Abstract
Patients with Marfan syndrome and related disorders are at risk for aortic dissection and aortic rupture and therefore require appropriate monitoring. Computed tomography (CT) and transthoracic echocardiography (TTE) are routinely used for initial diagnosis and follow-up. The purpose of this study is to compare whole-heart CT and TTE aortic measurement for initial work-up, 2-year follow-up, and detection of progressive aortic enlargement. This retrospective study included 95 patients diagnosed with Marfan syndrome or a related disorder. All patients underwent initial work-up including aortic diameter measurement using both electrocardiography-triggered whole-heart CT and TTE. Forty-two of these patients did not undergo aortic repair after initial work-up and were monitored by follow-up imaging within 2 years. Differences between the two methods for measuring aortic diameters were compared using Bland–Altman plots. The acceptable clinical limit of agreement (acLOA) for initial work-up, follow-up, and progression within 2 years was predefined as < ± 2 mm. Bland–Altman analysis revealed a small bias of 0.2 mm with wide limits of agreement (LOA) from + 6.3 to − 5.9 mm for the aortic sinus and a relevant bias of − 1.6 mm with wide LOA from + 5.6 to − 8.9 mm for the ascending aorta. Follow-up imaging yielded a small bias of 0.5 mm with a wide LOA from + 6.7 to − 5.8 mm for the aortic sinus and a relevant bias of 1.1 mm with wide LOA from + 8.1 to − 10.2 mm for the ascending aorta. Progressive aortic enlargement at follow-up was detected in 57% of patients using CT and 40% of patients using TTE. Measurement differences outside the acLOA were most frequently observed for the ascending aorta. Whole-heart CT and TTE measurements show good correlation, but the frequency of measurement differences outside the acLOA is high. TTE systematically overestimates aortic diameters. Therefore, whole-heart CT may be preferred for aortic monitoring of patients with Marfan syndrome and related disorders. TTE remains an indispensable imaging tool that provides additional information not available with CT.
Similar content being viewed by others
Introduction
Marfan syndrome is the most common genetic disorder affecting the aorta with an estimated incidence of 2–3 per 10,000 individuals. Less common inherited connective tissue disorders of the aorta include Loeys-Dietz syndrome and the vascular type of Ehlers-Danlos syndrome (EDS)1. Mutations underlying classic Marfan syndrome involve the FBN1 gene, whereas mutations in TGFBR1 and 2, SMAD2 and 3, and TGFB2 and 3 can be detected in Loeys-Dietz syndrome types 1 to 6, and a Col3A1 gene mutation has been described for the vascular type of EDS2,3. Patients with untreated genetic aortic disorders have a dramatically lower life expectancy as they are at risk for aortic dissection, aortic rupture, and heart failure4,5,6. Therefore, aortic monitoring is of great importance to reduce morbidity and mortality in these patients.
International guidelines recommend imaging of the aorta for initial aortic work-up and follow-up of patients with proven or suspected genetic aortic disease7,8. Correct measurement of aortic diameters is essential for risk stratification and preoperative evaluation before surgical repair. The 2017 ESC guideline states that surgery is indicated in all patients with Marfan syndrome and a maximal aortic diameter ≥ 50 mm, and that in patients with additional risk factors and in patients with a TGFBR1 or TGFBR2 mutations surgery should be considered at a maximal aortic diameter ≥ 45 mm9. One of these risk factors is as an increase in aortic diameter on repeated measurements using the same imaging technique. Therefore, correct measurement is also important during follow-up imaging to detect patients with progressive aortic enlargement. The availability of cardiovascular imaging techniques has greatly improved long-term survival especially in Marfan syndrome10.
The most commonly used imaging modalities for aortic monitoring are computed tomography (CT) and transthoracic echocardiography (TTE). Alternatively, magnetic resonance imaging (MRI) can be performed, though its use is often reserved for younger patients due to higher cost, longer examination time, and limited availability11. As measurement conventions of aortic diameters differ between institutions and imaging modalities, patients with genetic aortic disease should undergo follow-up imaging preferably at the same institution using the same imaging modality, especially if previous cross-sectional imaging did not correlate sufficiently12. The guideline on aortic disease endorsed by the American College of Cardiology (ACC) and the American Heart Association (AHA) recommends measurement of the external aortic diameter perpendicular to the axis of blood flow when CT or MRI is used and measurement of the internal aortic diameter perpendicular to blood flow when TTE is used8,13. There are three typical CT measurements of the aortic root: aortic annulus, aortic sinus and sinotubular junction. The ACC/AHA guideline states that the widest diameter, typically at the mid-sinus level, should be used. This CT measurement of the aortic root has shown to have the best reproducibility14.
State-of-the-art CT scanners allow ECG-triggered aortic imaging with high diagnostic accuracy. Hence, CT is the modality of choice for preoperative imaging in patients scheduled for transcatheter structural heart interventions and has been used as the “gold standard” in earlier comparative studies15,16. Exact measurement of aortic diameters is crucial for both initial work-up and follow-up monitoring, as patients with aortic aneurysm (defined by these measurements) and progressive aortic enlargement are at risk for life-threatening aortic rupture and dissection17.
In patients with genetic aortic disease, detecting progressive enlargement of the aorta is extremely important as they may require surgical aortic repair18. Even if follow-up imaging is performed at a different institution with different measurement conventions, progressive aortic enlargement can be detected more objectively if prior CT scans are provided to the radiologist rather than TTE, which has larger inter- and intraobserver variability19.
This study aims at comparing state-of-the-art whole-heart CT and TTE aortic measurements for initial work-up, follow-up, and detection of progressive aortic enlargement in patients with Marfan syndrome or a related disorder.
Materials and methods
Study design
The study was approved by the institutional review board (Ethikkommission der Charité – Universitätsmedizin Berlin), which waived the need for patient consent. In this single-center comparative cohort study, we compared measurements of aortic diameters in a retrospective dataset of patients who underwent CT and TTE of the aorta. In a subgroup of patients who did not undergo surgical repair and were monitored by follow-up aortic imaging within 2 years, we analyzed measurement accuracy by comparing measurements taken at initial work-up and follow-up and evaluated increases in aortic diameters between the two time points as a measure of disease progression. The study was in compliance with the Declaration of Helsinki.
Patient population and characteristics
We enrolled patients diagnosed with Marfan syndrome or a related disorder including Loeys-Dietz syndrome, Ehlers-Danlos syndrome, and familial aortic dissection at the outpatient Marfan Center of Charité University Hospital in Berlin. Marfan syndrome was diagnosed using the 2010 revised Ghent Criteria, which are based on physical examination, aortic imaging, family history, and genetic testing in some cases4. The diagnosis of Marfan syndrome requires presence of clinical criteria including aortic root dilatation/dissection and ectopia lentis. If genetic testing was performed, the presence of FBN1 mutation was confirmed in all patients diagnosed with Marfan syndrome. Patients diagnosed with Loeys-Dietz syndrome or Ehlers-Danlos syndrome had undergone genetic testing, which confirmed typical mutations.
All patients were referred to the Department of Radiology between 2015 and 2020. All patients underwent ECG-triggered CT angiography of the aorta and TTE of the proximal thoracic aorta for initial assessment. Our additional follow-up analysis was performed in a subgroup of patients who did not undergo surgical treatment within 2 years after initial work-up. Instead, the subgroup was monitored and underwent follow-up imaging with both CT and TTE due to suspected progression of aortic enlargement, suspected aortic dissection, or preoperative evaluation for surgical repair or replacement. Exclusion criteria were prior interventional or surgical aortic repair. As aortic enlargement may be due to causes other than Marfan syndrome or a related disorder, patients aged above 55 years were excluded from this study.
Image acquisition and aortic diameter measurement
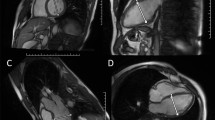
All patients referred to the Department of Radiology were examined in the same single-source 256-row CT scanner (Revolution CT, General Electric, Milwaukee, USA). Intravenous bolus injection of iodinated contrast medium was administered. In diastole an axial ECG-triggered whole-heart scan including the aortic root was obtained, directly followed by a helical scan of the entire aorta. Bolus tracking with SmartPrep (General Electric, Milwaukee, USA) was used to ensure adequate opacification of the aorta. Aortic diameters were determined from double oblique multiplanar reconstructions perpendicular to the course of the vessel20. All measurements were taken at the levels of the aortic sinus and the largest diameter of the ascending aorta (between aortic arch and sinotubular junction) using Visage (Visage Imaging, Berlin, Germany). At the level of aortic sinus, the maximal (non-anatomically landmarked) diameter at the mid-sinus level was used as recommended by the ACC/AHA guideline8. During serial follow-up imaging, aortic diameter was consistently measured from outer edge to outer edge. All measurements were performed by the same radiologist with more than 7 years of experience in cardiovascular imaging. An example illustrating aortic diameter measurement from double oblique multiplanar CT reconstructions perpendicular to the course of the aorta is shown in Fig. 1.
Example illustrating the importance of double oblique multiplanar reconstruction perpendicular to the course of the vessel for correct aortic diameter measurement: (a) from unreconstructed CT, a wrong ascending aortic diameter of 57.2 mm is measured; (b) measurement from reconstructed CT yields the correct aortic diameter of 53.4 mm. Like TTE, unreconstructed CT may preclude correct diameter measurement perpendicular to the course of the vessel in patients with an elongated aorta and/or thoracic deformities. CT = computed tomography; TTE = transthoracic echocardiography.
All patients also underwent two-dimensional TTE with the Epic 700 cardiac ultrasound machine (Philips, Amsterdam, the Netherlands) at initial work-up and at follow-up. Aortic diameters at the aortic sinus and the ascending aorta were measured in diastole from inner edge to inner edge. All examinations and measurements were performed by an attending-level cardiologist and an attending-level cardiac surgeon, both board-certified, with over 20 years of experience in echocardiography, using an established and standardized protocol based on recent echocardiography guidelines21.
Comparison of measurement methods
Pearson’s correlation coefficient was used to measure correlation between CT and TTE. For further analysis, Bland–Altman plots were used to compare the two measurement methods regarding initial work-up, follow-up, and change in diameter over time. The acceptable clinical limit of agreement for the difference between the two methods for initial work-up, follow-up, and progression within 2 years was predefined as < ± 2 mm. Diameters of the aortic sinus and ascending aorta obtained by CT- and TTE-based measurement were compared for initial work-up and follow-up. Measurement differences showing an increase in aortic diameters between initial and follow-up imaging were compared between CT and TTE using Bland–Altman plots.
Statistical analysis
All data analyses were performed using IBM SPSS Statistics version 27 (IBM, Armonk, New York, USA).
Results
Baseline data
A total of 95 patients with a mean age of 35 ± 10 years were included in this study, among them 60 men (63%) and 35 women (37%). A majority of 77 patients (81%) were diagnosed with Marfan syndrome, 10 patients (10%) with Loeys-Dietz syndrome, 6 patients (6%) with Ehlers-Danlos syndrome, and 2 patients (2%) with familial aortic dissection at the outpatient Marfan Center of Charité University Hospital in Berlin. For initial work-up, all 95 patients underwent aortic imaging with CT and TTE. In a subgroup of 42 patients (44%) who did not undergo aortic repair within 2 years and were instead monitored by follow-up imaging, aortic diameters were measured again with both CT and TTE. The mean interval between initial and follow-up imaging was 581 ± 170 days. Seventy-nine patients (83%) were on medication including a renin-angiotensin system antagonist or a beta-blocker, whereas 16 patients (17%) did not receive any medication. The mean dose-length product (DLP) of the aortic CT scan was 502 ± 193 mGy * cm. Baseline data are summarized in Table 1.
Diameters of aortic sinus and ascending aorta
At initial work-up, the mean diameter of the aortic sinus was 41.1 ± 5.9 mm using CT and 41.0 ± 5.3 mm using TTE. The mean diameter of the ascending aorta was 33.9 ± 7.3 mm using CT and 35.6 ± 7.3 mm using TTE.
At follow-up, the mean diameter of the aortic sinus was 43.9 ± 5.0 mm using CT and 43.4 ± 5.6 mm using TTE. The mean diameter of the ascending aorta was 37.3 ± 8.0 mm using CT and 38.4 ± 9.4 mm using TTE.
Progressive aortic enlargement
In the subgroup of 42 patients who underwent follow-up imaging, progressive aortic enlargement was defined as an increase in the diameter of the aortic sinus or the ascending aorta of at least 2 mm within 2 years. Follow-up aortic imaging for monitoring patients with Marfan syndrome or a related disorder detected progressive aortic enlargement in 24 patients (57%) using CT versus 17 patients (40%) using TTE. In these patients, we found a mean increase in aortic diameter of 2.6 ± 1.1 mm using CT and of 3.8 ± 2.8 mm using TTE.
Pearson’s correlation between CT and TTE measurement
The two measurement methods, CT and TTE, showed statistically significant correlation. For initial measurement, Pearson’s correlation coefficients were 0.85 for the aortic sinus and 0.87 for the ascending aorta. For follow-up measurement, Pearson’s correlation coefficients were similar with 0.83 for the aortic sinus and 0.87 for the ascending aorta. All measurement correlations were statistically significant at the p < 0.01 level.
Diameters of the aortic sinus: comparison of measurement methods
For initial measurement of the aortic sinus, Bland–Altman analysis showed a small discrepancy of the two measurement methods with a bias of 0.2 mm. There were wide limits of agreement ranging from + 6.3 to − 5.9 mm. Thirty-two patients (34%) were outside the clinically acceptable limits of agreement regarding the difference between the two measurement methods (> 2 mm), whereas 63 patients (66%) were within the limits of agreement (Fig. 2a).
Bland–Altman plots for analysis of measurement differences in aortic sinus diameter in patients with Marfan syndrome or a related disorder for (a) initial work-up, (b) follow-up, and (c) progression of aortic disease. Red lines: upper and lower limits of agreement. Dotted red line: clinically acceptable limit of agreement for difference. Green line: agreement bias.
For the subgroup of patients who underwent follow-up aortic imaging, Bland–Altman analysis showed a small discrepancy of the two measurement methods with a bias of 0.5 mm. There were wide limits of agreement ranging from + 6.7 to − 5.8 mm. Sixteen patients (38%) were outside the clinically acceptable limits of agreement regarding the difference between the two measurement methods (> 2 mm), whereas 26 patients (62%) were within the limits of agreement (Fig. 2b).
Bland–Altman plots analyzing differences in progression of aortic enlargement from initial work-up and follow-up showed a bias of 0.1 mm with wide limits of agreement ranging from + 3.8 to -3.5 mm. Five patients (12%) were outside the clinically acceptable limits of agreement regarding this parameter (Fig. 2c).
Diameters of the ascending aorta: comparison of measurement methods
For initial measurement of the ascending aorta, Bland–Altman analysis showed a relevant discrepancy of the two measurement methods with a bias of − 1.6 mm. There were wide limits of agreement ranging from + 5.6 to − 8.9 mm. Thirty-eight patients (40%) were outside the clinically acceptable limits of agreement for the difference (> 2 mm) between the two measurement methods, whereas 58 patients (60%) were within the limits (Fig. 3a).
Bland–Altman plots for analysis of measurement differences in ascending aorta diameter in patients with Marfan syndrome or a related disorder for (a) initial work-up, (b) follow-up, and (c) progression of aortic disease. Red lines: upper and lower limits of agreement. Dotted red line: clinically acceptable limit of agreement for difference. Green line: agreement bias.
For the subgroup of patients who underwent follow-up aortic imaging, Bland–Altman analysis showed a relevant discrepancy of the two measurement methods with a bias of 1.1 mm. There were wide limits of agreement ranging from + 8.1 to − 10.2 mm. Fifteen patients (36%) were outside the clinically acceptable limits of agreement for the difference (> 2 mm) between the two measurement methods, whereas 27 patients (64%) were within the limits (Fig. 3b).
Bland–Altman plots analyzing measurement differences regarding progressive aortic disease showed a bias of 0.1 mm with wide limits of agreement ranging from + 4.5 to − 4.3 mm. Thirteen patients (31%) were outside the clinically acceptable limits of agreement for the difference (Fig. 3c).
Discussion
In this study we analyzed differences in aortic diameter measurement using ECG-triggered whole-heart CT and TTE in patients with Marfan syndrome or a related disorder for initial work-up, follow-up, and detection of increases in aortic diameter within 2 years. Even though the two measurement methods showed good correlation and small overall differences of mean, Bland–Altman plots revealed that the individual differences in aortic diameters measured using CT and TTE show wide limits of agreement in both initial work-up and follow-up, especially for measurement of ascending aortic diameter. For both initial and follow-up aortic imaging, Bland–Altman analysis exposed a relevant measurement bias regarding the ascending aorta. TTE detected fewer cases of progressive aortic enlargement within 2 years of initial measurement compared with CT. For both the ascending aorta and the aortic sinus, we found high rates of measurement differences outside the acceptable clinical limits of agreement for differences in initial work-up, follow-up, and identification of increases in aortic diameter.
Imaging of the aorta using TTE is widely available at relatively low cost and offers the additional benefit of allowing assessment of cardiac function. For example, echocardiography provides information on the severity of mitral valve prolapse and aortic regurgitation, which is common in Marfan syndrome 22. However, accurate aortic diameter measurement might be hampered by thoracic deformity and an elongated ascending aorta23. Another disadvantage of TTE compared to CT is that only the aortic root and ascending aorta are visualized, meaning no information on the descending aorta can be obtained.
A disadvantage of using CT for aortic imaging is radiation exposure. Patients with Marfan syndrome or a related disorder require aortic imaging for initial work-up and serial follow-up monitoring, ultimately leading to a cumulative dose of potentially harmful ionizing radiation in often young patients. However, average radiation dose for aortic imaging has substantially decreased with state-of-the-art CT scanners and adjusted CT protocols24,25. Additionally, adverse effects related to intravenous contrast medium administration can occur, even though severe reactions have been reported in less than 0.01% of cases26.
Enlargement of the aortic root is a major criterion for diagnosing genetic aortic disease and is associated with a high risk of aortic dissection and hence cardiovascular mortality2,4,27. Life expectancy of patients with Marfan syndrome or a related disorder has improved to near-normal due to better monitoring including aortic imaging and timely surgery28,29. Given the increased cardiovascular mortality and morbidity, aortic repair is usually recommended in patients with progression of aortic enlargement30,31.
Accurate measurement of aortic diameters is of utmost importance for patients diagnosed with Marfan syndrome or a related disorder because aortic size and progressive aortic enlargement are crucial parameters for predicting fatal rupture of thoracic aortic aneurysm or dissection32,33. CT is the modality of choice for measuring aortic diameters for preoperative or preinterventional planning as it is widely available and has proven to be highly accurate34,35. Several studies have shown that, while adequate monitoring of the aortic root is also possible with TTE or MRI, CT is superior in detecting aortic dissection36. Another advantage of ECG-triggered CT is the option to rule out significant coronary artery disease, which is relevant for preoperative planning37. Moreover, additional information including CT body composition parameters relevant for prediction of progressive aortic enlargement and further manifestations of Marfan syndrome or related disorders can be derived20.
All aortic measurements during both initial work-up and follow-up were taken by the same observer, as intraobserver variability of aortic measurement is low while interobserver variability may be relevant38. Our results show relevant measurement differences between CT and TTE with a relevant bias regarding the ascending aorta. We think this finding is most likely attributable to systematic overestimation of aortic diameters when TTE is used. In most cases in which TTE shows measurement differences outside the acceptable clinical limit of agreement, CT reveals typical thoracic deformity and/or an elongated ascending aorta, which may preclude adequate aortic diameter measurement perpendicular to the axis of blood flow using TTE. In CT, aortic diameters are determined from double oblique multiplanar reconstructions perpendicular to the course of the aorta. Moreover, TTE-based measurement of the aortic sinus may falsely include an ectatic proximal coronary artery—a common phenomenon in patients with Marfan syndrome or a related disorder39.
Previous studies have compared CT and TTE measurements of aortic diameter. Bons et al. conclude that there may be large measurement differences in the ascending aorta and therefore also opt for CT or MR angiography40. In contrast, a retrospective comparison performed by Ocak et al. suggests that TTE yields substantially lower or even normal diameter measurements when CT demonstrates a dilated aortic root41, while Frazo et al. report that TTE underestimates aortic root diameters compared with CT42. Unlike our study, the investigators just quoted did not measure aortic diameters in patients with Marfan syndrome and related disorders. Ascending aortic aneurysms in patients with Marfan syndrome have been studied by Amsallem et al. who showed that CT measurements should be obtained on strict transverse plane instead of on three-cavity view or left ventricle-aorta view43. Even though we agree that the transverse plane should be used, their conclusion was based on overall differences of the mean which is an important limiting factor for comparing two measurement methods. As a good example in our study the mean diameter of the aortic sinus was 41.1 mm using CT and 41.0 mm using TTE at initial work-up. However, Bland–Altman plots revealed important individual measurement differences which are very relevant in a potentially life-threatening disease. Similarly, on the basis of overall differences of the mean Rodríguez-Palomares et al. concluded that the leading edge to leading edge convention should be used if TTE was compared to CT or MRI44. Both studies have used internal diameters for CT measurements, whereas we have used external diameters as recommended by the AHA/ACC guideline8,13.
Despite its limited availability, MRI has proven to be an important alternative in several studies showing good correlation with TTE and CT measurements45. Especially in young patients with suspected genetic aortic disease, MRI is a good modality for aortic imaging46. However, MRI is inferior to CT in terms of accurate multiplanar reconstruction and is less well established in the preoperative or preinterventional assessment of the ascending aorta47. Especially in patients with Marfan syndrome or a related disorder, even small differences in aortic diameters may have significant implications for clinical management48. Unlike the above-quoted earlier studies, we did not only perform correlation analyses because a high correlation coefficient alone does not automatically mean that there is good agreement between two methods49. Instead, by additionally using Bland–Altman plots, we here for the first time show true measurement differences between CT and TTE in patients with Marfan syndrome or a related disorder, who require particularly accurate monitoring.
As the ESC guideline for the management of valvular heart disease states that aortic repair should be considered in patients with Marfan syndrome and risk factors (family history of dissection, size increase of > 3 mm/year in serial examinations), we think that, to ensure robust aortic monitoring, measurement differences at the aortic root should be below 3 mm9. However, reliable measurement of aortic diameter using CT comes with a price: radiation in a typically young patient population. State-of-the-art CT scanners have helped to reduce the effective dose of CT angiographies considerably50. Considering the risk of rupture and dissection in case of undetected aortic enlargement, the benefits of CT outweigh the risks of radiation exposure even in younger patients. In our study, mean DLP was comparatively low for a potentially life-threatening disease51. The high rate of measurement differences between CT and TTE outside the clinically acceptable limits of agreement for initial work-up, follow-up, and detection of progressive disease in serial imaging suggests that the benefit of obtaining more accurate measurements with ECG-triggered CT might justify radiation exposure.
Conclusion
Highly accurate measurement of aortic diameters in patients at risk for fatal aortic rupture and dissection is warranted. CT and TTE measurements show good correlation, but the frequency of measurement differences outside the acceptable clinical limit of agreement is high, and TTE systematically overestimates aortic diameters. Therefore, monitoring of patients with Marfan syndrome and related disorders may preferably be done using ECG-triggered CT of the aorta for initial work-up, follow-up, and detection of aortic diameter changes over time to ensure reliable measurement for detection of aortic enlargement and identification of progressive disease. Nevertheless, TTE remains an indispensable imaging tool that provides additional information not available with CT.
Limitations
Our study is limited by the use of a retrospective dataset. Analysis of follow-up aortic measurement was performed in a relatively small subgroup because only a few patients undergo repeated CT imaging before surgery. As this study only included patients who did not yet have aortic repair, there might have been a selection bias toward patients with less severe disease. Finally, even though CT is widely considered the “gold standard” in clinical routine, even CT-based measurements are only approximations of true aortic diameters.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available because they include confidential data pertaining to human subjects but are available from the corresponding author on reasonable request.
Abbreviations
- ACC:
-
American College of Cardiology
- acLOA:
-
Acceptable clinical limit of agreement
- AHA:
-
American Heart Association
- CT:
-
Computed tomography
- Col3A1 :
-
Gene encoding for type III collagen
- ECG:
-
Electrocardiography
- EDS:
-
Ehlers-Danlos syndrome
- FBN1 :
-
Gene encoding for fibrillin-1
- LOA:
-
Limits of agreement
- MRI:
-
Magnetic resonance imaging
- SMAD2/3 :
-
Gene encoding for mothers against decapentaplegic homolog 2/3
- TTE:
-
Transthoracic echocardiography
- TGFB2/3 :
-
Gene encoding for transforming growth factor beta 2/3
- TGFBR1/2 :
-
Gene encoding for transforming growth factor beta receptor I/II
References
Judge, D. P. & Dietz, H. C. Marfan’s syndrome. Lancet (London, England) 366, 1965–1976. https://doi.org/10.1016/s0140-6736(05)67789-6 (2005).
Cook, J. R., Carta, L., Galatioto, J. & Ramirez, F. Cardiovascular manifestations in Marfan syndrome and related diseases; multiple genes causing similar phenotypes. Clin. Genet. 87, 11–20. https://doi.org/10.1111/cge.12436 (2015).
Akhurst, R. J. TGF beta signaling in health and disease. Nat. Genet. 36, 790–792. https://doi.org/10.1038/ng0804-790 (2004).
Loeys, B. L. et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 47, 476–485. https://doi.org/10.1136/jmg.2009.072785 (2010).
Nienaber, C. A. & Von Kodolitsch, Y. Therapeutic management of patients with Marfan syndrome: Focus on cardiovascular involvement. Cardiol. Rev. 7, 332–341. https://doi.org/10.1097/00045415-199911000-00011 (1999).
Pyeritz, R. E. Marfan syndrome: Improved clinical history results in expanded natural history. Genet. Med. 21, 1683–1690. https://doi.org/10.1038/s41436-018-0399-4 (2019).
Baumgartner, H. et al. ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehaa554 (2020).
Hiratzka, L. F. et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 121, e266-369. https://doi.org/10.1161/CIR.0b013e3181d4739e (2010).
Baumgartner, H. et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 38, 2739–2791. https://doi.org/10.1093/eurheartj/ehx391 (2017).
Finkbohner, R., Johnston, D., Crawford, E. S., Coselli, J. & Milewicz, D. M. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation 91, 728–733. https://doi.org/10.1161/01.cir.91.3.728 (1995).
Mokashi, S. A. & Svensson, L. G. Guidelines for the management of thoracic aortic disease in 2017. Gen. Thorac. Cardiovasc. Surg. 67, 59–65. https://doi.org/10.1007/s11748-017-0831-8 (2019).
Keane, M. G. & Pyeritz, R. E. Medical management of Marfan syndrome. Circulation 117, 2802–2813. https://doi.org/10.1161/circulationaha.107.693523 (2008).
Asch, F. M. et al. The need for standardized methods for measuring the Aorta: Multimodality core lab experience from the GenTAC registry. JACC Cardiovasc. Imaging 9, 219–226. https://doi.org/10.1016/j.jcmg.2015.06.023 (2016).
Leong, K. et al. Aortic root measurement on CT: Linear dimensions, aortic root area and comparison with echocardiography. A retrospective cross sectional study. Br. J. Radiol. 94, 20201232. https://doi.org/10.1259/bjr.20201232 (2021).
Harowicz, M. R., Shah, A. & Zimmerman, S. L. Preoperative planning for structural heart disease. Radiol. Clin. North Am. 58, 733–751. https://doi.org/10.1016/j.rcl.2020.02.005 (2020).
Ng, A. C. et al. Comparison of aortic root dimensions and geometries before and after transcatheter aortic valve implantation by 2- and 3-dimensional transesophageal echocardiography and multislice computed tomography. Circ. Cardiovasc. Imaging 3, 94–102. https://doi.org/10.1161/circimaging.109.885152 (2010).
Isselbacher, E. M., Lino Cardenas, C. L. & Lindsay, M. E. Hereditary influence in thoracic aortic aneurysm and dissection. Circulation 133, 2516–2528. https://doi.org/10.1161/circulationaha.116.009762 (2016).
Milewicz, D. M., Dietz, H. C. & Miller, D. C. Treatment of aortic disease in patients with Marfan syndrome. Circulation 111, e150-157. https://doi.org/10.1161/01.Cir.0000155243.70456.F4 (2005).
Park, J. Y. et al. Transthoracic echocardiography versus computed tomography for ascending aortic measurements in patients with bicuspid aortic valve. J. Am. Soc. Echocardiogr. 30, 625–635. https://doi.org/10.1016/j.echo.2017.03.006 (2017).
Beetz, N. L. et al. Artificial intelligence-based analysis of body composition in Marfan: Skeletal muscle density and psoas muscle index predict aortic enlargement. J. Cachexia Sarcopenia Muscle https://doi.org/10.1002/jcsm.12731 (2021).
Saura, D. et al. Two-dimensional transthoracic echocardiographic normal reference ranges for proximal aorta dimensions: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 18, 167–179. https://doi.org/10.1093/ehjci/jew053 (2017).
Brown, O. R. et al. Aortic root dilatation and mitral valve prolapse in Marfan’s syndrome: An ECHOCARDIOgraphic study. Circulation 52, 651–657. https://doi.org/10.1161/01.cir.52.4.651 (1975).
Magid, D., Pyeritz, R. E. & Fishman, E. K. Musculoskeletal manifestations of the Marfan syndrome: Radiologic features. AJR Am. J. Roentgenol. 155, 99–104. https://doi.org/10.2214/ajr.155.1.2112876 (1990).
Shnayien, S. et al. Radiation dose reduction in preprocedural CT imaging for TAVI/TAVR using a novel 3-phase protocol: A single institution’s experience. Rofo 192, 1174–1182. https://doi.org/10.1055/a-1150-7646 (2020).
Bittner, D. O. et al. Contrast volume reduction using third generation dual source computed tomography for the evaluation of patients prior to transcatheter aortic valve implantation. Eur. Radiol. 26, 4497–4504. https://doi.org/10.1007/s00330-016-4320-8 (2016).
Cha, M. J. et al. Hypersensitivity reactions to iodinated contrast media: A multicenter study of 196 081 patients. Radiology 293, 117–124. https://doi.org/10.1148/radiol.2019190485 (2019).
Desai, M. Y. et al. Adult patients with marfan syndrome and ascending aortic surgery. J. Am. Coll. Cardiol. 73, 733–734. https://doi.org/10.1016/j.jacc.2018.12.010 (2019).
Conway, A. M. et al. Endovascular abdominal aortic aneurysm repair in patients with Marfan syndrome. Vascular 28, 48–52. https://doi.org/10.1177/1708538119858045 (2020).
Gott, V. L. et al. The Marfan syndrome and the cardiovascular surgeon. Eur. J. Cardiothorac. Surg. 10, 149–158. https://doi.org/10.1016/s1010-7940(96)80289-2 (1996).
Jondeau, G. et al. Aortic event rate in the Marfan population: A cohort study. Circulation 125, 226–232. https://doi.org/10.1161/circulationaha.111.054676 (2012).
Erbel, R. et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2873–2926. https://doi.org/10.1093/eurheartj/ehu281 (2014).
Davies, R. R. et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann. Thorac. Surg. 73, 17–27; discussion 27–18, https://doi.org/10.1016/s0003-4975(01)03236-2 (2002).
Zafar, M. A. et al. Natural history of descending thoracic and thoracoabdominal aortic aneurysms. J. Thorac. Cardiovasc. Surg. 161, 498-511.e491. https://doi.org/10.1016/j.jtcvs.2019.10.125 (2021).
Blanke, P., Schoepf, U. J. & Leipsic, J. A. CT in transcatheter aortic valve replacement. Radiology 269, 650–669. https://doi.org/10.1148/radiol.13120696 (2013).
Binder, R. K. et al. The impact of integration of a multidetector computed tomography annulus area sizing algorithm on outcomes of transcatheter aortic valve replacement: A prospective, multicenter, controlled trial. J. Am. Coll. Cardiol. 62, 431–438. https://doi.org/10.1016/j.jacc.2013.04.036 (2013).
Flachskampf, F. A. & Daniel, W. G. Aortic dissection. Cardiol. Clin. 18(807–817), ix. https://doi.org/10.1016/s0733-8651(05)70181-8 (2000).
Gohmann, R. F. et al. Combined coronary CT-angiography and TAVI-planning: A contrast-neutral routine approach for ruling-out significant coronary artery disease. J. Clin. Med. 9, 8. https://doi.org/10.3390/jcm9061623 (2020).
Knobloch, G. et al. Inter- and intra-observer repeatability of aortic annulus measurements on screening CT for transcatheter aortic valve replacement (TAVR): Implications for appropriate device sizing. Eur. J. Radiol. 105, 209–215. https://doi.org/10.1016/j.ejrad.2018.06.003 (2018).
Mariucci, E. et al. Coronary artery aneurysms in patients with marfan syndrome: Frequent, progressive, and relevant. Can. J. Cardiol. 37, 1225–1231. https://doi.org/10.1016/j.cjca.2021.03.002 (2021).
Bons, L. R. et al. Intermodality variation of aortic dimensions: How, where and when to measure the ascending aorta. Int. J. Cardiol. 276, 230–235. https://doi.org/10.1016/j.ijcard.2018.08.067 (2019).
Ocak, I. et al. The aortic root: Comparison of measurements from ECG-gated CT angiography with transthoracic echocardiography. J. Thorac. Imaging 24, 223–226. https://doi.org/10.1097/RTI.0b013e31819f9369 (2009).
Frazao, C. et al. Multimodality assessment of thoracic aortic dimensions: Comparison of computed tomography angiography, magnetic resonance imaging, and echocardiography measurements. J. Thorac. Imaging 35, 399–406. https://doi.org/10.1097/rti.0000000000000514 (2020).
Amsallem, M. et al. Comparative assessment of ascending aortic aneurysms in Marfan patients using ECG-gated computerized tomographic angiography versus trans-thoracic echocardiography. Int. J. Cardiol. 184, 22–27. https://doi.org/10.1016/j.ijcard.2015.01.086 (2015).
Rodríguez-Palomares, J. F. et al. Multimodality assessment of ascending aortic diameters: Comparison of different measurement methods. J. Am. Soc. Echocardiogr. 29, 819-826.e814. https://doi.org/10.1016/j.echo.2016.04.006 (2016).
Koos, R. et al. Evaluation of aortic root for definition of prosthesis size by magnetic resonance imaging and cardiac computed tomography: Implications for transcatheter aortic valve implantation. Int. J. Cardiol. 158, 353–358. https://doi.org/10.1016/j.ijcard.2011.01.044 (2012).
Oddone, M., Granata, C., Vercellino, N., Bava, E. & Tomà, P. Multi-modality evaluation of the abnormalities of the aortic arches in children: Techniques and imaging spectrum with emphasis on MRI. Pediatr. Radiol. 35, 947–960. https://doi.org/10.1007/s00247-005-1436-4 (2005).
Freeman, L. A. et al. CT and MRI assessment of the aortic root and ascending aorta. AJR Am. J. Roentgenol. 200, W581-592. https://doi.org/10.2214/ajr.12.9531 (2013).
Milewicz, D. M. et al. Marfan syndrome. Nat. Rev. Dis. Prim. 7, 64. https://doi.org/10.1038/s41572-021-00298-7 (2021).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1, 307–310 (1986).
Den Harder, A. M. et al. Dose reduction with iterative reconstruction for coronary CT angiography: A systematic review and meta-analysis. Br. J. Radiol. 89, 20150068. https://doi.org/10.1259/bjr.20150068 (2016).
Halliburton, S. S. et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J. Cardiovasc. Comput. Tomogr. 5, 198–224. https://doi.org/10.1016/j.jcct.2011.06.001 (2011).
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité—Universitätsmedizin Berlin. Nick Lasse Beetz is participant in the BIH Charité Clinician Scientist Program funded by Charité – Universitätsmedizin Berlin and the Berlin Institute of Health at Charité (BIH).
Author information
Authors and Affiliations
Contributions
N.L.B. conceptualized this study, wrote the manuscript and prepared all tables and figures. K.P., C.M., and T.W. supported data curation. Supervision was led by S.S. and P.G. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beetz, N.L., Trippel, T.D., Philipp, K. et al. Discrepancy of echocardiography and computed tomography in initial assessment and 2-year follow-up for monitoring Marfan syndrome and related disorders. Sci Rep 12, 15333 (2022). https://doi.org/10.1038/s41598-022-19662-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19662-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.