Abstract
Bryozoans were common benthic invertebrates in the Silurian seas. The large biodiversity among Silurian benthic organisms prompted diversified interactions, and as a result bryozoans hosted many other organisms as symbionts. Here we analyse the cystoporate bryozoan Fistulipora przhidolensis and unidentified trepostomes intergrown with auloporid tabulate corals and putative hydrozoans. The material comes from the uppermost Přídolí Series (Late Silurian) of the Sõrve Peninsula, Saaremaa, Estonia. Our analysis shows that the interaction was beneficial for both organisms—cnidarians benefited from feeding currents created by the host bryozoan, while the latter benefited from the protection from predators by cnidae, it can thus be classified as mutualism. Such associations are common in modern seas. The analysed organisms are typically encrusting when the symbiosis is absent, when intergrown they display erect, branching morphologies, raised over the substratum, thus exploiting a higher suspension-feeding tier. While similar associations were known from the Devonian, we demonstrate that this novel ecological strategy for greater resource exploitation started as early as the latest Silurian.
Similar content being viewed by others
Introduction
Bryozoans were among the most common Silurian benthic organisms. A large number of diverse bryozoans have been described from the tropical shelves of the palaeocontinent Baltica1,2,3,4,5. Besides bryozoans, large numbers (both in terms of diversity and biomass) of other organisms competed for seafloor space in tropical Silurian seas. Such competition prompted interactions between benthic organisms that resulted in a diverse network of interactions6,7. For example. representatives of the common cystoporate bryozoan genus Fistulipora have been observed to host diversified symbionts, such as rugose corals, cornulitids and others8,9.
Substrate space is an important and limited resource for benthic organisms10. Population size, survival and reproductive success are correlated with the area occupied by given organisms11. While diverse benthic species may use aggressive chemicals or toxins to repel potential predators or competitors12, the abilities of bryozoans to produce repellent chemical agents are probably rare13, and therefore these organisms are generally prone to overgrowths by other organisms. As a result, bryozoan–cnidarian associations are common in modern seas14,15, and numerous cases have been described from the fossil record 16,17,18. Among these, representatives of Hydrozoa are particularly common symbionts of bryozoans. In general, such hydrozoan-bryozoan associations are mutualistic11, where the bryozoan receives protection from the hydrozoan cnidae, while the hydrozoan profits from the feeding currents generated by the bryozoan14. Moreover, it has been shown that association with other organisms (in this case, other species of bryozoans) may influence the feeding current strength, and neighbouring colonies can profit from each other’s presence19, thus such an association may be desirable for both organisms involved. This can likely be extended to other bryozoan–cnidarian associations. While several such associations are known from the Devonian e.g.,16,18,20,21 they are less frequent in older strata e.g.,9,22.
It has been demonstrated that the association of two taxa can create a new ecological niche unavailable for each of the organisms separately. An instructive case was described by McKinney et al.16, who detailed mutualism between the tabulate coral Aulopora sp. and the trepostome bryozoan Leioclema sp. from the Lower Devonian of Tennessee, USA. While both taxa were generally encrusting, their intergrowth resulted in branching colonies, which enabled greater penetration of the water column for each organism than would have been available without the intergrowth. It therefore created a new ecological niche and allowed partial escape from the limited bottom surface. A similar case was described by Suárez-Andrés et al.18,21 from the Lower Devonian of Spain, who interpreted the relationship as commensalism.
The aim of this paper is to describe and analyse examples of Fistulipora przhidolensis from the latest Přídolí Epoch exposed along Ohesaare cliff, Sõrve Peninsula, Saaremaa, Estonia (Fig. 1), which are intergrown with modular organisms of cnidarian affinities. Our material shows remarkable similarities to the Lower Devonian cases outlined above but is older by at least 5–7 Ma, thus pushing the appearance of such symbioses and the new niche deeper in time. While part of this material was briefly mentioned and illustrated by Vinn et al.9, it has not been separately analysed until now. In addition, we analyse two specimens of trepostome bryozoans from the same beds, hosting modular endosymbionts.
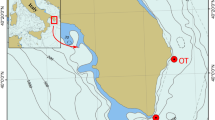
Location of the Ohesaare cliff on the map of Estonia and Europe. Based upon Vinn et al.9.
Geological setting and palaeogeographical context
During the Ohesaare age (latest Přídolí), the palaeocontinent of Baltica was at tropical latitudes, spanning from the Equator down to about 30°S23,24. A shallow epicontinental sea covered southernmost part of the today Saaremaa Island and its Sõrve Peninsula (Fig. 1). This shallow sea was characterized by tropical environments and diverse biotas25. Nestor and Einasto26 have described facies of the Baltic Silurian basin, including the Přídolí. They found five depositional environments in the Silurian of Estonia: tidal flat/lagoonal, shoal, open shelf, basin slope, and a basin depression. The first three environments formed a carbonate shelf, whereas sediments deposited in shoal and in open shelf environments are exposed on Saaremaa Island27. On the Sõrve Peninsula, the uppermost Přídolí strata (Ohesaare Formation) contain shallow to deeper shelf carbonate rocks, rich in shelly faunas. The best exposures of the Ohesaare Formation on Saaremaa Island are located on the west coast of the Sõrve Peninsula; the only uppermost Přídolí exposure is at the Ohesaare cliff (Fig. 1). The Ohesaare cliff is approximately 600 m long and has a maximum height of about 4 m (Fig. 2). The total thickness of the bedrock section is 3.5 m, whereas the thicknesses of individual beds are variable throughout the cliff25. The exposed rocks at Ohesaare cliff are typically an intercalation of thin-bedded limestones and marlstones28. The material used in this study originates from the clay-rich beds that are exposed at the base of the cliff, from the modern sea floor, and from skeletal packstones exposed directly above the lower hardground (Fig. 2).
Results
The specimens represent small fragments of bryozoan colonies, usually not exceeding 2–3 cm. These colonies are in most cases branching, rarely irregular fragments. There are two groups of endobionts of possible cnidarian origin.
Auloporid endobionts
Three bryozoan colonies contain auloporids, most likely Aulopora amica (GIT 403–419, GIT 403–261, GIT 403–44529). One of the bryozoan colonies (GIT 403–419) contains an auloporid, visible on a small fragment as an encrusting colony on its surface; in its later astogenetic stages it was overgrown by the bryozoan. The diameters of the auloporid calyces in the free-living parts of colony are 0.8–1.3 mm; in the parts overgrown by the bryozoan these diameters reach 1.6 mm. A thin section (GIT 403–261) shows a similar situation where the auloporid was encrusting earlier growth stages of the host F. przhidolensis and was subsequently overgrown by the host colony (Fig. 3).
Auloporid corals (probably A. amica) intergrown with the bryozoan F. przhidolensis. Ohesaare cliff, Saaremaa, Estonia; Ohesaare Fm. Přídolí Series, Silurian. (A,B) Two sides of the same specimen. Note that the auloporid is partly encrusting the surface and partly embedded in the host bryozoan. Specimen GIT 403–419. (C) Thin section showing cross sections of auloporid corallites (arrow). Specimen GIT 403–261.
Modular endobionts
Other specimens usually (F. przhidolensis: GIT 403–304; 403–415; 403–417; 403–418; 403–421; 403–422; 403–445; 403–451; 403–474; 403–634; 403–697; TUG 1743–135; Trepostomes: GIT 403–416; 403–420; Fig. 4) display usually regularly distributed holes, 0.6–0.9 mm in diameter and usually spaced 15–25 mm apart (measured as border to border), rather evenly distributed within a given host bryozoan colony. In one case, these holes form two, quite regular, parallel rows (specimen GIT 403–416, Fig. 4B). On a clearly branching specimen the openings are distributed along the axis, alternating from one side to the other, thus geniculate (e.g., GIT 403–421, Fig. 4F). In thin sections and slabs, the budding is visible, which proceeded at the base of the parent individual (Fig. 5). The structures possess their own walls of variable thickness, 0.012–0.016 mm, which is not significantly different from the thickness of the host’s walls. As shown by EDS analysis, their composition is carbonate (domination of Ca and locally Si) and does not show signs of phosphatization (entire lack of P). Thin section and specimen examination did not reveal any carbonization.
Modular endobionts, putative hydrozoans on F. przidolensis (A–C,F,G) and a trepostome bryozoan (D,E). Ohesaare cliff, Saaremaa, Estonia; Ohesaare Fm. Přídolí Series, Silurian. (A) Specimen GIT 403–261; (B) specimen GIT 403–416, figured by Vinn et al.9. (C) Specimen GIT 403–415. (D,E) two sides of the specimen GIT 403–420. (F) GIT 403–421, (G) thin section GIT 403–416. Scale bars 2 mm.
Modular endobionts, putative hydrozoans. Ohesaare cliff, Saaremaa, Estonia; Ohesaare Fm. Přídolí Series, Silurian. Polished slab. Note proximal budding (arrow). Specimen GIT 403–418, figured by Vinn et al.9.
Discussion
The biological affinity of the endobionts
The endobionts of the first group clearly belong to auloporids, identified here by their similar morphometric characteristics to Aulopora amica29. Auloporids are classified within Anthozoa, subclass Tabulata30,31.
The affinities of the second group of specimens are more interesting. Such modular morphology suggests cnidarian, bryozoan or hemichordate affinities. The investigated organisms of the second group display very simple morphology. They are geniculate, with openings alternating in many cases on both sides of the branch. This pattern commonly occurs in the material but is not consistent; it might be possible that our material represents more than one taxon. Geniculate colony morphology with distal budding and funnel-shaped calyces was described from fossil Cladochonus organisms30,32,33. This genus is characterized by funnel-shaped “calices”, uneven walls and lack of internal structures, such as tabulae or pores33. Our material shows strong resemblance to Cladochonus. Its taxonomic position has been discussed for a long time32, and it was traditionally assigned to Pyrgiidae within the subclass Tabulata30, but Stasińska32 pointed out deep anatomical differences between Cladochonus and tabulates on one hand, on the other she demonstrated its resemblance to modern hydrozoans. Król33 classified it as Incertae Sedis, while Coronado pointed microstructural features and concluded that Cladochonus is likely to be a calcifying hydrozoan34, a point of view we adopt here. It can be further supported by the fact that this kind of colony structure commonly occurs in various modern hydrozoans, such as representatives of Campanulariidae (e.g., Obelia) or Tiarannidae (e.g., Stegolaria)35. Apart from pyrgiids, such colony structure is unknown in both tabulate and rugose corals30.
The mode of preservation of our material does not show any signs of carbonization. While the budding pattern may suggest hemichordatan affinity36, the EDS analysis did not reveal any signs of phosphorus. Phosphorus presence could suggest chitinous remains37, and organic tubes are typical for hemichordates38, which are often preserved as carbonized remains39,40. Also, hemichordates, such as graptolites, have their thecae much smaller than in the material discussed here. Hemichordatan affinity therefore seems unlikely.
Also, bryozoan affinity can be ruled out, as the studied endobionts show no similarities to bryozoans. At first, the size of the modules (0.6–0.9 mm in diameter) exceeds the usual size of autozooecia in bryozoans. Their spacing is also too large for bryozoans (15–25 mm). Bryozoans are suspension feeders, therefore they need optimal distance between the tentacle crowns, so that tentacles can effectively operate in this space. The large spacing would assume presence of very long tentacles (more than 7–12 mm) which are unknown in bryozoans.
To sum up, it seems that our material resembles representatives of the Pyrgiidae family, notably Cladochonus. Following Stasińska32 and Coronado34, we accept its putative hydrozoan affinity, therefore our endobionts belong to Anthozoa (Auloporida) and Hydrozoa (?Pyrgiidae).
The interaction between host bryozoan and the endobionts
The presence of the endobiont apparently does not cause positive or negative modifications of the host bryozoan. Its skeleton is modified in the sense that zooids surround the endobiont, but without other type of modification. Tapanila41 published a list of criteria to distinguish between various kinds of symbioses; skeletal modifications are needed in order to choose from any of those. In cases of lacking modifications, Tapanila41 proposed commensalism as a null hypothesis in palaeoecology. While commensalism is often reported in both recent and fossil communities (see reviews42,43) it has been shown that detecting commensalism is unlikely in the fossil record42 due to lack of evidence of the lack of interaction. According to Mathis and Bronstein43, many studies on recent interactions have shown that evidence of any kind of interaction is truly absent. As a result, it is likely that it occurs in modern associations, despite lack of evidence.
While commensalism cannot be shown in this studied case, the nature of the relationship can be inferred from general knowledge of the biology of both organisms. While auloporids and pyrgiids were common parasites of echinoderms in the Palaeozoic44 it seems that this is not the case here. Bryozoans are suspension feeders45,46,47,48. They can create feeding currents, and as a result food particles flow towards their colony when it is active19,48. Therefore, an organism associated with the bryozoan colony can benefit from feeding currents, either impoverishing the particle composition, or capturing particles too large for the host bryozoan to swallow and digest. On the other hand, an endobiont, assuming that it is cnidarian, can protect the host with its cnidae14. As such an interaction is beneficial for both involved organisms and can thus be classified as mutualism. Research on hydrozoan-bryozoan associations demonstrated that such host-symbiont interactions evolved independently in several groups of hydroids14, and such associations are relatively common in recent seas49,50,51.
Mutualistic interrelations between colonial (or modular) organisms are widespread because of their ecological plasticity and similar ecological needs52. As seen from the previous discussion, two main factors are considered in mutualistic interrelations between bryozoans and cnidarians: feeding and protection. Colonial animals may also undergo constructional modifications in the process of adapting to their substrates to achieve mutual benefit. Encrusting organisms face substantial problems on the substrate such as space and food limitations (e.g.,53). Bryozoans are poor competitors for space on the substrate against other animals54,55; space and food limitations on the substrate can be avoided by developing erect forms and thus achieving higher feeding tiers52,56.
An example of mutual intergrowth similar to that represented here was described by McKinney et al.16. The trepostome bryozoan Leioclema sp. and the coral Aulopora sp. from the Lower Devonian of USA produced erect constructions, with a coral inside branches of the host bryozoan colony. It is supposed that the bryozoan and the coral benefitted from this association, first to escape from the limited space on the substrate and second to obtain tiered space for feeding. The material described by McKinney16 comes from the Birdsong Shale Member of the Ross Formation, which is middle Lochkovian57. The example presented here shows that such an ecological innovation appeared as early as in the Late Silurian, therefore it is 5–7 million years older than previously described.
Similar interrelations can be proposed for the material from the Silurian of Saaremaa. The bryozoans involved in the symbiosis were normally encrusting species. The species Fistulipora przhidolensis often produced unilaminar encrusting or globular multilayered colonies. Due to intecactions with the auloporid coral and a hydrozoan, erect colonies appeared which allowed occupation of higher tiers for feeding. Such a strategy benefits both involved organisms and helps them limit substrate competition and to exploit new food resources higher in the water column. As shown in Recent examples, cnidarians can limit the number of predators on bryozoans whereas the latter protect hydroids by enveloping their soft parts with calcitic skeleton11,14,49,58,59. It can therefore be assumed that protection by cnidae also played an important role in this interaction. The surface of the coral/hydrozoan was covered by the encrusting bryozoan, whereas the bryozoan might be protected by action of the cnidae of the cnidarian tentacles. Moreover, the cnidarian can profit from the feeding currents produced by the bryozoan. In contrast to cnidarians, bryozoans are active suspension feeders which produce feeding currents due to orchestrated movement of cilia on their tentacles (e.g.,60 and references herein). Cnidarians are incapable of actively creating feeding currents; they only catch the prey within their reach. Bryozoans and cnidarians are not food competitors: the former feed on smaller phytoplankton, whereas the latter utilize the larger zooplankton61.
We can easily rule out the alternative explanation that the observed tubes are a result of borings in the host bryozoan skeletons. If that was the case the bryozoan zooecia would be cut by the boring randomly62,63 and would not encircle the endobiont as they do in our material (Fig. 4G).
Bryozoan symbiotic endobionts in the Early Palaeozoic
Bryozoans are known to have formed symbiotic associations with other invertebrates since the Tremadoc64. Endobiotic invertebrates with phosphatic tubes and some with entirely soft bodies formed symbiotic associations with the trepostome Orbiramus in the Tremadoc of China64. These earliest bryozoan symbiotic endobionts were solitary animals with unknown biological affinities. However, phosphatic tubes are characteristics of the presumed cnidarian Sphenothallus known from the Tremadoc, though the tubes described by Ma et al.64 are slightly too small for Sphenothallus. Nevertheless, tubicolous morphology and Sphenothallus-like composition could indicate a cnidarian affinity.
The Great Ordovician Biodiversification Event resulted in appearance of dense ecological interactions in benthic communities and in consequence a number of new ecological niches, or ecospaces appeared65. As a result of biodiversity increase, and following increase of competition, Ordovician bryozoans often formed symbiotic associations with cnidarians such as solitary rugose corals and conulariids. These cnidarians were more common bryozoan symbionts than the suspension feeding cornulitid tubeworms22,66. While the latter were solitary forms, the earliest colonial bryozoan endobionts interpreted as hydroids or ascidiacian tunicates appeared in the early Late Ordovician of Laurentia67. It must be emphasized that the Ordovician record of bryozoan endobionts was dominated by solitary organisms. On the other hand, in the Přídolí, colonial animals became much more prevalent, with still significant contributions from rugose corals and Cornulites8,9. The abundance of colonial endobionts among the Přídolí bryozoans from Saaremaa could have resulted from locally favourable environmental conditions, faunal composition and lack of antifouling agents in bryozoans. Nevertheless, it is also possible that the importance of colonial bryozoan endobionts increased from the Ordovician to Silurian. It seems that cnidarians were dominant bryozoan endobionts in the Přídolí. One could hypothesize that cnidarian biology fit well with bryozoans as they likely consumed food particles of different sizes and cnidarian symbionts could protect the host bryozoan with its cnidae. Last, but not least, a successful mutualism, allowing the use of a new feeding tier could prompt its more common appearance, as evidenced by a number of similar associations described from various parts of the World—e.g., the Early Devonian of Spain18, and Czechia68 or Middle Devonian of Russia (Kuznetsk Basin69). For a review on palaeogeographical distribution of such forms, see18. The appearance of similar symbiosis in cystoporates and trepostomes also shows the success of the newly created niche.
Conclusions
We have shown that during the Přídolí the bryozoan Fistulipora przhidolensis and unidentified trepostomes formed associations with two different representatives of cnidarians. Auloporid tabulate corals belong the first group, while the representatives of the other most probably belong to “Cladochonus-like” fossils, which were most likely hydrozoans. Both organisms (bryozoans and cnidarians) usually formed flat, encrusting colonies when growing separately; when intergrown, they formed branches. Such a modification between free-living and symbiotic mode of life shows the appearance of a new ecological niche for both involved organisms. While the skeletal modifications of the host bryozoans are absent, it can be inferred that the interaction between them was mutualistic, where cnidarians profited from the feeding currents generated by the host bryozoan, and the bryozoan benefited from the protection by the cnidarian cnidae. Such mutualistic associations are common in modern seas. This mutualism therefore introduced a structural innovation, where both organisms started to exploit a new, higher tier of suspension feeding unavailable for them separately. Such an association is known from several Devonian sites around the world, which demonstrates its ecological success,. Our study shows that this innovation appeared 5–7 Ma earlier than the oldest known example, in the Přídolí (Late Silurian).
Material and methods
A collection of about 500 bryozoan colonies from Přídolí sediments of the Sõrve Peninsula, Saaremaa, Estonia (Ohesaare Formation), was searched for the intergrowth of different invertebrates. The present work analyses the material of 17 specimens, which contain 20 fragments of the bryozoan Fistulipora przhidolensis hosting modular bioclaustrations. The material comes from the Ohesaare cliff on Saaremaa, Estonia (Fig. 1). Five thin sections of selected specimens were prepared to investigate their internal structure. Specimens and thin sections were studied under a Zeiss Discovery.V20 stereoscopic microscope under reflected and transmitted light. The specimens were photographed using ammonium chloride coating with a Canon EOS 70D camera either using Zeiss Discovery.V20 stereoscopic microscope or using Canon EF 100 mm f/2.8L Macro IS USM Lens. Specimens photographed under the microscope were uncoated. Helicon software was used to stack photos of selected specimens in order to obtain the best depth-of-field. Thin sections were photographed using transmitted light and dark field. Selected specimens were also photographed using SEM—ZEISS AURIGA 60, Energy Dispersive Spectroscopy (EDS) analyses on one uncoated specimen was performed with Zeiss Sigma VP SEM at the Faculty of Geology, University of Warsaw. Brightness, contrast and sharpness of images was adjusted in Corel Photo Paint software, each time with the whole image.
Data availability
The investigated material that supports this study is available at the Natural History Museum of the University of Tartu (collection numbers with a prefix TUG) and Department of Geology of the Tallinn University of Technology (institutional abbreviation GIT).
References
Pushkin, V. I., Nehkorosheva, L. V., Kopaevich, G. V. & Yaroshinskaya, A. M. Přídolian Bryozoa of the USSR 1–125 (Nauka, 1990) (in Russian).
Kopaevich, G. V. Silurian Bryozoa of Estonia and Podolia (Cryptostomata and Rhabdomesonata). Trudy Paleontol. Inst Akad. Nauk SSSR 151, 5–153 (1975) (in Russian).
Tuckey, M. E. Biogeography of Ordovician bryozoans. Palaeogeogr. Palaeoclimatol. Palaeoecol. 77, 91–126 (1990).
McCoy, V. E. & Anstey, R. L. Biogeographic associations of Silurian bryozoan genera in North America, Baltica and Siberia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 297, 420–427 (2010).
Bassler, R. S. The early Paleozoic Bryozoa of the Baltic provinces. Bull. U. S. Natl. Museum 77, 1–382 (1911).
Vinn, O. & Wilson, M. A. Symbiotic interactions in the Silurian of Baltica. Lethaia 49, 413–420 (2016).
Vinn, O. Symbiotic interactions in the Silurian of North America. Hist. Biol. 29, 341–347 (2017).
Vinn, O., Ernst, A., Wilson, M. A. & Toom, U. Symbiosis of cornulitids with the cystoporate bryozoan Fistulipora in the Přídolí of Saaremaa, Estonia. Lethaia 54, 90–95 (2021).
Vinn, O., Ernst, A., Wilson, M. A. & Toom, U. Intergrowth of bryozoans with other invertebrates in the late Přídolí of Saaremaa, Estonia. Ann. Soc. Geol. Poloniae 91, 101–111 (2021).
Jackson, J. B. C. & Buss, L. Allelopathy and spatial competition among coral reef invertebrates. Proc. Natl. Acad. Sci. USA 72, 5160–5163 (1975).
Osman, R. W. & Haugsness, J. A. Mutualism among sessile invertebrates: A mediator of competition and predation. Science 211(4484), 846–848 (1981).
Pawlik, J. R. Marine invertebrate chemical defenses. Chem. Rev. 93, 1911–1922 (1993).
Figuerola, B., Núñez-Pons, L., Moles, J. & Avila, C. Feeding repellence in Antarctic bryozoans. Naturwissenschaften 100, 1069–1081 (2013).
Puce, S., Bavestrello, G., Di Camillo, C. G. & Boero, F. Symbiotic relationships between hydroids and bryozoans. Symbiosis 44, 137–143 (2007).
López-Gappa, J. & Liuzzi, M. G. An unusual symbiotic relationship between a cyclostome bryozoan and a thecate hydroid. Symbiosis 85, 217–223 (2021).
McKinney, F. K., Broadhead, T. W. & Gibson, M. A. Coral-bryozoan mutualism: Structural innovation and greater resource exploitation. Science 248(4954), 466–468 (1990).
McKinney, F. K. Bryozoan-hydroid symbiosis and a new ichnogenus, Caupokeras. Ichnos 16, 193–201 (2009).
Suárez-Andrés, J. L., Sendino, C. & Wilson, M. A. Life in a living substrate: Modular endosymbionts of bryozoan hosts from the Devonian of Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 559, 109897 (2020).
Okamura, B. The influence of neighbors on the feeding of an epifaunal bryozoan. J. Exp. Mar. Biol. Ecol. 120, 105–123 (1988).
Sendino, C., Suárez-Andrés, J. L. S. & Wilson, M. A. A rugose coral–bryozoan association from the Lower Devonian of NW Spain. Palaeogeogr. Palaeoclimatol. Palaeoecol. 530, 271–280 (2019).
Suárez-Andrés, J., Sendino, C. & Wilson, M. A. Caupokeras badalloi, a new ichnospecies of impedichnia from the Lower Devonian of Spain. Palaeoecological significance. Hist. Biol. 34, 62–66 (2021).
Vinn, O., Ernst, A., Wilson, M. A. & Toom, U. Symbiosis of conulariids with trepostome bryozoans in the Upper Ordovician of Estonia (Baltica). Palaeogeogr. Palaeoclimatol. Palaeoecol. 518, 89–96 (2019).
Melchin, M. J., Cooper, R. A. & Sadler, P. M. The Silurian period. In A Geologic Time Scale 2004 (eds Gradstein, F. M. et al.) 188–201 (Cambridge University Press, 2004).
Torsvik, T. H. & Cocks, L. R. M. New global palaeogeographical reconstructions for the Early Palaeozoic and their generation. Geol. Soc. Lond. Memoirs 38, 5–24 (2013).
Hints, O. The Silurian system in Estonia. in The Seventh Baltic Stratigraphical Conference. Abstracts and Field Guide (Hints, O. Ainsaar, L. Männik, P. & Meidla, T. eds.). 1–46. (Geological Society of Estonia, 2008).
Nestor, H. & Einasto, R. Facies-sedimentary model of the Silurian Paleobaltic pericontinental basin. in (Kaljo, D. ed.) Facies and Fauna of the Baltic Silurian. 89–121 (Academy of Sciences of the Estonian S. S. R. Institute of Geology, 1977) (in Russian, English summary).
Nestor, H. & Einasto, R. Ordovician and Silurian carbonate sedimentation basin. In Geology and Mineral Resources of Estonia (eds Raukas, A. & Teedumäe, A.) 192–205 (Estonian Academy Publishers, 1997).
Nestor, H. Locality 7: 4 Ohesaare cliff. in Field Meeting, Estonia 1990. An Excursion Guidebook (Kaljo, D. & Nestor, H. eds.). 175–178. (Institute of Geology, Estonian Academy of Sciences, 1990).
Klaamann, E. R. Tabulate corals of the Upper Silurian of Estonia. Trudy Inst. Gieol. AN Estonskoi SSR 9, 25–74 (1962) (in Russian).
Hill, D. Tabulata. in Treatise on Invertebrate Paleontology, Part F, Coelenterate, Supplement 1, Rugosa and Tabulata (Teichert, C. ed.). F430–F762 (The Geological Society of America, Inc./The University of Kansas, 1981).
Zapalski, M. K. Tabulate corals from the Givetian and Frasnian of the southern region of the Holy Cross Mountains (Poland). Spec. Pap. Palaeontol. 87, 1–100 (2012).
Stasińska, A. Colony structure and systematic assignment of Cladochonus tenuicollis McCoy, 1847 (Hydroidea). Acta Palaeontol. Pol. 27, 59–64 (1982).
Król, J., Zapalski, M. K. & Berkowski, B. Emsian tabulate corals of Hamar Laghdad (Morocco): Taxonomy and ecological interpretation. Neues Jahrbuch Geol. Palaontol.-Abhandlungen 290, 75–102 (2018).
Coronado, I. Biomineral analysis of the enigmatic fossil Cladochonus Mccoy, 1847: A representative of calcifiying hydrozoa? In New Perspectives on the Evolution of Phanerozoic Biotas and Ecosystems (Manzanares, E. et al. eds.). Vol. 24.
Bouillon, J., Gravili, C., Gili, J. M. & Boero, F. An Introduction to Hydrozoa (ResearchGate, 2006).
Tassia, M. G. et al. The global diversity of Hemichordata. PLoS ONE 11(10), e0162564 (2016).
Zapalski, M. K. & Clarkson, E. N. Enigmatic fossils from the Lower Carboniferous shrimp bed, Granton, Scotland. PLoS ONE 10(12), e0144220 (2015).
Sato, A. Seasonal reproductive activity in the pterobranch hemichordate Rhabdopleura compacta. J. Mar. Biol. Assoc. UK 88, 1033–1041 (2008).
Underwood, C. J. Graptolite preservation and deformation. Palaios 7, 178–186 (1992).
Maletz, J. Hemichordata (Enteropneusta & Pterobranchia, incl. Graptolithina): A review of their fossil preservation as organic material. Bull. Geosci. 95(1), 41–80 (2020).
Tapanila, L. Direct evidence of ancient symbiosis using trace fossils. Paleontol. Soc. Pap. 14, 271–287 (2008).
Zapalski, M. K. Is absence of proof a proof of absence? Comments on commensalism. Palaeogeogr. Palaeoclimatol. Palaeoecol. 302, 484–488 (2011).
Mathis, K. A. & Bronstein, J. L. Our current understanding of commensalism. Annu. Rev. Ecol. Evol. Syst. 51, 167–189 (2020).
Zapalski, M. K., Berkowski, B. & Klug, C. Subepidermal Emsian" auloporids" on crinoids from Hamar Laghdad (Anti-Atlas, Morocco). N. Jb. Geol. Paläont. 290, 103–110 (2018).
Winston, J. E. Feeding in marine bryozoans. In Biology of Bryozoans (eds Wollacott, W. S. & Zimmer, R. L.) 233–271 (Academic Press, 1977).
Okamura, B. & Partridge, J. C. Suspension feeding adaptations to extreme flow environments in a marine bryozoan. Biol. Bull. 196, 205–215 (1999).
Ernst, A. Fossil Record and Evolution of Bryozoa. Handbook of Zoology. Bryozoa 11–55 (De Gruyter, 2020).
Riisgård, H. U. & Manríquez, P. Filter-feeding in fifteen marine ectoprocts (Bryozoa): Particle capture and water pumping. Mar. Ecol. Prog. Ser. 154, 223–239 (1997).
Boero, F. & Hewitt, C. L. A hydrozoan, Zanclella bryozoophila n. gen, n.sp. (Zancleidae) symbiotic with a bryozoan, and a discussion of the Zancleidae. Can. J. Zool. 70, 1645–1651 (1992).
Piraino, S., Bouillon, J. & Boero, F. Halocoryne epizoica (Cnidaria, Hydrozoa), a hydroid that “bites”. Sci. Mar. 56(2), 141–147 (1992).
Maggioni, D. et al. Evolution and biogeography of the Zanclea-Scleractinia symbiosis. Coral Reefs 12, 1–17 (2020).
Taylor, P. D. Competition between encrusters on marine hard substrates and its fossil record. Palaeontology 59, 481–497 (2016).
Taylor, P. D. & Wilson, M. A. Palaeoecology and evolution of marine hard substrate communities. Earth Sci. Rev. 62, 1–103 (2003).
Gordon, D. P. Biological relationships of an intertidal bryozoan population. J. Nat. Hist. 6, 503–514 (1972).
Jackson, J. B. C. & Winston, J. E. Ecology of cryptic coral reef communities. I. Distribution and abundance of major groups of encrusting organisms. J. Exp. Mar. Biol. Ecol. 57, 135–147 (1982).
McKinney, F. K. & Jackson, J. B. C. Bryozoan Evolution 238 (Unwin Hyman, 1989).
Wicander, R. & Playford, G. Acritarchs and prasinophytes from the Lower Devonian (Lochkovian) Ross Formation, Tennessee, USA: Stratigraphic and paleogeographic distribution. Palynology 46(2), 1–50 (2022).
Ristedt, H. & Schuhmacher, H. The bryozoan Rhynchozoon larreyi (Audouin, 1826)—A successful competitor in coral reef communities of the Red Sea. Mar. Ecol. 6, 167–179 (1985).
Puce, S., Cerrano, C., Di Camillo, C. & Bavestrello, G. Hydroidomedusae (Cnidaria: Hydrozoa) symbiotic radiation. J. Mar. Biol. Assoc. U.K. 88(8), 1715–1721 (2008).
Winston, J. E. & Migotto, A. E. Behavior. In Phylum Bryozoa (ed. Schwaha, T.) 143–187 (De Gruyter, 2020).
Cadée, G. C. & McKinney, F. K. A coral-bryozoan association from the Neogene of northwestern Europe. Lethaia 27, 59–66 (1994).
Jackson, P. N. W. & Key, M. M. Jr. Borings in trepostome bryozoans from the Ordovician of Estonia: Two ichnogenera produced by a single maker, a case of host morphology control. Lethaia 40, 237–252 (2007).
Jackson, P. N. W. & Key, M. M. Epizoan and endoskeletozoan distribution across reassembled ramose stenolaemate bryozoan zoaria from the Upper Ordovician (Katian) of the Cincinnati Arch region, USA. Aust. Palaeontol. Memoirs 52, 169–178 (2019).
Ma, J., Taylor, P. D. & Buttler, C. J. Sclerobionts associated with Orbiramus from the Early Ordovician of Hubei, China, the oldest known trepostome bryozoan. Lethaia 54, 443–456 (2020).
Bambach, R. K., Bush, A. M. & Erwin, D. H. Autecology and the filling of ecospace: Key metazoan radiations. Palaeontology 50, 1–22 (2007).
Vinn, O., Ernst, A. & Toom, U. Symbiosis of cornulitids and bryozoans in the Late Ordovician of Estonia (Baltica). Palaios 33, 290–295 (2018).
Palmer, T. J. & Wilson, M. A. Parasitism of Ordovician bryozoans and the origin of pseudoborings. Palaeontology 31, 939–949 (1988).
Ernst, A. Trepostome and cryptostome bryozoans from the Koněprusy Limestone (Lower Devonia, Pragian) of Zlatý Kůň (Czech republic). Riv. Ital. Paleontol. Stratigr. 114(3), 329–348 (2008).
Morozova, I. P. Devonskie mshanki Minusinskikh i Kuznetskoy kotlovin. Trudy Paleontol. Inst. Akad. Nauk SSSR 86, 1–207 (1961) (in Russian).
Acknowledgements
Support for OV and UT was provided by Estonian Research Council Grant (PRG836) and a Sepkoski Grant from the Paleontological Society. OV was also supported by a research grant from Institute of Ecology and Earth Sciences, University of Tartu and UT by the Estonian Research Council grant PUTJD1106. We are grateful to Mare Isakar for access to the Tartu University collection.
Author information
Authors and Affiliations
Contributions
M.K.Z.: Conceptualized the study, conducted the analysis, led the writing of the manuscript; O.V.: assisted with conceptualization, provided expertise on the analysis and interpretation of results; U.T.: made the primary investigation of the entire bryozoan collection, provided expertise on the analysis and interpretation of results; A.E.: conducted the analysis and provided expertise on the interpretation of results; M.W.: provided expertise on the interpretation of results, reviewed and validated results. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zapalski, M.K., Vinn, O., Toom, U. et al. Bryozoan–cnidarian mutualism triggered a new strategy for greater resource exploitation as early as the Late Silurian. Sci Rep 12, 15556 (2022). https://doi.org/10.1038/s41598-022-19955-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19955-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.