduction
Current lifestyles in developed countries have been affected and modified by multiple aspects of the 24/7 (24 h a day and 7 days a week) society. In society, daily exposure to artificial light at night, multimedia device usage in bedrooms, staying up late, skipping breakfast, late dinner time, gaps between non-working and working day schedules, working overtime, and flexible work arrangements are prevalent. In such an environment, circadian misalignments (i.e. inappropriate behavioural cycles relative to biological rhythms) may be a general phenomenon. They could occur by exposure to an irregular daily routine, mainly determined by daily sleep and meal schedules1.
Recently, several experimental studies have shed light on the pivotal role of circadian alignment between the circadian clock in the suprachiasmatic nucleus and the sleep–wake cycle, light–dark cycle, and other peripheral clocks as a potential benefit against health problems2,3,4,5,6,7,8,9. Moreover, because of the circadian patterns of all cardiovascular functions and time-of-day variations in adverse cardiovascular events, a large degree of circadian misalignment (e.g. day-night reversal) has been reported to be associated with cardiovascular disease (CVD) risk in a meta-analysis of cohort studies10,11,12,13.
An irregular daily routine could be associated with CVD risk. For example, in the Multi-Ethnic Study of Atherosclerosis, regular sleep–wake schedules were assessed based on seven-day actigraph data collected under a free-living situation and were associated with increased levels of cardiometabolic outcomes, such as obesity, hypertension, and metabolic syndrome, as well as with increased risk of CVD in a cross-sectional14,15 and prospective study (4.9-year follow-up period) performed in a large population of older adults16. Moreover, breakfast frequency has also been associated with the risk of CVD17,18,19,20. Those who consider themselves as following an irregular daily routine might be more likely to indulge in behaviours, such as the instability of sleep–wake and eating schedules21, that lead to circadian misalignments. However, to the best of our knowledge, the association between a self-reported irregular daily routine, which is mainly indicative of the stability of sleep–wake and meal schedules, and the CVD risk is still unknown.
Therefore, this study aimed to elucidate the association between a self-reported irregular daily routine and the risk of incident CVD in a large-scale, middle- and older-aged population-based prospective study. Furthermore, some individuals with an irregular daily routine cannot change their behavioural patterns or schedules, even if they want to, because of work-related, physical, or environmental reasons. Because dietary habits are one of the changeable factors and vegetable and fruit consumption has been associated with CVD risk22, this may lead to new insights into these populations. Therefore, we also investigated whether other potential factors, such as vegetable and fruit consumption or demographic characteristics, could modify the association between a self-reported irregular daily routine and CVD risk. We hypothesised that an irregular daily routine was associated with the increased risk of CVD and that these associations were modified by vegetable and dietary intake or demographic characteristics.
Materials and methods
Study population
The Japan Public Health Center-Based Prospective Study (JPHC Study) is a prospective study performed using a population-based sample of 140,420 Japanese adults (68,722 men and 71,698 women) in two cohorts based on public health centre (PHC) areas: Cohort I (started in 1990, five PHC areas) and Cohort II (started in 1993, six PHC areas).
The residents of these 11 PHC areas were identified through population registries managed by local municipalities. A previous study has presented the details of the JPHC Study protocol23. Since participants from the Tokyo and Osaka areas were excluded from the present study due to unavailable incidence data, 116,896 participants (57,713 men and 59,183 women) from nine PHC areas were considered eligible for follow-up. Informed consent was obtained from each participant implicitly when they completed the baseline questionnaire, where the study purpose and follow-up procedures were described. This study followed the tenets of the Declaration of Helsinki and was approved by the institutional review boards of Azabu University, Toyo University, and the National Cancer Center, Tokyo, Japan.
A self-administered questionnaire was distributed to all residents in the study areas for Cohorts I and II. The questionnaire included items that captured demographic characteristics, medical history, smoking and drinking habits, and a simplified version of dietary habits. A 5-year follow-up survey was conducted for Cohort I in 1995 and Cohort II in 1998 using a questionnaire that included items regarding irregular routine in daily life and demographic characteristics, in addition to a more comprehensive food frequency questionnaire (FFQ). Therefore, we used these questionnaires as the starting point to assess the association between irregular daily routine and CVD incidence risk.
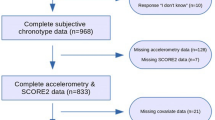
We excluded participants with non-Japanese nationality, migration procedures that had not been completed before the starting point, incorrect birth date, duplicate registration, and those who refused follow-up, died, moved out of the study areas, or were lost to follow-up before the starting point. Finally, 116,068 participants were eligible for this study. Among them, 92,558 participants responded to the 5-year follow-up questionnaire, yielding a response rate of 79.7%, and were included. Among these participants, we excluded those who were diagnosed with CVD in the period from the baseline survey to the 5-year follow-up survey (n = 2394) and those who had a past history of cancer (e.g. stomach, lung, bowel, liver, breast, uterine cancer, or other cancers), stroke, and ischaemic heart disease (or coronary heart disease [CHD]), as identified by the questionnaire (n = 5548). Furthermore, 1008 participants who did not complete the diet section of the questionnaire, 2025 participants with extreme total energy intake (lower and upper 1.0 percentile for men and women: 785 and 5096 kcal/day, and 656 and 4472 kcal/day, respectively), and 3468 participants who did not respond to the questions on irregular daily routine were excluded. Finally, 78,115 participants (36,210 men and 41,905 women) were included in the statistical analyses (Fig. 1).
Assessment of irregular daily routine and dietary intake
The self-reported irregular daily routine was assessed by the response to the question, ‘Is your daily routine or activity schedule regular?’ in the questionnaire. The possible response to this question was ‘yes’ or ‘no’. Participants who responded with ‘no’ were considered to have an irregular daily routine; participants who responded with ‘yes’ were included as the reference group. Dietary intake was assessed using the FFQ, consisting of 138 food and beverage items. The 5-year follow-up questionnaire was based on nine frequency categories (‘almost never’ to ‘seven or more times per day’) for vegetables and fruits and nine frequency choices (‘almost never’ to ‘10 or more glasses per day’) for juices. Standard portion sizes for each food item were categorised as follows: small (50% smaller than standard), medium (same as standard), and large (50% larger than standard). The quantity (g/day) of consumed vegetables and fruits was calculated from the data obtained. The FFQ for assessing vegetable and fruit consumption has already been validated in previous studies24,25.
Potential confounding factors
Potential confounding factors included age, sex, body mass index (BMI) (quartile), smoking status (never smoker, ex-smoker, or current smoker), sleep duration (6–8 h/day, ≤ 5 h/day, ≥ 9 h/day), breakfast (eating every day or other frequencies), working hours, perceived mental stress (low, medium, or high), living arrangement (living alone or others), job type (agriculture, forestry, fishery, or self-employed; salaried or professional; housework or unemployed), study area, alcohol intake (0, 1–150, 150–300, or ≥ 301 g/week), quartiles of metabolic equivalent task-hours per day (METs), total energy intake, vegetable, fruit, fish, meat, and sodium intake. The consumption of each food group was adjusted by the total energy intake using the residual method26.
Follow-up survey
Participants in Cohorts I and II were followed from 1995 to December 31, 2009, and from 1998 to December 31, 2012, respectively. Information about any changes in residence status was obtained annually from data on moving out of the area, identified by residency registration inside and outside the PHC areas. All mortality data of participants in the residential registry were forwarded to the Ministry of Health, Labour and Welfare and were coded for inclusion in the National Vital Statistics. Since residency and death registrations are required by the Basic Resident Registration Law and Family Registration Law, respectively, data from each registration were considered complete.
Ascertainment of stroke and CHD incidence
The medical records were reviewed by hospitals or PHC physicians in each registered major hospital within the PHC areas27,28. Diagnoses of stroke were based on focal neurologic symptoms and confirmed using computed tomography scan and/or magnetic resonance imaging according to the criteria of the National Survey of Stroke29. Diagnoses of myocardial infarction were confirmed in the medical records according to the criteria of the MONICA project30. Sudden cardiac death was defined as death of unknown origin that occurred within 1 h of the onset of the event. These medical data were extracted according to cohort-specific registration forms.
For the analyses, only the first-ever CVD events during the follow-up were regarded as incident CVD. If one person suffered both stroke and CHD, CVD was defined, whichever occurred first. Person-years of follow-up were calculated for each participant from baseline to the date of death, diagnosis, emigration from the PHC area, or end of the follow-up period (December 31, 2009, or December 31, 2012), whichever occurred first. Participants lost to follow-up were censored at the last confirmed date of their presence in the PHC area. A total of 1,038,087 person-years for the CVD analysis was accrued.
Statistical analysis
All statistical analyses were divided by sex, and the participants were divided into two groups according to their daily routine: the regular and irregular groups. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model, according to the SAS PHREG Procedure (SAS Institute, Inc., Cary, NC, USA). Multivariable Cox regression models with a covariate adjustment approach were used: model l included age at baseline and study area; model 2 included model 1 plus covariates, including living arrangement (living alone, other, or missing), alcohol intake (0, 1–150, 151–300, 301–450, and ≥ 451 g/week), cigarette smoking status (current [< 20 or ≥ 20 cigarettes/day], never, former, or missing), perceived mental stress (low, medium, high, or missing), working hours (< 5 h, ≥ 5 h and < 9 h, ≥ 9 h, or missing), job type (agriculture, forestry, fishery, and self-employed; office work, profession, and other work; housework and unemployed; or missing), breakfast (eating every day, other frequencies, or missing), sleep duration (≤ 5 h, ≥ 6 h and ≤ 8 h, ≥ 9 h, or missing), quartile of BMI, METs, and dietary factors (i.e. quartiles of energy intake and energy-adjusted dietary intakes of vegetables, fruits, meat, fish, and sodium). Missing covariate data were adjusted using dummy variables. Since hypertension, hypercholesterolaemia, and diabetes mellitus could be considered potential mediators of the association between an irregular daily routine and the increased risk of CVD, we included multivariable analysis data concerning the medication use for hypertension, hypercholesterolaemia, and diabetes mellitus in Table S1.
Since a significant inverse association between vegetable and fruit consumption and CVD risk has been reported22, we further explored the interaction between an irregular daily routine and vegetable and fruit consumption (dichotomised variable) on CVD risk by adding cross-product terms into the multivariable model. Moreover, stratified analyses according to selected demographic characteristics (i.e. BMI, living arrangement, smoking and drinking habits, working hours, type of job, total physical activity, perceived mental stress, sleep duration, and skipping breakfast) were also conducted to determine the heterogeneity of association between an irregular daily routine and CVD risk. A two-tailed p-value of < 0.05 was considered statistically significant.
Ethics approval and consent to participate
Informed consent was obtained from each participant implicitly when they completed the baseline questionnaire, where the study purpose and follow-up procedures were described. The study protocol was approved by the institutional review boards of Azabu University, Toyo University, and the National Cancer Center, Tokyo, Japan.
Results
The associations between a self-reported irregular daily routine and demographic characteristics are shown in Table 1. The self-reported irregular daily routine was significantly associated with sex, and the percentage of individuals with an irregular daily routine was 26.0% and 21.7% among men and women, respectively. In both men and women, an irregular daily routine was significantly associated with lower age, lower consumption of vegetables, fruits, fish, and fibre, higher BMI, and higher consumption of meat. Both men and women with an irregular daily routine were more likely to live alone, smoke and drink, work long hours, have higher levels of mental stress, sleep ≤ 5 h a day, and skip breakfast. The percentage of the job type category ‘office work, profession, or other work’ and ‘homemaker or unemployed’ was the highest in men and women, respectively, with an irregular daily routine.
We confirmed 3,763 stroke cases (2184 men and 1579 women) and 878 CHD cases (626 men and 252 women) during a mean follow-up duration of 13.3 years. The results of age- and study area-adjusted and multivariable-adjusted HRs (95% CIs) of incident CVD according to the self-reported irregular daily routine stratified by sex are shown in Table 2. Significant positive associations were found between an irregular daily routine and risk of total CVD and total stroke in the age- and study area-adjusted models in men. However, these significant associations disappeared after further adjustment for living arrangement, alcohol intake, smoking status, mental stress, working hours, job type, breakfast, sleep duration, BMI, METs, and dietary factors. In women, multivariable adjustment attenuated the significant associations between an irregular daily routine and increased risk of total CVD, total stroke, and CHD, but the following positive associations remained significant: total CVD (HR 1.24, 95% CI 1.11–1.39), total stroke (HR 1.21, 95% CI 1.07–1.36), and CHD (HR 1.49, 95% CI 1.11–2.00). These results were almost the same after adjustment for possible mediator variables, such as hypertension, hypercholesterolaemia, and diabetes mellitus (Table S1). When we performed a sensitivity analysis after excluding cases that occurred within 3 years after the baseline, significant associations were observed between an irregular daily routine and increased risks of total CVD (HR 1.25, 95% CI 1.10–1.41), total stroke (HR 1.19, 95% CI 1.04–1.36), and CHD (HR 1.60, 95% CI 1.17–2.20) in women.
To evaluate the interaction between an irregular daily routine and vegetable and fruit consumption on CVD risk, stratified analyses with cross-product terms were also conducted (Table 3). We observed a positive and significant association between an irregular daily routine and the risk of total CVD only in the population with vegetable and fruit consumption lower than the median value in men (HR 1.15, 95% CI 1.02–1.29) and detected an interaction between an irregular daily routine and vegetable and fruit consumption (p for interaction = 0.032). Similarly, multivariable HRs for total stroke and CHD in the lower vegetable and fruit consumption groups were slightly higher in men with irregular daily routine than in those with regular routine, but the interactions were not statistically significant in total stroke (p for interaction = 0.109) and CHD (p for interaction = 0.119). In women with lower vegetable and fruit consumption, multivariable HRs (95% CI) of total CVD and stroke were 1.32 (1.14–1.54) and 1.36 (1.15–1.60), respectively, and a significant interaction between an irregular daily routine and vegetable and fruit consumption was detected for total stroke (p for interaction = 0.039), but not for total CVD (p for interaction = 0.231). With regard to CHD in women, while multivariable HR (95% CI) in the higher vegetable and fruit consumption group was 1.90 (1.29–2.80), the interaction was not significant (p for interaction = 0.073).
In the analyses stratified by other demographic factors, such as BMI, living arrangement, smoking and drinking habits, working hours, total physical activity, perceived mental stress, sleep duration, and skipping breakfast, no significant interaction with an irregular daily routine was detected (Table S2), while multivariable HRs (95% CI) were 1.16 (1.01–1.33) in men and 1.50 (1.17–1.93) in women with the job type category ‘office work, profession, or other work’.
Discussion
This study aimed to elucidate the association between the self-reported irregular daily routine and the risk of incident CVD in a middle- and older-aged population-based prospective study. We found that an irregular daily routine is significantly associated with an increased risk of CVD in women but not in men. Moreover, in the stratified analysis on total vegetable and fruit consumption, significant interactions were found for total CVD in men and total stroke in women. Notably, higher vegetable and fruit consumption might weaken the positive association between irregular daily routine and CVD risk.
No previous study has investigated the associations between a self-reported irregular daily routine and CVD risk in middle- and older-aged men and women. Those who reported their daily routine as irregular had characteristics such as long working hours, higher perceived mental stress, short sleep, and skipping breakfast. Some findings, mainly related to sleep schedules, have suggested that circadian misalignments could increase CVD risk. For example, a previous cross-sectional study, using actigraphy-assessed rest-activity cycle, has demonstrated that more regular activity rhythms were associated with lower odds of metabolic syndrome, hypertension, dyslipidaemia, and CVD31. Recent studies have also indicated that irregular sleep duration and timing increased the risk of cardiometabolic factors or CVD events14,15,16,32,33. Increasing cardiometabolic risks were also observed among other specific populations or settings with circadian misalignments, such as rotating-shift workers10,11,34, daylight-saving time35, and individuals with higher social jetlag36,37,38. Moreover, greater eveningness, which is closely related to a larger degree of irregular daily routine39,40,41, has been associated with an increased risk of CVD mortality42. These findings on circadian misalignments support our results in examining the association between a self-reported irregular daily routine and CVD risk.
The association between a self-reported irregular daily routine and the risk of CVD may be explained by several potential physiological mechanisms underlying them. Given the characteristics of those who reported their daily routine as irregular, it is assumed that the 24-h behavioural cycles, such as the sleep–wake and feeding schedules, are not aligned with endogenous circadian rhythms4. Previous studies have indicated an association between exposure to experimentally-imposed circadian misalignments and increased blood pressure4,6. Other studies have shown that day-to-day circadian misalignments are related to metabolic risk factors, such as a lower high-density lipoprotein-cholesterol level, higher triglyceride level, fasting plasma insulin level, insulin resistance, and adiposity7,14. Considering that our results were almost the same after adjustment for possible mediator variables, such as hypertension, hypercholesterolaemia, and diabetes mellitus (although we should note that the adjustment could be inadequate because these mediators were self-reported variables), other potential candidates should also be discussed. For example, elevated inflammatory markers, such as serum interleukin-6, C-reactive protein, and tumour necrosis factor-α, have been observed among those with circadian misalignments5,6. Given that low-grade inflammation is not negligible43,44 and may also be involved in pathways between shift workers and CVD risk45,46, inflammation may become one of the targeted factors to address the positive association between an irregular daily routine and the risk of CVD. Moreover, since it is well recognised that the autonomic nervous system47 and thrombogenic properties, such as platelet aggregability48 and plasminogen activator inhibitor 149, have a robust circadian rhythm, these related factors may remain possible mechanisms between an irregular daily routine and the risk of CVD. Although the extent of these effects may be less than the impact of circadian misalignments caused by day-night reversal, the cumulative effects of an irregular daily routine (i.e., slight circadian misalignments) on health status would be noteworthy.
Our results show the possibility that the positive association between irregular daily routine and total CVD or total stroke may be weakened among men or women with a higher amount of vegetable and fruit consumption, indicating significant multiplicative interactions. A previous study has demonstrated the association between higher vegetable and fruit consumption and lower levels of inflammatory markers50, which are closely related to stroke and CHD51,52. Moreover, more pro-inflammatory diets may partially explain the increased inflammation-related chronic disease risk among those with an irregular daily routine due to day-night reversal53. Therefore, assuming that our population with a higher vegetable and fruit consumption had lower circulating levels of inflammatory markers, those with higher vegetable and fruit consumption may weaken the positive associations between an irregular daily routine and CVD or stroke among men and women. Although further investigations are warranted to understand the interaction (or effect modification) between an irregular daily routine and other potential factors of CVD, these angles may be important for those who inevitably have an irregular daily routine due to socio-environmental reasons. On the other hand, there was a tendency of interaction between an irregular daily routine and vegetable and fruit consumption on the risk of CHD among women, although it was not significant. However, the number of CHD cases in women was relatively small, and the confidence interval was especially wide for women in the higher vegetable and fruit consumption group.
In the present study, the lack of a positive association between an irregular daily routine and CVD risk among men could be partially explained by the results of stratified analysis by job type. The percentage of men with ‘office work, profession, or other work’ (excluding farming, forestry, fishery, self-employed) was twice that of women, and men with ‘homemaker or unemployed’ was one-fifth that of women, indicating a difference in the distribution of job type. Therefore, when we performed stratified analysis by job type, significant positive associations between an irregular daily routine and CVD risk were consistent in both men and women with job type ‘office work, profession, or other work’. However, since the detailed structures of circadian misalignments strongly depend on working environments, further research reflecting the current socio-environmental conditions among men and women is necessary.
The percentage of participants who ate breakfast every day was significantly lower in men and women who considered themselves to have an irregular daily routine in this study. A previous study reported that habitual skipping breakfast was associated with increased levels of inflammation54. Additionally, the frequency of breakfast intake was inversely associated with the risk of haemorrhagic stroke17. These studies suggest the importance of taking into account circadian misalignments between biological rhythms, sleep–wake cycles, and temporal irregularity in dietary habits. In fact, individuals with a more irregular pattern of energy intake, especially at breakfast, have an increased cardiometabolic risk55,56. Therefore, further studies are needed to confirm the validity of a self-reported irregular daily routine by objective measurements and examine the effects of dietary and sleep irregularity separately.
This study has several limitations. First, some participants might have changed their daily routines during the follow-up period. However, the percentage of individuals with the same response to the question, ‘Is your daily routine or activity schedule regular?’ in both the 5-year and 10-year follow-up surveys was 80.6%, and the Spearman rank correlation coefficients between the two surveys were 0.45 and 0.43 in men and women, respectively (data not shown). Second, the validity of a self-reported irregular daily routine was not confirmed. A self-reported irregular daily routine report may contain some ambiguity. Since this may have involved the association between an irregular daily routine and the risk of CVD toward the null in men and away from null in women, estimates without a bias could be obtained by objectively assessing what a self-reported irregular daily routine reflects (e.g. circadian misalignment due to weekday-weekend differences, day-to-day variation over a week, and the timing of dietary intake). Moreover, as inter-individual variation in lifestyles, which was included in the multivariable-adjusted model, would reflect presence/absence of irregular daily routine to a certain degree, the multivariable model could explain how it influences CVD risk. Especially, the fact that multivariable adjustment did not attenuate the association in women indicates that unmeasured lifestyle or unknown factors related to irregularity, such as circadian misalignment, would result in an increased risk of CVD development in women. However, an irregular daily routine was associated with poorer sleep quality in the 10-year follow-up data (i.e. longer sleep latency, arousal during night sleep, and early morning awakening) (data not shown), which corresponds well to the deleterious effect of circadian misalignment4,57. Therefore, although the validity of a self-reported irregular daily routine may not be low, a well-validated exposure is desirable58. Third, the assessment of an irregular daily routine was based on binary data. Further research on the effects of different degrees of irregularity should be more meaningful. Fourth, the results stratified by demographic characteristics should be interpreted as exploratory because analyses with several stratified variables may give rise to the potential for type I error inflation due to multiplicity issues. Finally, although we measured and adjusted for several confounders, there could still be potential residual confounding due to unmeasured variables, such as rotating shift work.
Conclusion
This prospective cohort study revealed that a self-reported irregular daily routine was significantly and positively associated with the risk of incident CVD, especially in women, and that these significant associations may be weakened in the populations with higher vegetable and fruit consumption. Daily routine as well as vegetable and fruit intake might be considered for the prevention of CVD in the middle-aged and older populations.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ramsey, K. M. & Bass, J. Obeying the clock yields benefits for metabolism. Proc. Natl. Acad. Sci. USA 106, 4069–4070 (2009).
Takeda, N. & Maemura, K. The role of clock genes and circadian rhythm in the development of cardiovascular diseases. Cell. Mol. Life Sci. 72, 3225–3234 (2015).
Potter, G. D. et al. Circadian rhythm and sleep disruption: Causes, metabolic consequences, and countermeasures. Endocr. Rev. 37, 584–608 (2016).
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 106, 4453–4458 (2009).
Leproult, R., Holmbäck, U. & Van Cauter, E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes 63, 1860–1869 (2014).
Morris, C. J., Purvis, T. E., Hu, K. & Scheer, F. A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 113, E1402–E1411 (2016).
Wong, P. M., Hasler, B. P., Kamarck, T. W., Muldoon, M. F. & Manuck, S. B. Social jetlag, chronotype, and cardiometabolic risk. J. Clin. Endocrinol. Metab. 100, 4612–4620 (2015).
Morris, C. J., Yang, J. N. & Scheer, F. A. J. L. The impact of the circadian timing system on cardiovascular and metabolic function. Prog. Brain Res. 199, 337–358 (2012).
Erren, T. C. & Lewis, P. Hypothesis: ubiquitous circadian disruption can cause cancer. Eur. J. Epidemiol. 34, 1–4 (2019).
Wang, D., Ruan, W., Chen, Z., Peng, Y. & Li, W. Shift work and risk of cardiovascular disease morbidity and mortality: A dose-response meta-analysis of cohort studies. Eur. J. Prev. Cardiol. 25, 1293–1302 (2018).
Torquati, L., Mielke, G. I., Brown, W. J. & Kolbe-Alexander, T. Shift work and the risk of cardiovascular disease: A systematic review and meta-analysis including dose-response relationship. Scand. J. Work Environ. Health 44, 229–238 (2018).
Vyas, M. V. et al. Shift work and vascular events: Systematic review and meta-analysis. BMJ 345, e4800 (2012).
Vetter, C. et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA 315, 1726–1734 (2016).
Huang, T. & Redline, S. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: The multi-ethnic study of atherosclerosis. Diabetes Care 42, 1422–1429 (2019).
Lunsford-Avery, J. R., Engelhard, M. M., Navar, A. M. & Kollins, S. H. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci. Rep. 8, 14158 (2018).
Huang, T., Mariani, S. & Redline, S. Sleep irregularity and risk of cardiovascular events: the multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 75, 991–999 (2020).
Kubota, Y., Iso, H., Sawada, N., Tsugane, S., JPHC Study Group. Association of breakfast intake with incident stroke and coronary heart disease: The Japan Public Health Center-based study. Stroke 47, 477–481 (2016).
Smith, K. J. et al. Skipping breakfast: Longitudinal associations with cardiometabolic risk factors in the childhood determinants of adult health study. Am. J. Clin. Nutr. 92, 1316–1325 (2010).
Ofori-Asenso, R., Owen, A. J. & Liew, D. Skipping breakfast and the risk of cardiovascular disease and death: A systematic review of prospective cohort studies in primary prevention settings. J. Cardiovasc. Dev. Dis. 6, 30 (2019).
Cahill, L. E. et al. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 128, 337–343 (2013).
Campos, T. F., Galvão Silveira, A. B. & Miranda Barroso, M. T. Regularity of daily activities in stroke. Chronobiol. Int. 25, 611–624 (2008).
Wang, X. et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349, g4490 (2014).
Tsugane, S. & Sawada, N. The JPHC study: Design and some findings on the typical Japanese diet. Jpn. J. Clin. Oncol. 44, 777–782 (2014).
Sasaki, S., Kobayashi, M., Tsugane, S., JPHC. Validity of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I: Comparison with dietary records for food groups. J. Epidemiol. 13, S57–S63 (2003).
Ishihara, J. et al. Validity and reproducibility of a self-administered food frequency questionnaire in the JPHC Study Cohort II: Study design, participant profile and results in comparison with Cohort I. J. Epidemiol. 13, S134–S147 (2003).
Willett, W. C. Nutritional Epidemiology 2nd edn. (Oxford University, 1998).
Iso, H. et al. Alcohol consumption and risk of stroke among middle-aged men: The JPHC study cohort I. Stroke 35, 1124–1129 (2004).
Iso, H. et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: The Japan public health center-based (JPHC) study cohort I. Circulation 113, 195–202 (2006).
Walker, A. E., Robins, M. & Weinfeld, F. D. The national survey of stroke: Clinical findings. Stroke 12, I13–I44 (1981).
Tunstall-Pedoe, H. et al. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 90, 583–612 (1994).
Sohail, S., Yu, L., Bennett, D. A., Buchman, A. S. & Lim, A. S. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol. Int. 32, 802–813 (2015).
Patel, S. R. et al. The association between sleep patterns and obesity in older adults. Int. J. Obes. 38, 1159–1164 (2014).
Taylor, B. J. et al. Bedtime variability and metabolic health in midlife women: the SWAN sleep study. Sleep 39, 457–465 (2016).
Cheng, M. et al. Shift work and ischaemic heart disease: Meta-analysis and dose-response relationship. Occup. Med. 69, 182–188 (2019).
Manfredini, R., Fabbian, F., Cappadona, R. & Modesti, P. A. Daylight saving time, circadian rhythms, and cardiovascular health. Intern. Emerg. Med. 13, 641–646 (2018).
Parsons, M. J. et al. Social jetlag, obesity and metabolic disorder: investigation in a cohort study. Int. J. Obes. 39, 842–848 (2015).
Chellappa, S. L., Vujovic, N., Williams, J. S. & Scheer, F. A. J. L. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol. Metab. 30, 767–779 (2019).
Islam, Z. et al. Association of Social jetlag with metabolic syndrome among Japanese working population: The Furukawa Nutrition and Health Study. Sleep Med. 51, 53–58 (2018).
Levandovski, R. et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol. Int. 28, 771–778 (2011).
Roepke, S. E. & Duffy, J. F. Differential impact of chronotype on weekday and weekend sleep timing and duration. Nat. Sci. Sleep 2, 213–220 (2010).
Wittmann, M., Dinich, J., Merrow, M. & Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 23, 497–509 (2006).
Knutson, K. L. & von Schantz, M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol. Int. 35, 1045–1053 (2018).
Danesh, J. et al. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ 321, 199–204 (2000).
Lüscher, T. F. Cardio-oncology: Low-grade inflammation as a common pathway of cancer and cardiovascular disease. Eur. Heart J. 40, 3871–3874 (2019).
Puttonen, S., Viitasalo, K. & Härmä, M. Effect of shiftwork on systemic markers of inflammation. Chronobiol. Int. 28, 528–535 (2011).
Sookoian, S. et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J. Intern. Med. 261, 285–292 (2007).
Boudreau, P., Dumont, G., Kin, N. M., Walker, C. D. & Boivin, D. B. Correlation of heart rate variability and circadian markers in humans. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 681–682 (2011).
Scheer, F. A. et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE 6, e24549 (2011).
Scheer, F. A. & Shea, S. A. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 123, 590–593 (2014).
Hosseini, B. et al. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 108, 136–155 (2018).
Kaptoge, S. et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 375, 132–140 (2010).
Luc, G. et al. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: The PRIME Study. Arterioscler. Thromb. Vasc. Biol. 23, 1255–1261 (2003).
Wirth, M. D. et al. Dietary inflammatory index scores differ by shift work status: NHANES 2005 to 2010. J. Occup. Environ. Med. 56, 145–148 (2014).
Zhu, S. et al. Habitually skipping breakfast is associated with chronic inflammation: A cross-sectional study. Public Health Nutr. 24, 2936–2943 (2021).
Pot, G. K., Hardy, R. & Stephen, A. M. Irregular consumption of energy intake in meals is associated with a higher cardiometabolic risk in adults of a British birth cohort. Int. J. Obes. 38, 1518–1524 (2014).
Pot, G. K., Hardy, R. & Stephen, A. M. Irregularity of energy intake at meals: prospective associations with the metabolic syndrome in adults of the 1946 British birth cohort. Br. J. Nutr. 115, 315–323 (2016).
Chellappa, S. L., Morris, C. J. & Scheer, F. A. J. L. Effects of circadian misalignment on cognition in chronic shift workers. Sci. Rep. 9, 699 (2019).
Monk, T. H., Kupfer, D. J., Frank, E. & Ritenour, A. M. The social rhythm metric (SRM): Measuring daily social rhythms over 12 weeks. Psychiatry Res. 36, 195–207 (1991).
Acknowledgements
We thank all the staff members for their contributions and efforts in the survey. The members of the JPHC Study Group are listed at https://epi.ncc.go.jp/en/jphc/781/index.html.
Funding
This study was supported by a grant from the National Cancer Center Research and Development Fund (23-A-31 [toku] and 26-A-2) (since 2011; the principal investigator is ST), a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (1989–2010; the principal investigator from 1997 to 2010 was ST).
Author information
Authors and Affiliations
Consortia
Contributions
T.Y.: Conceptualisation, Data Curation, Formal Analysis, Methodology, Software, Validation, Visualisation, Writing—original draft preparation. J.I.: Conceptualisation, Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Software, Supervision, Writing—original draft preparation. A.K.: Formal Analysis, Methodology. Y.K.: Formal Analysis, Investigation, Methodology. I.S.: Formal Analysis, Investigation, Methodology. H.Y.: Formal Analysis, Investigation, Methodology. K.Y.: Formal Analysis, Investigation, Methodology. N.S.: Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Resources, Supervision. M.I.: Formal Analysis, Investigation, Methodology. H.I.: Investigation, Methodology. S.T.: Funding Acquisition, Investigation, Methodology, Project Administration, Resources, Supervision. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshizaki, T., Ishihara, J., Kotemori, A. et al. Association between irregular daily routine and risk of incident stroke and coronary heart disease in a large Japanese population. Sci Rep 12, 15750 (2022). https://doi.org/10.1038/s41598-022-20019-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20019-8
This article is cited by
-
Circadian Factors in Stroke: A Clinician’s Perspective
Cardiology and Therapy (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.