Abstract
The primary function of heat shock transcription factor (HSF) in the heat shock response is to activate the transcription of genes encoding heat shock proteins (HSPs). The phloem-feeding insect Bemisia tabaci (Gennadius) is an important pest of cotton, vegetables and ornamentals that transmits several plant viruses and causes enormous agricultural losses. In this study, the gene encoding HSF (Bthsf1) was characterized in MED B. tabaci. The full-length cDNA encoded a protein of 652 amino acids with an isoelectric point of 5.55. The BtHSF1 deduced amino acid sequence showed strong similarity to HSF in other insects. Expression analyses using quantitative real-time PCR indicated that Bthsf1 was significantly up-regulated in B. tabaci adults and pupae during thermal stress. Although Bthsf1 was induced by both hot and cold stress, the amplitude of expression was greater in the former. Bthsf1 had distinct, significant differences in expression pattern during different duration of high but not low temperature stress. Oral ingestion of dsBthsf1 repressed the expression of Bthsf1 and four heat shock proteins (Bthsp90, Bthsp70-3, Bthsp20 and Bthsp19.5) in MED B. tabaci during hot and cold stress. In conclusion, our results show that Bthsf1 is differentially expressed during high and low temperature stress and regulates the transcription of multiple hsps in MED B. tabaci.
Similar content being viewed by others
Introduction
Insects are continually stressed by various environmental factors, and thermal stress is perhaps the most common and direct of these stressors. In response to thermal stress, insects deploy innate resistance mechanisms to alleviate the damage caused by temperature stress1,2,3. Among these, heat shock proteins (HSPs) directly respond to temperature stress and have a pivotal role in protecting insects from thermal damage4. In insects, HSPs can be subdivided into HSP100, HSP90, HSP70, HSP60, HSP40 and small heat shock proteins (sHSPs) depending on their structure, function and molecular weight5,6,7,8. Studies have shown that HSPs interact with heat shock elements (HSE) in the promoter region of genes via heat shock transcription factors (HSFs); this interaction facilitates the recruitment of other transcription factors and the formation of a transcription complex that promotes hsp expression9,10.
Heat shock transcription factors are crucial regulatory factors of the heat shock response that are conserved in eukaryotes10,11. HSFs are commonly divided into four types, including HSF1, HSF2, HSF3 and HSF4; of these HSF1 is considered to be the main regulator of hsp expression10,12. HSF1 is highly conserved in Drosophila melanogaster, yeast and vertebrates, and its function cannot be replaced by the other three HSF regulators. HSF1 is expressed in response to heat stress in most tissues and cells13 and has conserved domains: DNA-binding domain (DBD)14. After DNA binding, oligomerization, and nuclear localization, HSF1 regulates the expression of stress-induced hsps to foster the organismal response to environmental stressors such as high temperature, heavy metals, and protease inhibitors15. The function of HSF1 has been well-studied in insects16,17. In Drosophila, hsf is constitutively expressed in the cytoplasm and nucleus. In vitro studies have confirmed that Drosophila HSF can directly respond to high temperature and oxidative stress, thus indicating that HSF acts as an "thermometer" to regulate the stability of the intracellular environment when physiological tolerance is exceeded18. In addition, a few reports exist documenting HSF1 in other insect species including Helicoverpa armigera, Bombyx mori and Mamestra brassicae and explained the role of HSF1 in the process of resistance to the external environmental temperature stress19,20,21.
The whitefly, Bemisia tabaci (Gennadius), is a species complex that contains 44 cryptic species22. It is polyphagous and colonizes over 600 known host plants23,24. B. tabaci feeds directly on plants, secretes honeydew, and disseminates plant viruses; it is an invasive pest that causes damage to host plants and serious economic losses to crop production worldwide25,26. The invasive species represented by MED cryptic species (B. tabaci Q) is the most serious form of this pest. It can spread rapidly and competes to replace indigenous species, including the MEAM1 cryptic species (B. tabaci B). The adaptability of the MED cryptic species is related to many external factors, including pesticide sensitivity, behavioral interactions and host range27,28,29,30,31. The malleability of the MED cryptic species is the primary reason it can quickly adapt to different habitats, including those with temperature extremes32,33,34.
Several studies have demonstrated that thermotolerance of the MED cryptic species correlates with hsp expression, especially hsp90, hsp70 and shsps33,35,36. However, the relationship between these three hsp gene families and the whitefly heat shock transcription factor is not clear. In the present study, we cloned and identified the full-length gene encoding B. tabaci heat shock transcription factor 1 (Bthsf1) and analyzed its expression during temperature stress. RNA interference (RNAi) was used to further understand the role of BtHSF1 in the regulation of hsps in B. tabaci, which may ultimately lead to improved control methods for this important pest.
Results
Sequence analysis of Bthsf1
The full-length cDNA of Bthsf1 was 2500 bp and encoded a predicted protein containing 725 amino acids (GenBank accession no. MW478139) (Fig. S1). The predicted protein product of Bthsf1 was 80.23 kDa with an isoelectric point of 5.90. When the GenBank and PROSITE databases were compared, the BtHSF1 deduced protein showed high similarity to the HSF1 family; InterPro analysis indicated that BtHSF1 contained a conserved DNA-binding domain (DBD) at amino acid residues 10–114 (Fig. 1A). The 3D structure of HSF1 in B. tabaci was modeled using the DBD domain in D. melanogaster (SMTL ID: 1hkt.1) as a template (Fig. 1B); HSF1 showed 69.81% sequence identity to the D. melanogaster orthologue.
Multiple sequence alignment of HSF1 from various insect species and Structure of HSF1. (A) Alignment of the deduced amino acid sequences of Bthsf1, Dmhsf, Achsf, Bmhsf and Nlhsf. Abbreviations: Bt, Bemisia tabaci; Dm, Drosophila melanogaster; Ac, Apis cerana; Bm, Bombyx mori; Nl, Nilaparvata lugens. The DNA-binding (DBD) motif are underscored in black. Accession numbers of species are noted in Table S1. (B) 3D predicted structure of HSF1.
Phylogenetic analysis of BtHSF1
The BtHSF1 deduced amino acid sequence was compared with orthologous proteins in other insects. B. tabaci HSF1 showed high sequence identity with HSF in D. melanogaster, Apis cerana, Bombyx mori and Nilaparvata lugens (Fig. 1A). A phylogenetic tree was generated using the amino acid sequences of 15 HSF family members in orders Lepidoptera, Diptera, Coleoptera and Hemiptera (Table S1). BtHSF1 grouped in a well-supported cluster with other members of the Hemiptera and was well-separated from insects in other orders (Fig. 2).
Phylogenetic analysis of HSF1 in B. tabaci and other insect species. Numbers on the branches are bootstrap values obtained from 1000 replicates. Accession numbers and abbreviations for the insect species are listed in Table S1.
Bthsf1 expression during temperature stress
The expression of Bthsf1 was evaluated in response to temperature stress by qRT-PCR. The relative mRNA levels of Bthsf1 were compared at − 12, − 10, − 8, − 6, 26, 39, 41, 43, and 45 °C for 1 h. Bthsf1 expression levels were significantly increased at − 12 °C (but not − 10, − 8, and − 6 °C) relative to the control group at 26 °C, which was 2.5-fold great than the control in adults (F4,15 = 5.148, P < 0.05). Expression of Bthsf1 was significantly up-regulated at − 10, − 8, and − 6 °C (but not 12 °C) in pupae, which was highest at − 10 °C and was 7.28-fold greater than the control (F4,11 = 7.645, P < 0.05) (Fig. 3A, C).
Compared with the control group (26 °C), expression of Bthsf1 was significantly up-regulated at 41 °C and 43 °C (but not 39 and 45 °C) in adults and pupae (Adults: F4,14 = 20.324, P < 0.05; Pupae: F4,12 = 6.618, P < 0.05). Bthsf1 expression levels were highest at 41 °C, which were 3.6-fold and 3.8-fold greater than the control, respectively (Fig. 3B, D).
Bthsf1 expression at different duration of temperature stress
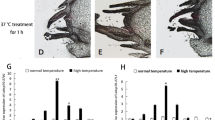
qRT-PCR was used to analyze expression of Bthsf1 during different duration of temperature stress. In this part, 31 °C, 37 °C and 43 °C were selected as high temperatures and the duration of exposure at each temperature was 15 min, 30 min, 1 h, 1.5 h and 2 h. Bthsf1 expression levels showed different patterns at the three temperatures. At 31 °C, expression levels in the 15 min and 1 h exposure period were 4.9- fold and 4.7- fold greater than the control, respectively (F5,17 = 19.282, P < 0.05). At 37 °C and 43 °C, expression levels were highest for the 1 h exposure period, where expression was 4.7- fold and 5.4-fold greater than the control, respectively (F5,17 = 16.166, P < 0.05; F5,15 = 15.266, P < 0.05) (Fig. 4A, B, C).
Low temperature treatments included exposure to − 10 °C, − 4 °C and 2 °C for 30 min, 1 h, 1.5 h, 2 h and 3 h. Expression levels of Bthsf1 were significantly increased after exposure to cold stress relative to the control group (ck, 26 °C) (− 10 °C: F5,17 = 7.825, P < 0.05;− 4 °C: F5,17 = 4.356, P < 0.05; 2 °C: F5,17 = 11.198, P < 0.05). However, the multiple of up-regulation is low, and the multiples of up-regulation under different duration treatments are relatively average. It was only found that the expression level of Bthsf1 was highest at − 10 °C for 1.5 h, at − °C for 2 h or 3 h and at 2 °C for 30 min, where expression was 1.53-, 1.26- and 1.29-fold greater than the control, respectively (Fig. 4D, E, F).
Expression of Bthsf1 and Bthsps in RNAi experiments
qRT-PCR analysis showed that mRNA levels of Bthsf1 were substantially lower when whitefly adults exposured to 41 °C (t = 8.456, P < 0.05) and − 6 °C (t = 6.226, P < 0.05) for 1 h (Fig. 5A, B) after whitefly were fed with dsBthsf1 for 1 day.
The expression levels of Bthsp90 (HM013710), Bthsp70-1 (HM013709), Bthsp70-3 (MK905884), Bthsp20 (HM013708), and BtHsp19.5 (MF114301) were evaluated after RNAi and thermal stress. When B. tabaci adults were fed with dsBthsf1 for 1 day, the expression levels of Bthsp90, Bthsp70-3, Bthsp20, and Bthsp19.5 were significantly down-regulated at − 6 °C relative to the dsGFP control (Bthsp90: t = 5.127, P < 0.05; Bthsp70-3: t = 5.491, P < 0.05; Bthsp20: t = 4.159, P < 0.05; Bthsp19.5: t = 4.334, P < 0.05) (Fig. 5A). The same four Bthsps were also down-regulated in response to 41 °C (Bthsp90: t = 6.705, P < 0.05; Bthsp70-3: t = 15.608, P < 0.05; Bthsp20: t = 6.318, P < 0.05; Bthsp19.5: t = 5.593, P < 0.05) (Fig. 5B). Interestingly, Bthsp70-1 was not significantly down-regulated after feeding with dsBthsf1 at either temperature stress ( − 6 °C: t = 2.334, P = 0.058; 41 °C: t = 0.695, P = 0.513).
Mortality of B. tabaci after RNAi
Mortality was measured after feeding B. tabaci with dsBthsf1 or dsGFP and then exposing adults to thermal stress. Mortality of B. tabaci fed with dsBthsf1 was 23% and 26% more than the dsGFP control at − 6 °C (t = 9.690, P < 0.05) and 41 °C (t = 6.759, P < 0.05), respectively (Fig. 6).
Discussion
A variety of internal and external stimuli can activate HSF, including heat shock. There are three key steps in HSF function in heat stress including the following: polymerization of HSF from monomer to trimer; recognition and binding of HSF to the HSE in hsp promoter regions; and transcriptional activation of the hsps37. Therefore, it is important to study how HSF regulates the expression of genes encoding HSPs when insects undergo thermal stress.
In this study, we cloned and identified the Bthsf1 in B. tabaci MED cryptic species. The deduced BtHSF1 contains the conserved motif (DNA-binding domain, DBD) of the HSF family. The predicted amino acid sequence of Bthsf1 shows considerable sequence similarity with HSF orthologues in D. melanogaster, B. mori, A. cerana and N. lugens. Phylogenetic analysis revealed that BtHSF1 resides within a phylogenetic group that includes HSF in other Hemiptera insects, including NlHSF in the brown planthopper (N. lugens), AgHSF in the cotton aphid (Aphis gossypii) and DnHSF in the Russian wheat aphid (Diuraphis noxia). The phylogenetic conservation of HSF within the Hemiptera indicates that BtHSF1 could be potentially useful in taxonomic studies. By modeling the 3D structure of BtHSF1, we found that it has a high degree of similarity at the conservative sequence (DBD) structure with Drosophila melanogaster, indicating that the BtHSF we obtained has the typical characteristics of the insect HSF1 family. Combined with multiple sequence comparison analysis results, it also shows that HSF1 family genes in insects have a strong conservativeness at the characteristic sequence (DBD) structure.
Under normal conditions, HSF exists as an inactive monomer in the cytoplasm and is bound to HSPs15. When cells are subjected to thermal stress, the internal environment shifts, which relieves the inhibition of HSF activity. Interestingly, there are relatively few studies documenting hsf expression patterns in insects during temperature stress. Our results show that Bthsf1 can be significantly activated and expressed constitutively by high and low thermal stress, It shows that this transcription factor can interact with the HSEs when the whitefly resists external temperature stress, and promote the expression of HSPs33,35,36. In D. melanogaster, the transcription of Dmhsf, Dmhsfb and Dmhsfd were upregulated during temperature stress17,38, and genes encoding HSF in Mamestra brassicae and Agasicles hygrophila was also induced by thermal stress21,39, However, it is important to note that there obvious differences in hsf expression among insects; for example, Cchsf encoding HSF in Cotesia chilonis was induced by low but not high temperature stress40.
Several studies have shown that the duration of temperature stress impacts the growth and development of organisms41,42,43. In this study, we analyzed Bthsf1 expression during different duration of temperature stress. Bthsf1 had obvious peak expression levels during high temperature stress; e.g. prominent peaks at 15 min (31 °C) and 1 h (31 °C, 37 °C, 43 °C). However, these spikes in Bthsf1 transcript levels were not observed during low temperature stress. Our results indicate that B. tabaci is conditioned by heat stress to activate HSF1 and promote hsp expression. Furthermore, our findings help explain why the fold increases in Bthsps transcription during high temperatures are so much higher than hsp expression levels during cold temperatures33,36. During prolonged periods of heat stress, Bthsf1 is gradually down-regulated; the protracted accumulation of HSPs becomes deleterious to the cell, which leads to the repression of HSF by HSP70 and other molecular chaperones44,45,46. Our results reveal the importance of studying the expression of hsf and hsp concurrently during thermal stress.
The feeding method of dsRNA delivery has been widely and successfully used to study gene function in hemipteran insects47,48,49,50,51,52. When B. tabaci was supplied with dsBthsf1 for 1 day, Bthsf1 expression was significantly downregulated after exposure to − 6 °C and 41 °C, and mortality increased relative to the dsGFP control. The contribution of HSF to hsp expression, fecundity and survival during adverse conditions has been studied in other organisms. For example, in Artemia franciscana, hsf1 knockdown decreased hsp expression in diapausing embryos53, and RNAi-mediated suppression of hsf in Haliotis diversicolor downregulated several hsps54. In A. hygrophila, microinjection of dsAhHsf into newly-emerging adults reduced the expression of two different hsps and decreased egg production and survival39. In our study, RNAi with dsBthsf1 resulted in a significant down-regulation of Bthsp90, Bthsp70-3, Bthsp20, and Bthsp19.5 at − 6 °C and 41 °C, indicating that Bthsf1 is involved in the regulation of multiple HSP genes in B. tabaci. In addition, we also found that the expression of Bthsp70-1 did not decrease as knockdown of Bthsf1, indicating that Bthsf1 may not be the most important regulatory path for Bthsp70-1, and there may be other ways to regulate the expression of the gene. Collectively these findings indicate that Bthsf1 can regulate the expression of some but not all hsps, and further studies are warranted to confirm BtHSF1 interactions and regulatory functions.
Materials and methods
Insects
B. tabaci were reared on tomato (The tomato seeds involved in this study are in line with the national seed quality standards in China, the implementation standard number is GB16715.3–2010, and the seed production and operation license number is: D (Jicangqing) Nongzhongxuzi (2016) No.0006, Xingyun Vegetable Breeding Center, Hebei, China) in controlled temperature chambers plants as described36. Identification of the B. tabaci MED cryptic species was determined using the mitochondrial cytochrome oxidase I (mtCOI) gene as described previously55.
Isolation of RNA, cloning and RACE
Total RNA was isolated from B. tabaci pupae and adults as described previously and stored at − 80 °C until needed36. cDNA was synthesized using an oligo(dT)18 primer (TaKaRa), and full-length cDNAs encoding HSF1 were obtained by 5′- and 3′-RACE (SMART RACE, Clontech) using the primers listed in Table 1. HSF sequences were confirmed by 5′ RACE.
Isolation and characterization of Bthsf1
The fragment of HSF1 was isolated and identified based on analysis of the published transcriptome data56. The primers used for amplifying fragment are provided in Table 1. PCR products were purified, cloned, and sequenced as described36.
Established methods were used for identifying ORFs and aligning amino acid sequences36,57. Bthsf1 sequences were analyzed with tools available at the ExPASy Molecular Biology Server (https://www.expasy.org/) including Compute pI/MW, BLAST, and Translate. Phylogenetic analyses were conducted as described previously36,58. The three-dimensional (3D) structure of the DBD domain was predicted by the SWISS-MODEL website (https://swissmodel.expasy.org/) using the Drosophila melanogaster DBD domain (SMTL ID: 1hkt.1) as a template.
Synthesis of dsRNA
Full-length B. tabaci HSF1 gene was identified using the online website (http://sidirect2.rnai.jp/); the regions for RNA silencing were determined, and primers for RNAi were designed. Sense and antisense primers included a T7 promoter sequence (TAATACGACTCACTATAGGG) at the 5′ ends to catalyze transcription from both cDNA strands (Table 1). dsRNA specific to the gene encoding green fluorescence protein (dsGFP) was used as a control (Table 1). PCR products were cloned in pGEM-T easy (Promega, Madison, WI, USA) and resulting constructs were used as template DNA in subsequent amplifications. The PCR product was used for preparation of double-stranded RNA (dsRNA) using the MEGAscript® RNAi kit according to the manufacturer's instructions (Thermo, Waltham, MA, USA)59. The quality of dsRNA was evaluated by spectrophotometry and gel electrophoresis and then diluted into 30% (w/v) sucrose for use in experiments.
Oral ingestion of dsRNA
Feeding chambers for delivering dsRNA were constructed as described previously60 with minor modifications. Two pieces of Parafilm membrane (2 × 2 cm2) were stretched out by hand until they were each twofold their original length. A tube was sealed with 2 layers of membrane containing 30% (w/v) sucrose solution (300 μL) between them. One side of the tube was covered with a piece of meshed net to allow aeration. Adult whiteflies (aged less than 12 h) were released into the Parafilm chamber before covering it with a meshed net. A Parafilm sandwich was positioned into the top of the tube and the tube was incubated at 25 °C For experiments, various amounts (500 ng/μL) of dsBthsf1 or dsGFP were diluted into 30% (w/v) sucrose solution. Experiments were conducted four times under identical conditions.
Temperature exposure
B. tabaci adults and pupae were collected, placed in glass tubes, and exposed to each of the following temperatures for 1 h: − 12,− 10,− 8,− 6, 39, 41, 43, and 45 °C. Adults and pupae that were maintained at room temperature (26 °C) were used as controls. Treated adults and pupae were allowed to recover at 26 °C for 1 h and were then frozen in liquid nitrogen and stored at − 80 °C (N = 4).
In experiments with different duration of temperature, B. tabaci adults (n = 60) were exposed to high temperatures (31, 37 and 43 °C) for 15 min, 30 min, 1 h, 1.5 h, and 2 h and low temperatures ( − 10, − 4 and 2 °C) for 30 min, 1 h, 1.5 h, 2 h and 3 h. Insects were then allowed to recover at 26 °C for the same duration as the temperature treatment (N = 4). Adults and pupae maintained at room temperature (26 °C) were used as controls.
For RNAi, newly emerged B. tabaci adults were supplied with dsBthsf or dsGFP for 1 day, exposed to -6 and 41 °C for 1 h, and then allowed to recover at 26 °C for 1 h. The mortality of B. tabaci was checked after temperature stress, and the surviving B. tabaci were frozen in liquid nitrogen and stored at − 80 °C. Each treatment included four biological replications.
Quantitative real-time PCR
The cDNA template was transcribed from RNA with the HiScript III RT SuperMix for qPCR (Vazyme, Nanjing, China) as recommended, and primers were designed with Primer 5.0 software (Table 1). Quantitative real-time PCR (qRT-PCR) was performed in 20 μL total reaction volumes comprised of 10 μL of 2 × ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China), 1 μL of each gene specific primer (Table 1), and 2 μL of cDNA templates. It was carried out that reactions on a CFX-Connect real-time PCR system (Bio-Rad, Berkeley, CA, USA) using the following conditions: 3 min at 95 °C, 40 cycles of denaturation at 95 °C for 30 s, and annealing (30 s) at 60 °C for each gene. And gene expression was calculated using the 2−ΔΔCt method and normalized to the abundance Elongation factor 1 alpha (EF-1α) and 60S ribosomal protein L29 (RPL29)61.
Data analysis
One-way ANOVA, followed by Tukey’s and Duncan’s multiple comparison, was used to detect significant differences among temperatures using SPSS v. 16.062. For ANOVA, data were transformed for homogeneity of variances, and differences were considered statistically significant when P < 0.05.
For RNAi, the relative abundance of target genes and survival rates were compared to the dsGFP control. Student’s t-test was used to compare differences in gene expression and mortality with SPSS v. 16.0, and differences were considered significant at P < 0.05.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Mcdonald, J. R. et al. Temperature, development and establishment potential of Thrips palmi (Thysanoptera: Thripidae) in the United Kingdom. Eur. J. Entomol. 96(2), 169–173 (1999).
Hoffmann, A. A. et al. Adaptation of Drosophila, to temperature extremes: Bringing together quantitative and molecular approaches. J. Therm. Biol. 28(3), 175–216 (2003).
Chidawanyika, F. & Terblanche, J. S. Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 57(1), 108–117 (2011).
Kang, L. et al. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol. 54, 127–145 (2009).
Moseley, P. L. Heat shock proteins and heat adaptation of the whole organism. J. Appl. Physiol. 83(5), 1413–1417 (1997).
Kim, K. K. et al. Crystal structure of small heat-shock protein. Nature 394(6693), 595–599 (1998).
Feder, M. E. & Hofmann, G. E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282 (1999).
Sørensen, J. G. et al. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6(11), 1025–1037 (2003).
Amin, J. et al. Key features of heat shock regulatory elements. Mol. Cell Biol. 8(9), 3761–3769 (1988).
Akerfelt, M. et al. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11(8), 545–555 (2010).
Wiederrecht, G. et al. Isolation of the gene encoding the S. cerevisiae heat shock transcription factor. Cell 54(841), 853 (1988).
Ito, T. et al. Isolation, structural elucidation, and biological evaluation of a 5-hydroxymethyl-2-furfural derivative, asfural, from enzyme-treated asparagus extract. J. Agric. Food Chem. 61(38), 9155–9159 (2013).
Triandafillou, C. G. & Drummond, D. A. Heat shock factor 1: From fire chief to crowd-control specialist. Mol. Cell 63(1), 1–2 (2016).
Kihara, F. et al. Heat shock factor binds to heat shock elements upstream of heat shock protein 70a and Samui genes to confer transcriptional activity in Bombyx mori diapause eggs exposed to 5 °C. Insect Biochem. Mol. 41(11), 843–851 (2011).
Pirkkala, L. et al. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15(7), 1118–1131 (2001).
Anckar, J. & Sistonen, L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv. Exp. Med. Biol. 594, 78–88 (2017).
Fujikake, N. et al. Alternative splicing regulates the transcriptional activity of Drosophila heat shock transcription factor in response to heat/cold stress. FEBS Lett. 579(17), 3842–3848 (2005).
Zhong, M. et al. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol. Cell 2(1), 101–108 (1998).
Chen, W. et al. Alternative splicing and expression analysis of HSF1 in diapause pupal brains in the cotton bollworm Helicoverpa armigera. Pest. Manag. Sci. 75, 1258–1269 (2019).
Kihara, F. et al. Heat shock factor binds to heat shock elements upstream of heat shock protein 70a and Samui genes to confer transcriptional activity in Bombyx mori diapause eggs exposed to 5 °C. Insect Biochem. Mol. 41, 843–851 (2011).
Sonoda, S. & Tsumuki, H. Characterization of alternatively spliced transcripts encoding heat shock transcription factor in cultured cells of the cabbage armywonn Mamestra brassicae. Arch. Insect Biochem. Physiol. 73(1), 49–60 (2010).
Kanakala, S. & Ghanim, M. Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PLoS ONE 14, e0213946 (2019).
Oliveira, M. R. V. et al. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 20, 709–723 (2001).
Xia, J. X. et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 184(7), 1693–1705 (2021).
Wan, F. H. et al. Invasive mechanism and management strategy of Bemisia tabaci (Gennadius) biotype B: Progress report of 973 program on invasive alien species in China. Sci in China Series C Life Sci 52, 88–95 (2009).
De Barro, P. J. et al. Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56, 1–19 (2011).
Crowder, D. W. et al. Mating behaviour, life history and adaptation to insecticides determine species exclusion between whiteflies. J. Anim. Ecol. 79(3), 563–570 (2010).
Sun, D. B. et al. Competitive displacement between two invasive whiteflies: insecticide application and host plant effects. Bull. Entomol. Res. 103(3), 344–353 (2013).
Frewin, A. J. et al. Demographic trends in mixed Bemisia tabaci (Hemiptera: Aleyrodidae) cryptic species populations in commercial poinsettia under biological control- and insecticide-based management. J. Econ. Entomol. 107(3), 1150–1155 (2014).
Horowitz, A. R. & Ishaaya, I. Dynamics of biotypes B and Q of the whitefly Bemisia tabaci and its impact on insecticide resistance. Pest Manag. Sci. 70(10), 1568–1572 (2014).
Sun, D. B. et al. Effects of reproductive interference on the competitive displacement between two invasive whiteflies. Bull. Entomol. Res. 104(3), 334–346 (2014).
Tsueda, H. & Tsuchida, K. Reproductive differences between Q and B whiteflies, Bemisia tabaci, on three host plants and negative interactions in mixed cohorts. Entomol. Exp. Appl. 141(3), 197–207 (2011).
Yu, H. et al. Different thermal tolerance and Hsp gene expression in invasive and indigenous sibling species of Bemisia tabaci. Biol. Invasions 14(8), 1587–1595 (2012).
Xiao, N. et al. Differential tolerance capacity to unfavourable low and high temperatures between two invasive whiteflies. Sci. Rep. 6, 24306 (2016).
Shim, J. K. et al. Oral ingestion of heat shock protein 70 dsRNA is lethal under normal and thermal stress conditions in the sweet potato whitefly Bemisia tabaci. J. Asia Pac Entomol. 18(4), 797–800 (2015).
Bai, J. et al. Characterization of genes encoding small heat shock proteins from Bemisia tabaci and expression under thermal stress. PeerJ 7, e6992 (2019).
Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 11, 441–469 (1995).
Jedlicka, P. et al. Multiple functions of Drosophila heat shock transcription factor in Vivo. EMBO J. 16(9), 2452–2462 (1997).
Jin, J. S. et al. Heat shock factor is involved in regulating the transcriptional expression of two potential Hsps (AhHsp70 and AhsHsp21) and its role in heat shock response of Agasicles hygrophila. Front. Physiol. 11, 562204 (2020).
He, F. J. et al. Molecular characterization of heat-induced HSP11.0 and master-regulator HSF from Cotesia chilonis and their consistent response to heat stress. Insects 12(4), 322 (2021).
Friant, S. et al. Increased ubiquitin-dependent degradation can replace the essential requirement for heat shock protein induction. EMBO J 22(15), 3783–3791 (2003).
Li, X. et al. Comparative physiological and proteomic analyses of poplar (Populus yunnanensis) plantlets exposed to high temperature and drought. PLoS ONE 9, e107605 (2014).
Lv, J. & Liu, S. Influence of acclimation to sublethal temperature on heat tolerance of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) exposed to 50˚C. PLoS ONE 12, e0182269 (2017).
Shi, Y. et al. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 12(5), 654–666 (1998).
Neef, D. W. et al. A direct regulatory interaction between chaperonin Tric and stress-responsive transcription factor HSF1. Cell Rep. 9(3), 955–966 (2014).
Sivery, A. et al. A minimal titration model of the mammalian dynamical heat shock response. Phys. Biol. 13(6), 066008 (2016).
Jain, R. G. et al. RNAi-Based functional genomics in Hemiptera. Insects 11(9), 557 (2020).
Lü, Z. C. & Wan, F. H. Using double-stranded RNA to explore the role of heat shock protein genes in heat tolerance in Bemisia tabaci (Gennadius). J. Exp. Biol. 214(5), 764–769 (2011).
Li, J. J. et al. RNA interference of the P450 CYP6CM1 gene has different efficacy in B and Q biotypes of Bemisia tabaci. Pest Manag. Sci. 71(8), 1175–1181 (2015).
Upadhyay, S. K. et al. Molecular characterization of vitellogenin and vitellogenin receptor of Bemisia tabaci. Plos ONE 11, e0155306 (2016).
Vyas, M. et al. Knock down of Whitefly Gut Gene Expression and Mortality by Orally Delivered Gut Gene-Specific dsRNAs. Plos ONE 12, e0168921 (2017).
Zhang, C. et al. RNAi knock-down of the Bemisia tabaci Toll gene (BtToll) increases mortality after challenge with destruxin A. Mol. Immunol. 88, 164–173 (2017).
Tan, J. B. & Macrae, T. H. Stress tolerance in diapausing embryos of Artemia franciscana is dependent on heat shock factor 1 (Hsf1). PLoS ONE 13, e0200153 (2018).
Zhang, X. et al. Regulatory effect of heat shock transcription factor-1 gene on heat shock proteins and its transcriptional regulation analysis in small abalone Haliotis diversicolor. BMC Mol. Cell Biol. 21(1), 83 (2020).
Xu, L. L. et al. Biotypes and phylogenetic analysis of Bemisia tabaci (Hemiptera: Aleyrodidae) in China. J. Appl. Ecol. 25(4), 1137–1144 (2014).
Bai, J. et al. Transcriptional profiling of MED Bemisia tabaci exposed to thermal stress and verification of HSP70 expression. Entomol. Res. 51, 251–262 (2021).
Thompson, J. D. et al. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. https://doi.org/10.1002/0471250953.bi0203s00 (2002).
Kumar, S. et al. (2016) MEGA7 molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7), 1874 (1870).
Chang, Y. W. et al. RNA interference of genes encoding the vacuolar-ATPase in Liriomyza trifolii. Insects 12(1), 41 (2021).
Jahan, S. M. H. et al. Upregulation of probing- and feeding-related behavioural frequencies in Bemisia tabaci upon acquisition of Tomato yellow leaf curl virus. Pest Manag. Sci. 70(10), 1497–1502 (2014).
Li, R. M. et al. Reference gene selection for qRT-PCR analysis in the sweet potato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 8, e53006 (2013).
Pallant, J. SPSS survival manual: A step by step guide to data analysis using SPSS for windows (Version 12) (Open University Press, 2005).
Acknowledgements
This research was supported by the Special Fund for Agro-scientific Research in the Public Interest of China (No. 201303019), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_2111) and the Jiangsu Agricultural Industry Technology System (JATS [2020] 309). We sincerely thank Dr. Carol L. Bender for editing English and helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Data curation: J.B., Y.C.L. and Y.Z.D; formal analysis: J.B., Y.C.L. and R.W.; software: J.B. and Y.C.W.; validation: R.W. and Y.C.W.; investigation: Y.C.L., R.W. and Y.C.W.; writing-original draft preparation: J.B. and Y.C.L.; writing-review and editing: Y.Z.D.; supervision, W.R.G.; funding acquisition, W.R.G and Y.Z.D. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bai, J., Liu, YC., Wei, R. et al. Knockdown of heat shock transcription factor 1 decreases temperature stress tolerance in Bemisia tabaci MED. Sci Rep 12, 16059 (2022). https://doi.org/10.1038/s41598-022-19788-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19788-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.