Abstract
Selecting a suitable catalyst for implementing the simultaneous production of hydrogen-rich syngas and multi-walled carbon nanotubes through the integration of dry reforming and methane decomposition reactions has recently gained great interests. In this study, a series of bimetallic (NiMo/MgO) and trimetallic (CoNiMo/MgO, FeNiMo/MgO, CoFeMo/MgO) catalysts was prepared and evaluated for a catalytic activity of CH4 and CO2 conversions of biogas in a fixed bed reactor at 800 °C and atmospheric pressure. Among the investigated catalysts, the bimetallic NiMo/MgO catalyst showed the outstanding catalytic performance with 86.4% CH4 conversion and 95.6% CO2 conversion as well as producing the highest syngas purity of 90.0% with H2/CO ratio = 1.1. Moreover, the characterization of the synthesized solid products proved that the well-aligned structured morphology, high purity, and excellent textural properties of CNTs were obtained by using NiMo/MgO catalyst. On the other hand, using trimetallic catalysts which have the composition of Co and Fe leads to the severe deactivation. This could be attributed the catalyst oxidation with CO2 in biogas, resulting in the transformation of metals into large metal oxides. The integrative process with NiMo/MgO catalyst is regarded as a promising pathway, which has a high potential for directly converting biogas into the high value-added products and providing a green approach for managing the enormous amounts of wastes.
Similar content being viewed by others
Introduction
Nowadays, the emissions of carbon dioxide (CO2), methane (CH4), and other greenhouse gases have all increased in the earth’s atmosphere more than at any time in the last 800,000 years. This is because of the dramatic growth of the world population and industrialization, resulting in huge energy consumption1. Fossil fuel combustion is found to be one of the greatest emitters of carbon dioxide which is a primary contribution of greenhouse gases that contributed to global warming, leading to unpredictable and negative changes in the environment2. These changes seem to become even crueler as time passes. Accordingly, to overcome these problems, renewable energy sources have drawn great interest in the research and development since they promise to reduce the environmental pollution while also increase the domestic energy supplies3.
Unlike other forms of renewable energy sources, biogas offers a very attractive route to utilize certain categories of biomass for meeting partial energy needs in both rural and industrial areas4. Biogas is a mixture primarily comprised of two components (e.g., CH4 and CO2) and is typically useful in a variety of ways including as vehicle fuel and to generate heat, electricity, combined heat, and power5. Another interesting option for utilizing biogas is to convert it directly into syngas (H2 and CO), which can be produced in different ways such as steam or partial oxidation reforming of methane (SRM or POM). Dry reforming of methane (DRM) as presented in Eq. (1), has been regarded as a favorable process to produce syngas from biogas because of its environmental and economic advantages6,7. It can provide a mixture of syngas with an H2/CO ratio close to unity which can be further beneficially upgraded to produce high-value-added products and is crucial for mitigating the greenhouse gas emissions6. Nevertheless, the coking issue is the main challenge for DRM because it can cause catalyst deactivation. Thus, a number of studies have been discussed on how to prevent carbon formation8,9,10. In this work, we proposed an approach to overcome this momentous concern by engineering the deposited carbon into carbon nanomaterials such as carbon nanotubes (CNTs) that can be also produced through catalytic decomposition of methane (CDM) as shown in Eq. (2). Since CNTs have raised a broad interest in the fields of science and technology. Because of their extraordinary physical and chemical properties, they can be used for a wide range of applications including energy storage11, electronic12, biomedical13, and environmental7.
In comparison with the noble metals, such as Rh, Ru, Pt, Pd, and Ir, from a practical point of view, the supported transition metals, especially Ni catalysts have been extensively applied as a promising alternative for large-scale use because of their comparable catalytic activity and relatively low cost14,15,16. Figueredo et al.17 reported that Ni catalyst supported on perovskite-type LaAlO3 could reach the maximum values of CH4 and CO2 conversions of 94.0 and 85.1% at 700 °C, respectively. Moreover, methane conversion can be significantly improved with the increased Ni content and the reaction temperature, as reported in the literatures17,18,19. Co and Fe catalysts have also been studied20,21,22. It has been observed that the performance of Co supported catalysts exhibited very similar to that of Ni-based catalysts23,24. Meanwhile, Fe-based catalysts showed a lower activity than those catalysts containing Ni or Co, but they have received more attention than Co catalysts due to their economical and environmentally friendly advantages. Besides, they can provide the interesting characteristics and can be used at elevated temperatures25,26.
Even though they showed the superior performance, the activity of the catalyst has dropped rapidly due to the carbon-deposition-induced deactivation and subsequent sintering from the agglomeration of the particles at high reaction temperatures, which are still considered to be the main challenge for the DRM procedure16. Therefore, to increase the durability of the catalyst, most research studies have concentrated on the inclusion of a second metal to form bimetallic catalysts. Kutteri et al.24 adopted Ni, Co, and Fe to examine the performance of mono and bimetallic (Ni–Co, Ni–Fe, and Co–Fe) catalysts over SiO2 support for methane decomposition. The results revealed that all bimetallic catalysts have been found to be more active and stable than the single metal catalysts ones. This may be attributed to the formation of bimetallic alloys during the reaction as reported by Pudukudy et al.27. They have successfully used the set of novel Ni, Co, and Fe based bimetallic catalysts supported over SBA-15 to improve the activity and stability. Other metals, such as Mo, Sn, Cu, and Ce can be also used as a promoter in combination with active metals to further enhance the catalytic properties of the catalysts23,28. These authors29,30 studied the synergistic effect of the mixture between Co and Mo. They claimed that only Co alone did not catalyze the growth of SWCNTs. By contrast, alloying Mo in Co-based catalyst with an appropriate proportion can efficiently increase the yield and the selectivity towards the synthesis of high-quality CNTs. Moreover, it has been published that the addition of 5–10% Mo to Co/MgO catalyst raised the carbon yield about 20 times higher than usual and also extended the lifespan of the catalyst for the CNTs growth even after 2 h of synthesis time31. In similar findings, Awadallah et al.31,32 employed the Ni–Mo and Co–Mo/Al2O3 as catalysts with different metal loadings for the decomposition of natural gas into H2 and CNTs production. They found that all catalysts exhibited the excellent activity and durability up to 9 h without deactivation, suggesting that a small amount of Mo addition can keep the catalyst being active for a long time.
In the DRM process, another important component that affects the catalytic performance is catalyst supports, which are beneficial for anti-sintering ability, mechanical strength, and large specific surface area16. Many efforts have been devoted to investigate MgO and Al2O3 as support materials because of their availability, high thermal stability, and low cost33,34,35,36. Takenaka et al.37 determined the activity and lifetime of the Co catalysts supported on different supports (MgO, Al2O3, SiO2, and TiO2), and the results showed that the Co/Al2O3 and Co/MgO were more effective catalyst than those of Co/SiO2 and Co/TiO2 which could be explained by the crystallite size of Co metal particles in Co/Al2O3 and Co/MgO have diameters in the range of 10–30 nm that more easily to form CNFs, while the larger ones (> 30 nm) were inactive for methane decomposition reaction.
Therefore, in this study, we attempted to find a suitable catalyst for implementing the simultaneous production of hydrogen-rich syngas and multi-walled carbon nanotubes through DRM and CDM reactions by preparing the MgO-supported bi-, tri-metallic catalysts (NiMo/MgO, CoNiMo/MgO, FeNiMo/MgO, and CoFeMo/MgO) to combine their individual advantages, and investigated the effect of introducing a third metal on the performance of the catalysts and the properties of the synthesized CNTs.
Experimental
Catalyst preparation
The bimetallic NiMo and the trimetallic CoNiMo, FeNiMo, and CoFeMo/MgO catalysts were prepared by wetness impregnation method using the solution of metal precursors (Sigma Aldrich) such as Ni(NO3)2·6H2O, Co(NO3)2·6H2O, Fe(NO3)3·9H2O, and (NH4)6Mo7O24·4H2O. The metal loading was fixed at 30 wt.%. Typically, the metal precursors with a mass ratio of 1:1 for bimetallic catalyst and 1:1:1 for trimetallic catalyst was dissolved in DI water to get a completely mixed solution. This mixture was then added dropwise to MgO nano-powder (Merk) at room temperature while stirring to form a homogenous slurry, which was then evaporated on a hotplate at 80 °C till obtaining dried catalysts powder. The resulting catalysts powder was further calcined at 500 °C with a ramp rate of 10 °C/min in a muffle furnace for 3 h.
Characterization
The calcined, reduced, and spent catalysts were characterized. The X-ray diffraction was conducted to examine the crystallinity and crystalline phases in calcined, reduced and spent catalysts using X-ray diffractometer (XRD, Bruker, D2 Phaser). The diffraction angle ranged from 2θ = 10° to 80°. The morphology and particle-sized distribution of synthesized CNTs were identified by a Field-emission scanning electron microscope (FE-SEM, JEOL, JSM-7610F) at the operating voltage of 10 kV. The internal structure of nano carbon deposited on the spent catalyst was characterized by a Field-emission transmission electron microscope (HR-TEM, JEOL, JEM-3100, Japan) at the operating voltage of 300 kV. The purity of the synthesized carbon nanotube was determined by thermogravimetric analysis (TGA, Metteler Toledo, TGA/DSC1) with an oxygen gas flow rate of 50 ml/min and a heating rate of 10 °C/min from room temperature to 800 °C. The hydrogen programmed reduction (H2-TPR, BEL JAPAN, BELCAT-B) method was used to assess the reducibility and metal-supported interaction of calcined catalysts. Prior to measurements, 100 mg of calcined catalysts was pretreated at 200 °C for 1 h under a He flowrate of 30 ml/min. Afterward, the H2-TPR profile was obtained using a mixture of 5% H2/Ar flowing at a rate of 30 ml/min with the sample heated from 100 to 900 °C with a fixed heating rate of 10 °C/min. The chemical composition of the reduced catalysts was analyzed by X-ray fluorescence (XRF, S8 TIGER, Series 2). The graphitic and disorder carbon of synthesized CNTs was examined by Raman spectrophotometer (PerkinElmerÒ Spectrum™ GX). The textural properties of spent catalysts were identified by the N2 sorption measurement (Micromeritics, TriStar II 3020).
Production process
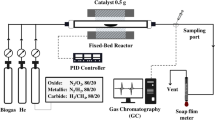
The evaluation of the catalytic activity was performed under atmospheric pressure. The 0.5 g catalyst held on a quartz boat (15 mm width, 100 mm length, 8 mm height) was inserted at the center of a fixed-bed horizontal quartz reactor (26 mm I.D. and 1100 mm length) with PID temperature controller (KP1000 series, Chino) and Type-K thermocouple inserted at the center of the reactor. The experimental apparatus is schematically shown in Fig. 1. Prior to the reaction, the catalyst was reduced by H2 (75 ml/min) with a ramp rate of 10 °C/min until the temperature reached 1000 °C. Once the reactor temperature reached 1000 °C, biogas with a composition of 60% CH4 and 40% CO2 was continually fed into the reactor at the flow rate of 400 ml/min, corresponding to GHSV = 48,000 ml/g-h. During the reaction, the gas composition of the outlet stream was examined every 10 min using Gas chromatograph (TCD, Shimadzu, GC-2014). The volumetric flow rate of the inlet and outlet streams was controlled and calibrated by a mass flow controller (HORRIBA METRON, S48-32HMMT) and a soap bubble meter, respectively. After 3 h of the reaction time, the biogas was switched to the He flow at 50 ml/min to cool down the reactor to room temperature. The spent catalyst with deposited carbon was then weighed and collected in a desiccator for further characterization.
Product analysis
The catalytic performance of the bimetallic and trimetallic catalysts in the integration of dry reforming.
For the gas analysis, the effluent gas of the outlet stream, H2 purity, and H2/CO ratio were calculated using Eqs. (5, 6), where Fi is the flow rate of component i, [i] represents the concentration of CH4, CO2, H2 or CO. Fin and Fout are the total gas volumetric flow rate of inlet and outlet from the reactor, in term of (ml/min). The production yield, purity of synthesized CNTs and gas hourly space velocity were calculated according to Eqs. (7–10).
whereas Fin is volumetric flow rate of reactant gas, mcatalyst is a mass of reduced catalyst, mproduct is a mass of solid product including catalyst and CNTs, mcarbon,in is a mass of methane from inlet stream.
Results and discussion
Characterization of catalyst
All catalysts were prepared by wetness impregnation method. The total loading of metals (Ni, Co, Fe, and/or Mo) on MgO was fixed at 30 wt.%. The equal mass ratio of metals was used. Each catalyst was reduced by H2 (75 ml/min) before the characterization. The actual elemental composition of the reduced catalyst was analyzed by X-ray diffractometer (XRF) as shown in Table 1. The total metal loading of bimetallic catalyst was observed to be 31.6 wt.%. Likewise, the total metal loading of trimetallic catalyst is in the range of 30.0–30.8 wt.%. All catalyst results have a total metal loading that is quite close to the prescribed values. Furthermore, a trace of Ca impurity in all of the metal catalysts was detected with 0.2 wt.%.
X-ray diffraction (XRD) measurement was principally carried out to identify the crystalline phases arising during the process. The results of the contemporary catalysts analyzed by XRD after calcination at 500 °C for 3 h are depicted in Fig. 2a. For all samples, many diffraction peaks that mainly ascribed to magnesium oxide support (JCPDS 45-0946) were observed at 2θ = 36.96°, 42.92°, 62.29°, 74.59°, and 78.58°, which is perfectly in accordance with (111), (200), (220), (311), and (222) planes. However, the crystal phase of NiO (JCPDS 47-1049) was identified only in the pattern of NiMo/MgO catalyst, showing a higher number of particles consisting of Ni species on the surface of the support. Moreover, the Co3O4 phases (JCPDS 42-1467) are almost absent as identified in the XRD results profile of CoNiMo and CoFeMo/MgO catalysts. The possible reason is that it can be well dispersed in the support and/or too small to be detected. The appearance of MoO3 species (JCPDS 05-0508) in these catalysts are known to form due to the oxidation of Mo at temperatures above 500 °C37. That can be attributed to a high composition of molybdenum oxide phases on the catalysts surface. The reflection peaks of MgMoO4 (JCPDS 72-2153) are also detected with very low or less intensity in the diffraction pattern of all catalysts, which formed via interaction between MgO and MoO3 at a temperature higher than 400 °C38.
Figure 2b illustrates XRD diffractograms of fresh catalysts obtained after carrying out the activation process by reducing under certain conditions, along with the structural parameters analyzed by the XRD technique are presented in Table 2. It was noticed that the characteristic peak of MgO phases appears extensively in all catalysts. Also, it can be clearly seen that the MgO crystal domain size significantly diminished in all catalysts compared to the reduced MgO. In addition, the reflections attributed to metallic forms such as Ni (JCPDS 04-0850), Co (ICPDS 15-0806), Fe (JCPDS 65-4899), and Mo (ICSD 64-3962) were detected. In comparison, the crystallinity of metal Mo peaks for using bi-metallic NiMo/MgO catalyst presents a higher than other tri-metallic catalysts, revealing a high percentage of Mo in the catalyst. Meanwhile, no other diffraction patterns reflexed to their oxides were observed, expecting that metallic phases can be completely reduced into the activated catalyst by the usage of hydrogen reducibility at a high temperature.
The reducibility of all the fresh calcined catalysts used in this study was determined by the H2-TPR technique and the results are shown in Fig. 3. For the bimetallic NiMo/MgO catalyst, the TPR profile illustrates two hydrogen consumption regions: in the first low-temperature region, between 300 and 600 °C, a broad peak with a slightly low intensity observed at 399 °C is assigned to the reduction of loosely attached NiO bound on the support surface, and the second, shoulder peak centered at 523 °C could be attributed to the reduction of NiO species strongly interacted with the support37,39. At the higher temperature region between ranges of 600–1000 °C, the profile shows the presence of two distinct peaks that appear centered at 630 and 720 °C, indicating two differentiated reduction processes. According to previous studies40,41,42, the former represents the reduction of octahedral Mo-oxo species (Mo6+ → Mo4+) together with the first reduction step of MgMoO4, and the following peak consumed in the high reduction temperature is associated with the second reduction step of MgMoO4 as well as the further reduction from tetrahedral Mo6+ to metallic Mo0 passing through the Mo4+ phase which has been reported that is more difficult to reduce than that of octahedral ones.
On the other hand, the H2 consumption peaks of the trimetallic with Co-containing catalysts appeared centered at temperatures of 372 °C are related to the reduction from Co3O4 to metallic Co0 phase. It is reported that if the size of these peaks is small, the relative amount of these crystals is very low. Thus, it can be said that the MgO surface held a small proportion of Co3O443,44. Also, the peaks at a temperature range of 400–500 °C were detected in the catalysts composed of Fe content, which reflected the formation of metallic iron from the two-step reduction of magnetite as a sequence of Fe3O4 to FeO and Fe21,41,45. In addition, the TPR profiles of all trimetallic catalysts display a single broad peak with a maximum at around 650 °C which is deconvoluted into mainly two peaks corresponding to the reduction of MgMoO4 and Mo species as mentioned before. These peaks begin with temperatures higher than 500 °C and seem to continue even up to 900 °C, especially in the profiles of FeNiMo/MgO and CoFeMo/MgO catalysts. The broad tail at high temperature is probably due to the result of a larger amount of Fe oxides were not completely reduced to Fe0, indicating a strong interaction between iron species and the support46. This suggests that both catalysts needed temperatures higher than 900 °C for the full reduction into the metallic form.
Evaluation of catalytic performance and durability
The performance of bimetallic and trimetallic catalysts was evaluated under the integration of dry reforming catalytic decomposition of methane. All catalytic has been carried out in a fixed bed reactor at 800 °C, 1 atm under biogas (CH4:CO2 = 1.5:1) feeding, with a corresponding space velocity of GHSV = 48,000 ml/gCat-h. The feed gas conversion and the amount of H2 and CO product were calculated based on the measurement of the effluent gas composition of the outlet stream during the reaction for 3 h. In this process, the decomposition of CH4 on the catalyst surface leads to the production of H2 and carbon nanotubes (CNTs)47,48. Meanwhile, CO2 interacts with CH4 through a dry reforming reaction, which is accompanied by a parallel reaction that produces syngas (H2 + CO)49. As a result, the outlet stream effluent gas contains H2, CO, and a small amount of unconverted CO2 and CH4. Figure 4a,b shows the CH4 and CO2 conversion as a function of time on stream. It is clearly observed that the coexistence of Ni and Mo reveals exceptional performance with achieving higher CH4 conversion of 86.4% and CO2 conversion of 95.6% in NiMo catalyst as well as showing excellent stability over 3 h. Besides, H2-rich syngas was produced over NiMo/MgO catalyst having H2/CO ratio of 1.1. In addition, the syngas purity of 90.0% was obtained as listed in Table 3. Meanwhile, the addition of Co and Fe in the catalyst is found to be that the conversion of CH4 and CO2 decreases gradually. This occurrence may imply that the catalyst surface of Co and Fe would have undergone oxidation while exposure to CO2 in biogas led to the catalyst deactivation26,50,51,52.
Table 4 compares the performance of NiMo/MgO catalyst, with respect to Ni-based catalysts from the previous reports for converting biogas in various circumstances. In the case of the CH4/CO2 ratio equal to 1.5/1, NiMo/MgO catalyst in the present study outperforms the competition in CH4 and CO2 conversions. Our catalyst possesses outstanding performance for syngas production having the H2/CO ratio > 1, a resource for producing high-value products such as methanol and liquid hydrocarbons by Fischer–Tropsch synthesis53,54. Furthermore, NiMo/MgO catalyst can perform superior for the formation of valuable carbon nanomaterials without encountering the deactivation of the catalyst during the tested time. These findings assure that our catalyst is regarded as a promising catalyst for biogas transformation into syngas and carbon nanomaterials through the integrative process of DRM and CDM. However, as indicated in Table 4, the concentrations of CH4 and CO2 in biogas are another important factor in the integration process of DRM and CDM, which influences the biogas conversion and the syngas production.
Characterization of CNTs products
CNTs generated from the decomposition of CH4 were examined for yield, purity, crystallinity, graphitization, morphology, and textural properties. Table 3 presents the production yield of CNTs in terms of gProduct/gCat-h was synthesized over bimetallic (NiMo/MgO) and trimetallic (CoNiMo/MgO, FeNiMo/MgO, CoFeMo/MgO). The bimetallic NiMo/MgO catalyst exhibited high performance for the CNTs production of 2.60 gProduct/gCat-h than all trimetallic catalysts. The production yield of CNTs obviously that the cohabitation of Ni and Mo particles drastically encouraged the creation of CNTs through CDM, Eq. (2)60 as compared to that obtained without Ni catalyst60 as compared to that obtained without Ni catalyst. On the contrary, the addition of Co and Fe to the catalyst induced a significant abatement yield of synthesized carbon nanotubes. This could be owing to the metals Co and Fe in biogas being easily oxidized by CO2 and forming metal oxides26,50,51,52.
Figure 5 provides more insight into the morphological differences of the deposited carbon over various catalysts used, as well as the analyzing parameters of synthesized CNTs which were calculated from FE-SEM and HR-TEM images are presented in Table 5. From the SEM images, it was clearly observed that the presence of condensed nanocarbon was observed on the catalyst surface, especially on catalysts that contain Ni metal particles while a small amount of CNTs detected on the surface of the catalyst with the absence of Ni (Fig. 5d). This indicates the high activity and capability of producing CNTs by using Ni-based catalysts. In addition, TEM images as shown in Fig. 5, confirm the formation of multi-walled carbon nanotubes (MWCNTs) on all catalysts used in this study. Also, it is obvious that the addition of Fe in the catalyst forming the bamboo type in carbon nanostructures21,25 which may be useful in some practical applications, such as hydrogen storage, electrochemical capacitors, and lithium-ion batteries61.
From Fig. 5 and Table 5, it was found that the narrowest and smallest carbon nanotubes can be achieved over NiMo catalyst without any further combination with other transition metals. Meanwhile, the addition of Fe and Co apparently gives the higher range of the wall numbers and diameters of CNTs. This might be attributed to the agglomeration of oxide species (Fe3O4 and Co3O4) on the catalyst surface which leads to an increase in metal particle size, consequently, large CNTs are produced44,62.
The graphitization of all synthesized CNTs produced onto bimetallic and trimetallic catalysts at 800 °C was evaluated by Raman spectroscopy. All of these spectra show three distinct peaks, including D-band, G-band, and G′-band. The D-band at around 1300–1400 cm−1 is associated with the structural defect and impurity which represents the disordered carbon or amorphous carbon deposited on the outer surface of carbon nanotubes63. The G-band at around 1500–1600 cm-1 is related to the tangential stretching mode of all pairs of sp2 atoms in both rings and chains which represents the graphitic carbon structure61, while G’-band observed at around 2600–2700 cm−1 is associated with the process of two-photon elastic scattering64. The appearance of both G-band and G’-band can be used as an indicator for assuring the formation of graphite nanotube. This data is in accordance with the morphology observation in Fig. 5. The relation between D-band and G-band in the aspects of intensity ratio Ig/Id is used to evaluate the quality of MWCNTs on the used catalyst, which the low value of the Ig/Id ratio (> 1) indicates a good degree of graphitization. The results from Raman analyze show that all synthesized CNTs (Ig/Id = 1.14–1.60) presented the Ig/Id ratio higher than commercial CNTs (Ig/Id = 0.74). As seen in Fig. 6, the synthesized CNTs over the FeNiMo catalyst had the highest graphitization carbon structure (Ig/Id = 1.60) due to Fe metal having a high carbon solubility47. Therefore, the addition of Fe into NiMo/MgO catalysts favors the formation of CNTs with a high graphitization and a small amount of defect on the wall surface, which corresponds to the TEM analysis shown in Fig. 5. On considering CoFeMo/MgO catalyst, the Raman shift appears at 260, 376, and 440 cm−1 reflecting the presence of MoO3. This peak shoulder could be confirmed the oxidation of Mo with CO2 on the surface catalyst, which may be converting Mo into MoO326.
Figure 7 displays XRD diffraction data for the CNTs generated after conducting in the isothermal test at 800 °C for 3 h. All the pattern results, besides the CoFeMo/MgO catalyst, the intensity of the 002 plane of the graphitic peaks at 2\(\uptheta\) = 26.1° are more prominent compared to the relevant peaks that are assigned to metallic and other phases. This peak is known to quantify the crystallinity of carbon materials. However, in our study, there seems to be more associated with the formation of CNTs, where the sharp diffraction peak shows the high activity of the catalysts for achieving the carbon products, which confirms the higher yield of the carbon nanofilaments grown over these catalysts. The interlayer distance (d002) or d-spacing value derived from the XRD data can be used to the structural degree of synthesized CNTs as well, which was calculated by applying the Bragg’s equation (\(d=nl/2\mathrm{sin}\theta\))65,66 and the results are presented in Table 6. It was found that the used catalysts have produced the CNTs with the interplanar spacing value in the range between 0.344 and 0.348 nm, which is very nearly the value obtained from commercial CNTs (0.343 nm). As shown in the table, the crystallite size of Ni, Fe, and Co particles decreased in all samples compared to the reduced catalysts except for the CoFeMo/MgO catalyst (Figs. 3b, 7). This could be implied that the portion of Ni in the catalyst makes the metal particles to be small, resulting in the increase of the active surface area thus improving the dispersion and catalytic activity of active nanoparticles.
After the reaction, The Mo2C peaks slightly appeared for all cases, which have been known to be generated during the formation of CNTs via the carbide cycle mechanism28. In addition, the presence of MoO3 in the consumed CoFeMo/MgO catalyst can be ascribed to the oxidation of Co as mentioned above. It might be corresponded to the conversion of Mo into their oxides26. The several reflection peaks of Co3O4 and Fe3O4 are detected in the XRD pattern of the Co- and Fe-containing catalysts. This certifies that Co and Fe metallic phases could be oxidized with CO2 to form metal oxides. Besides, some spot of Fe2O3, and Fe3C peaks were also identified with very low intensities especially on the surface of the CoFeMo/MgO catalyst. This suggested that some Fe particles were possibly converted into Fe2O3, and Fe3C through the oxidation of Fe with carbon dioxide (CO2 + Fe → Fe2O3 + Fe3C). In order to better understand what exactly happens, another two approaches are performed, and latter being thoroughly discussed in “The influence of CO2 oxidation” section. Moreover, the bigger diameter of CNTs generated with the CoNiMo/MgO, FeNiMo/MgO, and CoFeMo/MgO catalysts can be regarded as the huge proportion of their metal oxide forms hold on the surface of catalysts, which are responsible for the growing of large diameter CNTs. Since the crystal size of oxide species is normally higher than its usual metallic form as obviously seen in Table 6 and TEM images in Fig. 7.
Thermal gravimetric analysis (TGA) was used to determine the quality and the purity of CNTs formed over these catalysts, and the results are depicted in Fig. 8. The weight loss is ascribed to the burning of deposited carbon in oxygen and then corresponds to the yield of solid carbon in the catalysts67. Thus, as can be seen in Fig. 8a, the largest weight loss of about 84% was obtained by using the NiMo/MgO catalyst, indicating the presence of high carbon content in the sample, and hence high purity CNTs was observed. The impurities in the CNTs sample were possibly attributed to the residual catalysts that remained after the reaction and other species including oxygen from CO2 which would possibly be contained in the samples. In general, the classic chemical technique for separating CNTs from other entities such as residual catalysts has been done by treating the CNTs sample with acid leaching68. Moreover, the purity of CNTs can be further increased by extending the reaction time as well, as reported in our previous work26. The TGA profiles of the carbonaceous materials deposited on all catalysts show similar oxidation behaviors with single-step degradation, regarding the absence of amorphous carbon which is closely related with their textural and structural properties. Additionally, a thorough analysis of all the results derived from TGA data illustrated a high inflection at temperatures up to 550 °C, reflecting that thermal stability and structural degree of CNTs had improved67. Particularly again in the case of the NiMo catalyst, the CNTs was produced with remarkably outstanding thermal stability better than the commercial as demonstrated in Fig. 8b. However, the purity of synthesized CNTs has lower than the commercial CNTs ones.
The textural properties of CNTs were assessed by N2 sorption measurements, the data are listed in Table 7 and shown in Fig. 9 The highest surface area was obtained by synthesized CNTs over NiMo/MgO catalysts. In contrast, the addition of Co into NiMo/MgO catalysts is reduced the BET surface area of synthesized CNTs owing to the oxidation of Co leading to the deactivation of the catalyst. Additionally, the effect of the addition of Fe into NiMo/MgO catalysts results in a decrease in the BET surface area of synthesized CNTs due to Fe could be readily oxidized in exposure CO2 in Biogas, which could have caused the catalyst deactivation50,51,52. For these reasons, the synthesized CNTs over CoFeMo/MgO exhibit the lowest surface area. According to Fig. 9a, N2 absorption–desorption isotherms of all synthesized CNTs, present type IV isotherms according to IUPAC classification, indicating that the majority of porosity in the CNTs is mesoporous69. These results consistent with the mean pore size of synthesized CNTs is in 10–13 nm which is in the range of mesopore material (2–50 nm), as shown in Table 7. The hysteresis loops formed by the capillary condensation effect can be classified as H3 hysteretic loops, usually found in solids consisting of aggregates or agglomerates of particles forming slit-shaped pores, with a non-uniform size and/or shape70. The pore diameter distributions based on the BJH method as shown in Fig. 9b present the curves, which can be ascribed that all the synthesized CNTs exhibit a bimodal feature that has a pore size with a board distribution ranging from 2 to 150 nm, including a small pore-size fraction (2–5 nm) and a large pore-size fraction (5–150 nm).
The influence of CO2 oxidation
One aspect that the influence of CO2 oxidation was revealed in the former discussion. Herein, an effort is exerted to comprehend how CO2 oxidation influences Fe-based metal catalysts through performing under two different conditions including CH4/He (3/2), CH4/CO2 (3/2), and He/CO2 (3/2) feeding into the reactor. All this condition was operated over FeNiMo/MgO catalyst at 800 °C with the flow rate of 400 ml/min, corresponding to GHSV = 48,000 ml/gCat-h. The reaction time was set to 3 h. Figure 10 shows the effluent gas of the outlet stream for each condition. Considering the CH4 decomposition reaction as seen in Fig. 10a, H2 and CNTs are generally being product according to Eq. (2). Meanwhile, it is noteworthy that the CO concentration is slightly present in the effluent gas of the outlet stream. This observation can be clarified that the existence of iron oxide (hematite, Fe2O3) due to unaccomplished reduction under H2 at 1000 °C with a heating rate of 10 °C/min71 is reduced with CH4 to form CO according to Eq. (11)72. In this regard, CH4 is probably more effective than H2 as a reductant. For the condition under He/CO2, Fe could be readily oxidized with CO2 to form Fe2O3 and Fe3C which could have caused the activity loss according to Eq. (12). Furthermore, the CO composition was found to be slightly concentrated, as illustrated in Fig. 10c. This occurrence regarded as another form of Fe is being oxidized with CO2 to produce CO and Fe3O4 resulting in loss activity according to Eq. (13).
Figure 11a shows the XRD pattern over FeNiMo/MgO catalyst under CH4/CO2, CH4/He, and He/CO2 after the reaction. Some diffraction peaks attributed to Fe2O3, Fe3O4, and Fe3C were found in CH4/CO2 and He/CO2 conditions. In the case of He/CO2, the presence of Fe2O3 and Fe3O4 can be confirmed from the Raman spectra at 200–1400 cm−1, as seen in Fig. 11b. Meanwhile no characteristic peaks devoted to Fe2O3, Fe3O4, and Fe3C species were observed as to CH4/He condition. These findings assure that operating under the existence of Fe on the catalyst suffered from CO2 oxidation resulting in deactivation. The graphitization of CNTs produced by the mean of CH4 decomposition are shown in Fig. 11b. The results show that the synthesized CNTs on CH4/He had a higher graphitization degree of carbon structure (Ig/Id = 1.98) in comparison to that obtained by operating at normal CH4/CO2 condition (Ig/Id = 1.60). This may be explained by the decrease in the proportion of Fe metallic phase due to it can be oxidized into Fe oxides with CO2, corresponding to the lower level of graphitization in CNTs47.
To further observe the catalyst oxidation by CO2 in biogas (CO2 + CH4), the two experiments of sole CO2 and sole CH4 balanced with He were carried out as shown in Fig. 12. Figure 12c reveals that the CO2 causes higher degree of oxidation, resulting in the transformation of metal into larger metal oxide particles. The formation of metal oxide particles regards as another key factor that is correlated with CNTs diameter. Thus, the synthesized CNTs using FeNiMo/MgO catalysts had enlarged sizes of CNTs. This is evident by TEM analysis as shown in Fig. 5.
Conclusion
In this study, the bimetallic NiMo, trimetallic CoNiMo, FeNiMo, and CoFeMo supported on MgO were used as catalysts for CNTs growth and syngas production. The experimental results show that the introduction of the third composition (Co or Fe) to NiMo/MgO catalyst does not give any further increase in the catalytic performance. The NiMo/MgO catalyst can perform the highest activity for both reforming and methane cracking. In addition, only slight deactivation of NiMo/MgO catalyst was observed over 3 h. The high purity of syngas was obtained, while the narrowest distribution and the smallest diameter of CNTs with graphitization degree are comparable to those of commercial CNTs. This makes NiMo/MgO a remarkably outstanding catalyst to directly convert biogas into syngas and multi-walled carbon nanotubes. In the case of the Fe-containing catalysts, they are known to catalyze the carbon oxidation, causing the agglomeration of oxide phases, and the formation of large diameter CNTs. However, the existence of Fe in the catalyst greatly enhances the bamboo structure with high grades which may be useful in some practical applications.
Data availability
All data related to the finding of this study are accessible upon request from the corresponding author Sakhon Ratchahat.
References
MRaPR, H. R. CO2 and greenhouse gas emissions: Our world in data, https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (2017).
EUSEP, A. Causes of climate change https://www.epa.gov/climatechange-science/causes-climate-change
Janet, L. S., Freyr, S. & Anna L. Renewable energy and sustainable development: Accounting for impacts on the path to 100% RE. 1–62 (World Future Council, Oeding print GmbH, 2016).
Balat, M. & Balat, H. Biogas as a renewable energy source—A review. Energy Sources Part A Recovery Util. Environ. Eff. 31, 1280–1293 (2009).
Surendra, K. C., Takara, D., Hashimoto, A. G. & Khanal, S. K. Biogas as a sustainable energy source for developing countries: Opportunities and challenges. Renew. Sustain. Energy Rev. 31, 846–859 (2014).
Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2, 81 (2014).
Azonono. Application of carbon nanotubes, https://www.azonano.com/article.aspx?ArticleID=4842.Azonono (2018).
Zhang, X. et al. High-performance binary Mo–Ni catalysts for efficient carbon removal during carbon dioxide reforming of methane. ACS Catal. 11, 12087–12095 (2021).
Chaudhary, P. K., Koshta, N. & Deo, G. Effect of O2 and temperature on the catalytic performance of Ni/Al2O3 and Ni/MgAl2O4 for the dry reforming of methane (DRM). Int. J. Hydrog. Energy 45, 4490–4500 (2020).
Pakhare, D. & Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43, 7813–7837 (2014).
Komatsubara, K. et al. Highly oriented carbon nanotube supercapacitors. ACS Appl. Nano Mater. 5, 1521–1532 (2022).
Cruz-Arzon, A. J. et al. Ultraviolet light tunable single walled carbon nanotubes/n-Si junction diode. Synth. Met. 283, 116967 (2022).
Son, K. H., Hong, J. H. & Lee, J. W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int J Nanomed. 11, 5163–5185 (2016).
Papadopoulou, C., Matralis, H. & Verykios, X. Utilization of biogas as a renewable carbon source: Dry reforming of methane. Catal. Altern. Energy Gener. 57–127 (2012).
Abdulrasheed, A. et al. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 108, 175–193 (2019).
Sharifianjazi, F. et al. A review on recent advances in dry reforming of methane over Ni- and Co-based nanocatalysts. Int. J. Hydrog. Energy (2021).
Figueredo, G. P. et al. A comparative study of dry reforming of methane over nickel catalysts supported on perovskite-type LaAlO3 and commercial α-Al2O3. Int. J. Hydrog. Energy 43, 11022–11037 (2018).
Takanabe, K., Nagaoka, K., Nariai, K. & Aika, K.-I. Titani-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 232, 268–275 (2005).
Majewska, J. & Michalkiewicz, B. Carbon nanomaterials produced by the catalytic decomposition of methane over Ni/ZSM-5: Significance of Ni content and temperature. New Carbon Mater. 29, 102–108 (2014).
Avdeeva, L. B., Kochubey, D. I. & Shaikhutdinov, S. K. Cobalt catalysts of methane decomposition: Accumulation of the filamentous carbon. Appl. Catal. A 177, 43–51 (1999).
Torres, D., Pinilla, J. L., Lázaro, M. J., Moliner, R. & Suelves, I. Hydrogen and multiwall carbon nanotubes production by catalytic decomposition of methane: Thermogravimetric analysis and scaling-up of Fe–Mo catalysts. Int. J. Hydrog. Energy 39, 3698–3709 (2014).
Ibrahim, A. A., Fakeeha, A. H., Al-Fatesh, A. S., Abasaeed, A. E. & Khan, W. U. Methane decomposition over iron catalyst for hydrogen production. Int. J. Hydrog. Energy 40, 7593–7600 (2015).
Sukri, M. F. F., Khavarian, M. & Mohamed, A. R. Effect of cobalt loading on suppression of carbon formation in carbon dioxide reforming of methane over Co/MgO catalyst. Res. Chem. Intermed. 44, 2585–2605 (2018).
Ayillath Kutteri, D., Wang, I. W., Samanta, A., Li, L. & Hu, J. Methane decomposition to tip and base grown carbon nanotubes and COx-free H2 over mono- and bimetallic 3d transition metal catalysts. Catal. Sci. Technol. 8, 858–869 (2018).
Pinilla, J. L. et al. Ni- and Fe-based catalysts for hydrogen and carbon nanofilament production by catalytic decomposition of methane in a rotary bed reactor. Fuel Process. Technol. 92, 1480–1488 (2011).
Rattanaamonkulchai, R. et al. Simultaneous production of hydrogen and carbon nanotubes from biogas over mono- and bimetallic catalyst. J. Environ. Chem. Eng. 10, 107910 (2022).
Pudukudy, M., Yaakob, Z. & Akmal, Z. S. Direct decomposition of methane over SBA-15 supported Ni, Co and Fe based bimetallic catalysts. Appl. Surf. Sci. 330, 418–430 (2015).
Xu, X. et al. Controllable synthesis of carbon nanotubes by changing the Mo content in bimetallic Fe–Mo/MgO catalyst. Mater. Chem. Phys. 127, 379–384 (2011).
Kitiyanan, B., Alvarez, W. E., Harwell, J. H. & Resasco, D. E. Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co–Mo catalysts. Chem. Phys. Lett. 317, 497–503 (2000).
Flahaut, E., Peigney, A., Bacsa, W., Bacsa, R. & Laurent, C. CCVD synthesis of carbon nanotubes from (Mg Co, Mo)O catalysts: Influence of the proportions of cobalt and molybdenum. J. Mater. Chem. 14, 646–653 (2004).
Lobiak, E. V., Kuznetsova, V. R., Flahaut, E., Okotrub, A. V. & Bulusheva, L. G. Effect of Co-Mo catalyst preparation and CH4/H2 flow on carbon nanotube synthesis. Fuller. Nanotubes Carbon Nanostruct. 28, 707–715 (2020).
Awadallah, A. E., Aboul-Enein, A. A. & Aboul-Gheit, A. K. Various nickel doping in commercial Ni–Mo/Al2O3 as catalysts for natural gas decomposition to COx-free hydrogen production. Renew. Energy 57, 671–678 (2013).
Akbari, E., Alavi, S. M. & Rezaei, M. Synthesis gas production over highly active and stable nanostructured NiMgOAl2O3 catalysts in dry reforming of methane: Effects of Ni contents. Fuel 194, 171–179 (2017).
Feng, J., Ding, Y., Guo, Y., Li, X. & Li, W. Calcination temperature effect on the adsorption and hydrogenated dissociation of CO2 over the NiO/MgO catalyst. Fuel 109, 110–115 (2013).
Al-Swai, B. M. et al. Low-temperature catalytic conversion of greenhouse gases (CO2 and CH4) to syngas over ceria-magnesia mixed oxide supported nickel catalysts. Int. J. Hydrog. Energy 46, 24768–24780 (2021).
Abd Ghani, N. A. et al. Dry reforming of methane for syngas production over Ni–Co-supported Al2O3–MgO catalysts. Appl. Petrochem. Res. 8, 263–270 (2018).
Takenaka, S., Ishida, M., Serizawa, M., Tanabe, E. & Otsuka, K. Formation of carbon nanofibers and carbon nanotubes through methane decomposition over supported cobalt catalysts. J. Phys. Chem. B 108, 11464–11472 (2004).
Radwan, N. R. E., Ghozza, A. M. & El-Shobaky, G. A. Solid–solid interactions in Co3O4–MoO3/MgO system. Thermochim. Acta 398, 211–221 (2003).
Kordouli, E., Sygellou, L., Kordulis, C., Bourikas, K. & Lycourghiotis, A. Probing the synergistic ratio of the NiMo/γ-Al2O3 reduced catalysts for the transformation of natural triglycerides into green diesel. Appl. Catal. B 209, 12–22 (2017).
Liu, H. et al. Preparation of highly active unsupported nickel–zinc–molybdenum catalysts for the hydrodesulfurization of dibenzothiophene. Appl. Catal. B 174–175, 264–276 (2015).
Aboul-Enein, A. A. & Awadallah, A. E. Production of nanostructured carbon materials using Fe–Mo/MgO catalysts via mild catalytic pyrolysis of polyethylene waste. Chem. Eng. J. 354, 802–816 (2018).
Awadallah, A. E., Deyab, M. A. & Ahmed, H. A. Mo/MgO as an efficient catalyst for methane decomposition into COx-free hydrogen and multi-walled carbon nanotubes. J. Environ. Chem. Eng. 9, 106023 (2021).
Infantes-Molina, A. et al. Catalysts based on Co/zirconium doped mesoporous silica MSU for the hydrogenation and hydrogenolysis/hydrocracking of tetralin. Appl. Catal. A 286, 239–248 (2005).
Aboul-Enein, A. A. & Awadallah, A. E. Impact of Co/Mo ratio on the activity of CoMo/MgO catalyst for production of high-quality multi-walled carbon nanotubes from polyethylene waste. Mater. Chem. Phys. 238, 121879 (2019).
Zhang, C.-H., Wan, H.-J., Yang, Y., Xiang, H.-W. & Li, Y.-W. Study on the iron–silica interaction of a co-precipitated Fe/SiO2 Fischer-Tropsch synthesis catalyst. Catal. Commun. 7, 733–738 (2006).
Liu, K. et al. An active Fischer-Tropsch synthesis FeMo/SiO2 catalyst prepared by a modified sol–gel technique. Catal. Commun. 12, 137–141 (2010).
Awadallah, A., Abdel-Mottaleb, M., Aboul-Enein, A., Yonis, M. & Aboul-Gheit, A. Catalytic decomposition of natural gas to CO/CO2-free hydrogen production and carbon nanomaterials using MgO-supported monometallic iron family catalysts. Chem. Eng. Commun. 202, 163–174 (2015).
Liu, W. W., Azizan, A., Chai, S. -P., Mohamed, A., Hashim, U. Synthesis of single-walled carbon nanotubes: Effects of active metals, catalyst supports, and metal loading percentage. J. Nanomater. 2013 (2013).
Khavarian, M. & Mohamed, A. Effects of reaction temperature on CO2 reforming of CH4 over multi-walled carbon nanotubes catalyst with Co-Mo-MgO nanoparticles. Mater. Sci. Forum 756, 182–189 (2013).
Giesen, A., Herzler, J. & Roth, P. High temperature oxidation of iron atoms by CO2. Phys. Chem. Chem. Phys. 4, 3665–3668 (2002).
Theofanidis, S. A., Galvita, V. V., Poelman, H. & Marin, G. B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 5, 3028–3039 (2015).
Zhang, T. et al. Dry reforming of methane on Ni-Fe-MgO catalysts: Influence of Fe on carbon-resistant property and kinetics. Appl. Catal. B 264, 118497 (2020).
Gadalla, A. M. & Sommer, M. E. Synthesis and characterization of catalysts in the system A12O3-MgO-NiO-Ni for Methane Reforming with CO2. J. Am. Ceram. Soc. 72, 683–687 (1989).
Dry, M. E. The fischer-tropsch process: 1950–2000. Catal. Today 71, 227–241 (2002).
Chein, R. & Yang, Z. Experimental study on dry reforming of biogas for syngas production over ni-based catalysts. ACS Omega 4, 20911–20922 (2019).
Xu, J., Zhou, W., Li, Z., Wang, J. & Ma, J. Biogas reforming for hydrogen production over nickel and cobalt bimetallic catalysts. Int. J. Hydrog. Energy 34, 6646–6654 (2009).
Izquierdo, U. et al. Tri-reforming: A new biogas process for synthesis gas and hydrogen production. Int. J. Hydrog. Energy 38, 7623–7631 (2013).
Charisiou, N. D. et al. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 31, 164–183 (2016).
le Saché, E., Johnson, S., Pastor-Pérez, L., Amini Horri, B. & Reina, T. R. Biogas upgrading via dry reforming over a Ni-Sn/CeO2-Al2O3 catalyst: Influence of the biogas source. Energies 12, 1007 (2019).
Zhou, L.-P. et al. Catalytic functions of Mo/Ni/MgO in the synthesis of thin carbon nanotubes. J. Phys. Chem. B 109, 4439–4447 (2005).
Li, W. Z., Wen, J. G. & Ren, Z. F. Effect of temperature on growth and structure of carbon nanotubes by chemical vapor deposition. Appl. Phys. A 74, 397–402 (2002).
Awadallah, A. E., Aboul-Enein, A. A. & Aboul-Gheit, A. K. Effect of progressive Co loading on commercial Co–Mo/Al2O3 catalyst for natural gas decomposition to COx-free hydrogen production and carbon nanotubes. Energy Convers. Manag. 77, 143–151 (2014).
Al-swai, B., Osman, N., Alnarabiji, M., Adesina, A. & Abdullah, B. Syngas production via methane dry reforming over ceria-magnesia mixed oxide supported nickel catalysts. Ind. Eng. Chem. Res. 58, 539–552 (2018).
Yao, D., Zhang, Y., Williams, P. T., Yang, H. & Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B 221, 584–597 (2018).
Tan, W., Lee, S. & Chong, C. T. TEM and XRD analysis of carbon nanotubes synthesised from flame. Key Eng. Mater. 723, 470–475 (2016).
Flygare, M. & Svensson, K. Quantifying crystallinity in carbon nanotubes and its influence on mechanical behaviour. Mater. Today Commun. 18, 39–45 (2019).
Awadallah, A. E., Aboul-Enein, A. A. & Aboul-Gheit, A. K. Impact of group VI metals addition to Co/MgO catalyst for non-oxidative decomposition of methane into COx-free hydrogen and carbon nanotubes. Fuel 129, 27–36 (2014).
Pornsunthorntawee, O., Chuaybumrung, S., Kitiyanan, B. & Chavadej, S. Purification of single-walled carbon nanotubes (SWNTs) by acid leaching, NaOH dissolution, and froth flotation. Sep. Sci. Technol. 46, 2056–2065 (2011).
Rahbar Shamskar, F., Rezaei, M. & Meshkani, F. The influence of Ni loading on the activity and coke formation of ultrasound-assisted co-precipitated Ni–Al2O3 nanocatalyst in dry reforming of methane. Int. J. Hydrog. Energy 42, 4155–4164 (2017).
Meshkani, F., Golesorkh, S. F., Rezaei, M. & Andache, M. Nickel catalyst supported on mesoporous MgAl2O4 nanopowders synthesized via a homogenous precipitation method for dry reforming reaction. Res. Chem. Intermed. 43, 545–559 (2017).
Spreitzer, D. & Schenk, J. Reduction of iron oxides with hydrogen—A review. Steel Res. Int. 90, 1900108 (2019).
Poirier, M. G. & Sapundzhiev, C. Catalytic decomposition of natural gas to hydrogen for fuel cell applications. Int. J. Hydrog. Energy 22, 429–433 (1997).
Acknowledgements
This work (Grant No. RGNS 63-168) was supported by Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (OPS MHESI), Thailand Science Research and Innovation (TSRI) and Mahidol University. Authors would like to thank Nawapol Udpuay, Chawalit Takoon, Suwilai Chaveanghong, and Bancha Panyacharoen scientists at Mahidol University - Frontier Research Facility (MU-FRF) for their kind assistance in instrumental operation of XRD and Raman spectroscopy.
Author information
Authors and Affiliations
Contributions
S.S.: Conceptualization, Investigation, Methodology, Data curation, and Writing—Original draft preparation. N.S: Investigation, Methodology, Data curation, and Writing—Original draft preparation. A.S.: Resources, Visualization, and Validation. W.K.A.: Resources. S.A.: Resources, Visualization, and Validation. C.F. Resources, and Visualization. S.R. Conceptualization, Methodology, Validation, Visualization, Data curation, Supervision, Funding acquisition, and Writing—Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saconsint, S., Sae-tang, N., Srifa, A. et al. Development of high-performance nickel-based catalysts for production of hydrogen and carbon nanotubes from biogas. Sci Rep 12, 15195 (2022). https://doi.org/10.1038/s41598-022-19638-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19638-y
This article is cited by
-
Development of Ni–Mo carbide catalyst for production of syngas and CNTs by dry reforming of biogas
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.