Abstract
We have investigated the diversity and composition of gut microbiotas isolated from AD (Alzheimer's disease) patients (n = 41) and healthy seniors (n = 43) from Nur-Sultan city (Kazakhstan). The composition of the gut microbiota was characterized by 16S ribosomal RNA sequencing. Our results demonstrated significant differences in bacterial abundance at phylum, class, order, and genus levels in AD patients compared to healthy aged individuals. Relative abundance analysis has revealed increased amount of taxa belonging to Acidobacteriota, Verrucomicrobiota, Planctomycetota and Synergistota phyla in AD patients. Among bacterial genera, microbiotas of AD participants were characterized by a decreased amount of Bifidobacterium, Clostridia bacterium, Castellaniella, Erysipelotrichaceae UCG-003, Roseburia, Tuzzerella, Lactobacillaceae and Monoglobus. Differential abundance analysis determined enriched genera of Christensenellaceae R-7 group, Prevotella, Alloprevotella, Eubacterium coprostanoligenes group, Ruminococcus, Flavobacterium, Ohtaekwangia, Akkermansia, Bacteroides sp. Marseille-P3166 in AD patients, whereas Levilactobacillus, Lactiplantibacillus, Tyzzerella, Eubacterium siraeum group, Monoglobus, Bacteroides, Erysipelotrichaceae UCG-003, Veillonella, Faecalibacterium, Roseburia, Haemophilus were depleted. We have also found correlations between some bacteria taxa and blood serum biochemical parameters. Adiponectin was correlated with Acidimicrobiia, Faecalibacterium, Actinobacteria, Oscillospiraceae, Prevotella and Christensenellaceae R-7. The Christensenellaceae R-7 group and Acidobacteriota were correlated with total bilirubin, while Firmicutes, Acidobacteriales bacterium, Castellaniella alcaligenes, Lachnospiraceae, Christensenellaceae and Klebsiella pneumoniae were correlated with the level of CRP in the blood of AD patients. In addition, we report the correlations found between disease severity and certain fecal bacteria. This is the first reported study demonstrating gut microbiota alterations in AD in the Central Asian region.
Similar content being viewed by others
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by memory loss, dramatic changes in character and behavior, and the impossibility of carrying out normal daily activities in the latter stages of the disease. AD incidence increases with age and impacts approximately 10% of people aged 65–75 and 32% of the elderly aged 80 and above1,2. As indicated by the World Health Organization (WHO) frequency of AD is exacerbating each year; in this way, it is hypothesized that there could be a triple expansion in the number of AD patients by 2050, and the most significant increase in dementia will occur in low and middle-income countries3. In Kazakhstan and other countries of the World, there has been an increase in older adults over the past decades. A recent pilot study on cognitive impairment revealed that the prevalence of mild cognitive impairment (MCI) is about 40% among the elderly population in the Almaty city region, Kazakhstan4. Because up to 30% of MCI develops into AD2, we can approximate the prevalence of AD in Kazakhstan up to 15% among people aged 65 and over, which is projected to triple by 20503. However, despite the estimated high prevalence, the Kazakh population is not represented in global studies of dementia. The 2015 World Alzheimer's Disease Report states a constant lack of research on dementia in the Central Asian region (the region Kazakhstan belongs to)3.
Many risk factors have been recognized as contributors to the development of AD. These include non-modifiable (age, gender, family history, genetics) and modifiable factors such as low education, midlife hypertension, high cholesterol, physical inactivity, obesity, diabetes, etc. The gut microbiota is one of the most important factors influencing human health, and it has gotten a lot of attention from scientists in the last two decades. There are roughly 1000 species and 7000 strains of microscopic organisms that populate the human digestive tract (1013–1014 microorganisms altogether), among which the most widely recognized are Firmicutes (51%) and Bacteroidetes (48%)5. The remaining 1% of bacteria belong to other divisions such as Proteobacteria, Actinobacteria, Fusobacteria, Spirochaetes, Verrucomicrobia, and Lentispherae5. Not long ago, understanding the gut microbiota's role was limited to the processes that entirely occur in the intestine. Nonetheless, the composition of the intestinal microbiota has been studied during the last 15 years, resulting in a direct link between the density and species variety of the gut microbiota and a range of pathological disorders, including diabetes, obesity, and cardiovascular diseases. These disorders, in turn, are well-known risk factors for the development of sporadic AD, and there is data showing that the gut microbiota impacts brain functions6,7,8. Besides, ongoing investigations have uncovered the critical contrasts in the amount and nature of gut microbiota in AD patients compared to healthy seniors of a similar age9,10,11,12.
For instance, two case–control studies conducted at Wisconsin Alzheimer's infection Research Center (USA)10 and Chongqing Medical University (China)11 uncovered the differences in intestinal microbiotas composition in patients with AD compared to healthy individuals at the phylum and species levels. Though, personal changes in the intestinal microbiome in Chinese patients varied from those in the United States. These differences may be attributed to biogeography, ethnicity, lifestyle, and eating habits13. Therefore, more research is needed to reveal the relationships between microbiota and lifestyle in different ethnic populations and their influence on AD's cognitive functions and risks.
The Asian population is heterogeneous, and microbiota biomarkers in the Kazakhstani population differ from previously published ones, including those from other Asian people14. Our previous research has demonstrated that Kazakh microbiotas collected from healthy subjects and metabolic syndrome patients are relatively different from European and East Asian counterparts. This is important for developing prognostics and preventive measures14. Yet, no studies have been published on the association between gut microbiota and AD risks in the Central Asian region. Thus, in the present paper, we report the first pilot case–control study of the diversity and composition of gut microbiota isolated from local patients diagnosed with AD compared to healthy seniors. To our knowledge, this is the first study in the Central Asian region investigating gut microbiota biomarkers in association with AD.
Methods
Forty-one individuals diagnosed with Alzheimer's disease and forty-three cognitively normal controls were recruited from inpatient and outpatient treatment and prevention facilities in Nur-Sultan, Kazakhstan. For case selection, the following inclusion criteria were identified: (a) diagnosis of dementia due to Alzheimer's dementia according to the guidelines for diagnosis and statistics of mental disorders (DSM-IV) and the criteria of the National Institute of Neurological and Communicative Disorders, stroke, Alzheimer's disease and other related disorders (NINCDS-ADRDA)14; (b) age from 55 years and older at the time of diagnosis and data collection; (c) voluntary consent to participate in the study. The following inclusion criteria were identified for control selection: (a) absence of cognitive and memory impairment; (b) voluntary consent to participate in the study. We excluded subjects with severe somatic diseases of the kidneys, liver, severe chronic obstructive pulmonary disease, cancer, etc., and subjects with a mental disorder not related to AD.
Data collection and diagnosis of AD
The experienced neurologists confirmed the diagnosis of Alzheimer's disease according to the criteria of NINCDS-ADRDA (The National Institute of Neurological and Communicative Disorders and the Alzheimer's Disease and Related Disorders Association)15. Evaluation of cognitive functions was performed using a mini-mental state examination scale (MMSE) and a clock drawing test (CDT). The MMSE scores were distributed as the following: no dementia (30 points), questionable (26–29), mild and moderate (11–25), and severe dementia (0–10)16. Medical history data (history of diabetes, heart disease, hypertension, and any other health condition), complaints, socio-demographic data, and risk factors were collected from research participants and their guardians using questionnaires. The study protocol was approved by the National laboratory Astana Local Ethics Committee. Written informed consent was obtained from all subjects involved in the study.
Blood sample collection and biochemical analyses
The study participants were sampled fasting venous blood by qualified medical personnel in disposable plastic vacuum tubes with K2-EDTA (purple cap, 10 ml) and coagulation activator gel (yellow hat, 8 ml). Blood samples were left to clot at room temperature for 30 min and then centrifuged for 10 min at 4000 rpm to prepare serum. Determination of the serum levels of lipids was outsourced to the commercial clinical-diagnostic laboratory "Olymp," which routinely performs biochemical analyses. The adiponectin level of the samples was measured using a serum, plasma, and cell culture adiponectin quantification kit (Sigma-Aldrich) according to the manufacturer's protocol.
Fecal sample collection, DNA isolation, and sequencing
Study participants were given instructions and a special kit for self-collection of feces. The toilet was covered with a white bag labeled "Class A" according to the instructions. A filter paper-sized 9 × 12.5 was then placed in the center before defecation. The feces were placed in a special tube with a spoon, and the tubes were packed in a soft foil envelope and stored in a freezer at – 20 °C until a doctor's visit. Bacterial DNA was isolated according to the QIAamp DNA stool Mini Kit protocol (Qiagen, 51504). Sterile water served as a negative control. Following the standard Illumina protocols, samples were sequenced at Novogene (Beijing, China).
Sequence analysis
The final 84 samples from AD patients and the healthy group were pooled into four libraries according to 16S rRNA sequencing data. Raw data quality control was carried out using FastQC v0.11.7 programs (Andrews S. et al. FastQC: a quality control tool for high throughput sequence data—2010) и MultiQC v1.817. Sequence data were processed through the LotuS pipeline as previously described18 using following parameters: quality filtered (minimum length = 170, minAvgQuality = 27, TruncateSequenceLength = 170, maxAccumulatedError = 0.75) and demultiplexed with sdm (pdiffs = 1, bdiffs = 1). Chimera filtering was undertaken using USEARCH de novo chimera filtering (abundance annotation = 0.97, abskew = 2).
Statistical analysis
Non-bacterial domain OTUs and OTUs with a total read count of less than 0.001% of the total read count in all samples were removed. All statistical analyses were performed in R v. 4.1.219; graphics were conducted in R using the package ggplot220. Feature table, taxonomic assignment, and tree files for the OTU datasets were imported into phyloseq21 for downstream analyses. Alpha diversity metrics such as Shannon22, Simpson23, Chao1, Observed, ACE and Fisher were calculated with phyloseq on absolute sample abundance values. For ordination plots of beta diversity metrics, sampling counts were first transformed with the Hellinger standardization transformation method24. Then weighted Unifrac distance25 was calculated, and the graphs of the Principal Coordinate Analysis (PCoA) were generated from a distance26. ANOSIM and PERMANOVA tests27 with 9999 permutations compared the AD vs. Normal controls. LEfSe was used for differential taxa abundance testing, using default recommended settings according to the author's instructions28, at an adjusted p ≤ 0.05 for significance and requiring an LDA effect size of at least 2 for every significant call. Correlation analysis between blood biomarkers (absolute indicators) and OTU (relative indicators) was carried out on the basis of the Spearman test. The list of blood biomarkers included markers that had differences between patients and healthy people (ADPQ, TBIL, TRIG (p < 0.05)), as well as CRP (p = 0.058). The list of OTUs is represented by taxa derived from LEfSe analysis. Also, MaAsLin2 was used to determine specific OTUs associated with disease severity, with correlations considered significant at the 5% level29.
Ethics declarations
The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee of National Laboratory Astana Nazarbayev University (protocol code 05-2020 from 24.09.2020).
Results
Analysis of clinical and biochemical data in research groups
The characteristics of the study subjects are presented in Table 1. Individuals with AD had significantly lower MMSE and CDT scores as expected. The frequency of comorbidities such as hypertension, diabetes, and coronary health disease was not statistically different between groups. Body mass index (BMI) was lower in the AD group. Carriers of ApoE ɛ4 were more frequent in the AD group, albeit not statistically significant (possibly, due to the low sample size).
Also, the analysis did not reveal a statistically significant difference in the blood levels of total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), insulin, atherosclerotic index, blood glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and C-reactive protein (CRP) between the compared research groups. This indicates the relative homogeneity of the studied groups. However, levels of triglycerides were identified to be reduced while total bilirubin and serum adiponectin were observed to be eleveated amongst the diseased samples significantly (p < 0.05). (Table 2).
Fecal microbiota analysis
To determine the hallmarks of the gut microbiota in Alzheimer's disease, we have analyzed the composition and diversity of bacterial taxa in patients with a confirmed diagnosis versus controls at phylum, class, and genus levels (Figs. 1, 2, Supplement file Table 1). We have found that Firmicutes, Bacteroidota, Proteobacteria, and Actinobacteriota were the predominant phyla both in AD and controls (Fig. 1A). However, we found higher abundance of Acidobacteriota (p < 0.001), Latescibacterota (p = 0.036), Verrucomicrobiota (p = 0.0149), Synergistota (p = 0.0033), Planctomycetota (p = 0.0292), and Zixibacteria (p = 0.0678) in AD group compared to healthy controls (Fig. 2, Supplement file Table 1).
At class level the predominant bacteria in both groups were Clostridia, Bacteroidia, Gammaproteobacteria (Fig. 1B), while amount of Actinobacteria (p = 0.0159) were depleted, whereas Blastocatellia (p = 0.0001), Synergistia (p = 0.0033), Vicinamibacteria (p = 0.0139), lineages Pla4 (p = 0.0322), Phycishaerae (p = 0.0037) were enriched in AD group compared to the healthy controls (Figs. 1B, 2). At genus level, the relative abundance of Bifidobacterium (p = 0.0204), uncultured Clostridia bacterium (p = 0.0264), Castellaniella (p = 0.0019), Erysipelotrichaceae UCG-003 (p = 0.0066), Roseburia (p = 0.0098), Tuzzerella (p = 0.0038), genus related to family Lactobacillaceae (Lactiplantibacillus, Latilactobacillus, Levilactobacillus p < 0.05) and Monoglobus (p = 0.0019) were decreased in AD patients. At the same time, the microflora in the AD group was enriched with the taxa Akkermansia (p = 0.0197), Niastella (p = 0.0326), Oxalobacter (p = 0.024), Prevotella (p = 0.045), Flavobacterium (p = 0.0469) (Figs. 1C, 2).
Analysis of α-diversity using Shannon, Simpson, Chao1, Observed, ACE, and Fisher indices did not reveal any significant differences between AD individuals and healthy controls (Fig. 3A, Supplement file Table 2). Bacterial composition clustering using PCoA of weighted UniFrac distance via Hellinger-transformed data (β-diversity) has also not revealed group-dependent segregation between AD comparing control (p = 0.2313, R2 = 0.011) (Fig. 3B). Checking other axes also did not allow us to clearly separate the samples between groups (Supplementary file Fig. 1). The relatively low sample size could explain the absence of statistical significance; therefore, more studies are needed.
(A) α-diversity (Shannon, Simpson indexes) of fecal bacteria in individuals with Alzheimer's disease (AD) and normal controls. (B) β-diversity (weighted UniFrac distance) of fecal bacteria in individuals with Alzheimer's disease (AD) and healthy controls (Normal control). (C) The linear discriminant analysis (LDA) scores (LEfse plot). An LDA score (log 10) > 2 indicates a significantly different enrichment of bacteria taxa in the AD group (purple) compared to the control group (green). p phylum, c class, o order, f family, g genus, s species.
Next, we used a linear discriminant analysis effect size (LEfSe) and determined enriched taxa of Genus level Christensenellaceae R-7 group, Prevotella, Alloprevotella, Bifidobacterium (OTU140), Eubacterium coprostanoligenes group, Ruminococcus, Flavobacterium, Ohtaekwangia, Akkermansia, Bacteroides sp. Marseille-P3166 in AD patients, whereas Levilactobacillus, Lactiplantibacillus, Tyzzerella, Eubacterium siraeum group, Monoglobus, Bacteroides, Erysipelotrichaceae UCG-003, Veillonella, Faecalibacterium, Roseburia, Haemophilus were depleted (Fig. 3C). The opposite enrichment direction of Bifidobacterium may demonstrate that in patients with AD, the Bifidobacterium taxon at the genus level is reduced, while the number of sequences related to Bifidobacterium (OTU140) has increased. This discrepancy might be explained by the lower level of the identified taxon, namely, its species affiliation. Comparison of data obtained from two different methods of analysis (LefSe and MaaSlin2) revealed a consistent list of taxa Faecalibacterium (OTU209), Monoglobus (OTU271), Eubacterium siraeum group (OTU212), Bacteroides (OTU229), Gammaproteobacteria (OTU33) reduced in the AD group (Supplement file Table 3).
Correlation between gut microbiota community and blood biochemical parameters
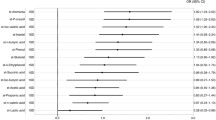
We investigated possible associations between the blood laboratory parameters and gut microbiota composition in AD patients and healthy aged individuals using correlation analysis between serum adiponectin, cholesterol, HDL, LDL, triglycerides, insulin, fasting glucose, ALT, AST, total bilirubin, C-reactive protein and different bacterial taxa found in control and AD groups (Fig. 4). In the AD group, we have seen a negative correlation of adiponectin with Acidimicrobiia (OTU21) at the class level and Faecalibacterium (OTU209) at the genus level. We have also observed positive correlations of adiponectin with Actinobacteria (OTU55), Oscillospiraceae (OTU8), Prevotella (OTU46) and Christensenellaceae R-7 group (OTU228). The Christensenellaceae R-7 group (OTU117) and Acidobacteriota (OTU249) were negatively correlated with total bilirubin. Firmicutes (OTU254), Acidobacteriales bacterium (OTU324), indeterminate taxon (OTU555), Castellaniella alcaligenes (OTU376), Lachnospiraceae family taxon (OTU18) were negatively correlated with CRP, while Christensenellaceae R-7 (OTU228) and Klebsiella pneumoniae (OTU585) were positively correlated with the level of CRP in the blood of AD patients. In controls, Bacteroides (OTU229), Gammaproteobacteria (OTU368), and Clostridia UCG-014 (OTU523) were positively correlated with blood levels of adiponectin. Blood bilirubin levels were positively correlated with Castellaniella alcaligenes (OTU376) and uncultured Ohtaekwangia (OTU622). The taxa Acidimicrobiia (OTU109) and Bifidobacterium (OTU140) were positively correlated while the taxa Alloprevotella (OTU40) and Monoglobus (OTU271) were negatively correlated with the level of triglycerides in control.
Correlation between gut microbiota community and disease severity
The moderate form of AD was correlated with Bacteroides (OTU3880), Methylomirabilota (OTU903), uncultured Clostridiales bacterium (OTU2480), Prevotellaceae (OTU726), and the severe AD was correlated with Symbiobacteraceae (OTU530), uncultured proteobacterium (OTU3544), Clostridia vadinBB60 group (OTU406), Collinsella (OTU2030), Caldilineales (OTU300), Latescibacterota uncultured prokaryote (OTU3172), Elusimicrobiota Lineage Iib uncultured archaeon (OTU5211), Christensenellaceae R-7 group uncultured Clostridia bacterium (OTU1726) and gut metagenome (OTU1452) (Supplement file Table 4).
Discussion
In the past decade, numerous studies on the relationship between the microbiota and the central nervous system (CNS) have been published, suggesting the conception of the "brain-gut-microbiota axis"8. There are a lot of published papers showing the impact of intestinal dysbiosis, caused by changes in diet, antibiotics, non-steroidal anti-inflammatory drugs, presence of pathogenic microorganisms on the cognitive capacities of the brain30,31. Furthermore, recent research reveals correlations between the number and quality of the gut microbiota and Alzheimer's disease9,10,11.
For instance, studies from animal models of AD revealed substantial differences in gut microbiota composition of transgenic mice expressing the human APP gene and PS1 from wild-type mice32. The number of Firmicutes, Verrucomicrobia, Proteobacteria, and Actinobacteria was significantly decreased, whereas Bacteroidetes and Tenericutes were increased in 8-month-old CONVR-APPPS1 mice compared to wildlife mice. Furthermore, in the brain of CONVR-APPPS1 mice bred under sterile conditions, there was a substantial decrease in Aβ deposits relative to animals of the same genotype generated under normal conditions. Furthermore, the level of Aβ expression was upregulated after the microbiota transplantation from the CONVR-APPPS1 mice bred under normal conditions to the CONVR-APPPS1 mice bred in an aseptic environment. In contrast, fecal transplantation from wild-type mice did not change Aβ levels in the CNS.
Evidence obtained from clinical studies verified study results on laboratory animals. For instance, the relationship of brain amyloidosis with intestinal bacterial taxa and peripheral markers facilitating inflammation in people of advanced age with dementia was described12. This examination showed that in dementia patients with amyloidosis, an expanded degree of blood pro-inflammatory cytokines (IL-6, CXCL2, NLRP3, and IL-1β) was positively correlated with a number of some pro-inflammatory gut bacteria such as Escherichia/Shigella and negatively associated with anti-inflammatory E.rectale taxon. These associations of pro-inflammatory gut bacteria with increased levels of pro-inflammatory cytokines in the blood suggest that the alterations of the gut microbiota might be one of the factors responsible for the chronic peripheral inflammation leading to the development of neuroinflammation and neurodegeneration. In support of this notion, studies of microbiota from PD (Parkinson's disease) patients have demonstrated that clinical phenotype and severity of the illness were somehow correlated with the alterations of the gut microbiota and elevated plasma cytokines33.
At the Alzheimer's Disease Research Center (Wisconsin Alzheimer's Infection Research Center, USA), researchers discovered significant differences in the composition of the gut microbiota in patients with AD and healthy individuals at the phylum and species levels10. In the intestinal microbiota of AD patients, these investigations found a decrease in the number of bacteria belonging to the Firmicutes and Actinobacteria phyla (particularly bacteria of the genus Bifidobacterium) and an increase in the number of bacteria belonging to the Bacteroidetes phylum. Furthermore, a differential connection appeared between the degrees of individual bacterial genera in the digestive tract and cerebrospinal markers of AD, for example, Aβ42/Aβ40, p-tau, just as the Aβ/p-tau proportion10. Bacteroides, Actinobacteria, Ruminococcus, Lachnospiraceae, and Selenomonadales were found to have substantial changes in the spectrum of bacteria present in the bowels of individuals with Alzheimer's disease, according to research conducted at Chongqing Medical University in China11. Though, personal changes in the intestinal microbiota in Chinese patients varied to some degree from those in the United States. According to Zhuang et al., the number of bacteria belonging to the phylum Bacteroidetes is decreased. In contrast, if compared to healthy controls, the number of bacteria in the phylum Firmicutes remained unchanged.
In our cohort, differences in bacterial taxa between AD and controls were characterized by the elevated abundance of Acidobacteriota, Latescibacterota, Verrucomicrobiota, Synergistota, Planctomycetota, and Zixibacteria at the phylum level. Our results also demonstrated a relatively lower abundance of Bifidobacterium and microorganisms belonging to a family of Lactobacillaceae. Thus, microbiota biomarkers of AD patients from Kazakhstani population differ from previously published reports. However, despite the close geographical location of Kazakhstan to China and the predominantly Asian origin of the Kazakh population, our data are more consistent with the results of Vogt et al.10 in comparison with findings of Zhuang et al.11, who reported an increased amount of Lactobacillaceae and did not report any changes of Bifidobacterium. These similarities could result from such factors as the multi-ethnicity of the Kazakh population, westernized eating preferences, and relatively similar climatic conditions of the Nur-Sultan city region and Wisconsin13. Interestingly, Bifidobacteria and Lactobacillaceae taxa are involved in the production of important metabolites (neurotransmitters, neuroactive metabolites, SCFA, etc.) that play a crucial role in maintaining healthy cognitive, neuropsychiatric function and the reduction of which may be associated with the risk of developing neurodegenerative disorders34.
Gut microbiota has been suggested to be actively involved in host metabolism. In particular, several studies have shown that fecal bacteria can influence the metabolism of fatty acids and lipids35,36 and affect the expression of adiponectin37, a hormone produced by white adipose tissue. For instance, in human studies, the family Clostridiaceae/Lachnospiracease was associated with LDL, Pasteurellaceae, Coprococcus, and genus Collinsella, and species Stercoris showed association with triglyceride levels38. The gut composition of individuals with hypercholesterolemia was characterized by a lower abundance of the genera Anaeroplasma and Haemophilus, while a higher presence of Odoribacter; Anaeroplasma, and Haemophilus were correlated with lipid profile39. The effect of intestinal microbiota on adiponectin expression has been shown in animal models using a high-fat diet in obese mice. Significant correlations have been revealed between lipid metabolism, adiponectin, and altered gut microflora in coronary heart disease37,40.
As mentioned, adiponectin is a protein hormone produced mainly by white adipose tissue that regulates insulin sensitivity, fatty acid catabolism, glucose homeostasis, and anti-inflammatory system through various mechanisms. Adiponectin is involved in the pathogenesis of several age-related diseases, such as atherosclerosis, type 2 diabetes, and cardiovascular disorders41. In the past ten years, considerable information has been accumulated on the importance of adiponectin activity in the pathogenesis of Alzheimer's disease42. According to our findings, the adiponectin level was as twice as elevated in the AD group. This is in line with several studies that demonstrated that the plasma concentration of adiponectin was elevated in patients with mild cognitive impairment (MCI) and AD43,44. In our sample of AD patients, elevated levels of serum adiponectin were strongly correlated with Actinobacteria, Acidomicrobiia at the class level, Prevotella, Faecalibacterium, and Christensenellaceae R-7 at the genus level, and Oscillospiraceae at the family level; however, further studies are needed to investigate if this correlation is a result of a causal relationship.
Over the last decade, it become evident that chronic peripheral inflammation is one of the important factors contributing significantly to the development and progression of AD. There is also an assumption that the development of sporadic AD might be driven by the microbiome-associated peripheral inflammation45. Previous studies investigating the links between gut microbiota and low-grade inflammation marker C-reactive protein found Ruminococcaceae, Akkermansia, and Lactobacillales to be associated with the risk of anxiety and depression46. In cardiovascular patients, peripheral C-reactive protein was linked to the abundance of Bifidobacterium, Faecalibacterium, Ruminococcus, and Prevotella47. In our study, we observed a correlation between CRP and Firmicutes, Acidobacteriales bacterium, Castellaniella alcaligenes, Lachnospiraceae, Christensenellaceae R-7, and Klebsiella pneumoniae among the AD group participants.
In conclusion, we have investigated gut microbiota composition in AD patients from the Central Asian region for the first time. Consistent with previous reports, our study has demonstrated that the gut microbiota is altered in individuals with AD. However, microbiota biomarkers of AD patients from Kazakhstan are not identical to microbiomes of AD individuals from other countries. Furthermore, we reported the correlations exciting between blood serum profile and some fecal bacteria taxa. Significant results were also obtained by correlation analysis between the severity of the disease and meta-omics features. However, there are several limitations in our study worth mentioning. The relatively low sample size is one of the limitations of the study, yet comparable with sample sizes in similar published papers. Besides, we have utilized well established 16S ribosomal RNA MiSeq sequencing in our study, although metagenomics sequencing instead of targeted amplicon sequencing is more preferable since they provide broader and more accurate microbial information. By focusing on the mechanism for the interactions between human microbiota, peripheral metabolism, and the brain, we can uncover new pathophysiological pathways leading to the onset and progression of AD and other neurodegenerative disorders. Studying the relationships between human microbiotas and AD in different ethnic populations will help evaluate the contribution of biogeography and lifestyle to the increase in the prevalence of dementia and develop practical recommendations for the prevention and treatment of this severe pathology. However, more study is needed to ascertain our findings and disclose new associations between lifestyle, gut microbiota, and neurodegenerative disorders in different ethnic populations.
Data availability
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI BioProject [accession number PRJNA811324].
References
Prince, M. et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimer’s Res. Ther. 8, 1–3 (2016).
Alzheimer’s Association, Thies, W. & Bleiler, L. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 9(2), 208–245 (2013).
Prince, M. J. et al. World Alzheimer Report 2015-the Global Impact of Dementia (Alzheimer’s Disease International, 2015) https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf.
Tsoy, R. T., Turuspekova, S. T., Klipitskaya, N. K., Mereke, A. & Cumming, R. G. Prevalence of mild cognitive impairment among older people in kazakhstan and potential risk factors: A cross-sectional study. Alzheimer Dis. Assoc. Disord. 33(2), 136–141 (2019).
The Human Microbiome Project C et al. Structure, function and diversity of the healthy human microbiome. Nature 486, 207 (2012).
Westfall, S. et al. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 74, 3769–3787 (2017).
Zhu, X. et al. Microbiota-gut-brain axis and the central nervous system. Oncotarget 8(32), 53829–53838 (2017).
Kowalski, K. & Mulak, A. Brain-gut-microbiota axis in alzheimer’s disease. J. Neurogastroenterol. Motil. 25(1), 48–60 (2019).
Larroya-García, A., Navas-Carrillo, D. & Orenes-Piñero, E. Impact of gut microbiota on neurological diseases: Diet composition and novel treatments. Crit. Rev. Food Sci. Nutr. 59, 1–39 (2019).
Vogt, N. M. et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7(1), 13537 (2017).
Zhuang, Z.-Q. et al. Gut microbiome is altered in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 63, 1–10 (2018).
Cattaneo, A. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 49, 60–68 (2017).
Tasnim, N., Abulizi, N., Pither, J., Hart, M. & Gibson, D. L. Linking the gut microbial ecosystem with the environment: Does gut health depend on where we live?. Front. Microbiol. 8, 1935 (2017).
Kushugulova, A. et al. Metagenomic analysis of gut microbial communities from a Central Asian population. BMJ Open 8(7), e021682 (2018).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease. Neurology 34(7), 939 (1984).
Perneczky, R. et al. Mapping scores onto stages: Mini-mental state examination and clinical dementia rating. Am. J. Geriatr. Psychiatry 14, 139–144 (2006).
Ewels, P., Magnusson, M., Lundin, S. & Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32(19), 3047–3048 (2016).
Hildebrand, F., Tadeo, R., Voigt, A. Y., Bork, P. & Raes, J. LotuS: An efficient and user-friendly OTU processing pipeline. Microbiome. 2(1), 30 (2014).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020).
Wickham H. tidyverse/ggplot2. tidyverse (2016) https://github.com/tidyverse/ggplot2/releases (дaтa oбpaщeния: July 29).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4), e61217 (2013).
Shannon, C. E. The Mathematical Theory of Communication 117 (University of Illinois Press, 1949).
Simpson, E. H. Measurement of diversity. Nature 163(4148), 688–688 (1949).
Legendre, P. & Gallagher, E. D. Ecologically meaningful transformations for ordination of species data. Oecologia 129(2), 271–280 (2001).
Hamady, M., Lozupone, C. & Knight, R. Fast UniFrac: Facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 4(1), 17–27 (2010).
Lozupone, C. & Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71(12), 8228–8235 (2005).
Aitchison, J., Barceló-Vidal, C., Martín-Fernández, J. A. & Pawlowsky-Glahn, V. Logratio analysis and compositional distance. Math. Geol. 32(3), 271–275 (2000).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12(6), R60 (2011).
Mallick, H.A.-O. et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 17(11), e1009442 (2021).
Gareau, M. G. Microbiota-gut-brain axis and cognitive function. Adv. Exp. Med. Biol. 817, 357–371 (2014).
Jiang, C., Li, G., Huang, P., Liu, Z. & Zhao, B. The gut microbiota and Alzheimer’s disease. J. Alzheimers Dis. 58(1), 1–15 (2017).
Harach, T. et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7, 41802 (2017).
Lin, C.-H. et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 16(1), 129 (2019).
Caspani, G. & Swann, J. Small talk: Microbial metabolites involved in the signaling from microbiota to brain. Curr. Opin. Pharmacol. 48, 99–106 (2019).
Matey-Hernandez, M. L. et al. Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol. Genomics 50(2), 117–126 (2017).
Wang, Z., Koonen, D., Hofker, M. & Fu, J. Gut microbiome and lipid metabolism: From associations to mechanisms. Curr. Opin. Lipidol. 27(3), 216–224 (2016).
Yao, H. et al. Alteration of gut microbiota affects expression of adiponectin and resistin through modifying DNA methylation in high-fat diet-induced obese mice. Genes Nutr. 15(1), 12–12 (2020).
Fu, J. et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 117(9), 817–824 (2015).
Granado-Serrano, A. B. et al. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 9(1), 1–13 (2019).
Peng, Y. et al. Correlations of changes in inflammatory factors, glucose and lipid metabolism indicators and adiponectin with alterations in intestinal flora in rats with coronary heart disease. Eur. Rev. Med. Pharmacol. Sci. 24(19), 10118–10125 (2020).
Achari, A. E. & Jain, S. K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 18(6), 1321 (2017).
Waragai, M. et al. Importance of adiponectin activity in the pathogenesis of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 4(8), 591–600 (2017).
Ma, J. et al. Peripheral blood adipokines and insulin levels in patients with Alzheimer’s disease: A replication study and meta-analysis. Curr. Alzheimer Res. 13(3), 223–233 (2016).
Waragai, M. et al. Possible involvement of adiponectin, the anti-diabetes molecule, in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 52(4), 1453–1459 (2016).
Sochocka, M. et al. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—A critical review. Mol. Neurobiol. 56(3), 1841–1851 (2019).
Chen, Y. et al. Assessing the effect of interaction between C-reactive protein and gut microbiome on the risks of anxiety and depression. Mol. Brain. 14(1), 133 (2021).
van den Munckhof, I. C. L. et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 19(12), 1719–1734 (2018).
Funding
This work was supported by funding provided by the Ministry of Education and Science of the Republic of Kazakhstan (research grant AP09259730) and by the Nazarbayev University CRP initiative (Funder Project Reference: 091019CRP2112).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.R.K. and S.A.; funding acquisition, A.R.K. and S.A.; investigation, A.K., S.K., D.B., G.Z., D.A., A.B., F.O.; Supervision, S.A., and A.R.K.; writing—original draft, S.A., A.K., S.K., A.R.K.; writing—review and editing, A.R.K. and S.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaiyrlykyzy, A., Kozhakhmetov, S., Babenko, D. et al. Study of gut microbiota alterations in Alzheimer's dementia patients from Kazakhstan. Sci Rep 12, 15115 (2022). https://doi.org/10.1038/s41598-022-19393-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19393-0
This article is cited by
-
Combination of Lactobacillus fermentum NS9 and aronia anthocyanidin extract alleviates sodium iodate-induced retina degeneration
Scientific Reports (2023)
-
Gut microbiota and circadian rhythm in Alzheimer’s disease pathophysiology: a review and hypothesis on their association
npj Aging (2023)
-
Dementia, infections and vaccines: 30 years of controversy
Aging Clinical and Experimental Research (2023)
-
Effect of gastrodin against cognitive impairment and neurodegeneration in APP/PS1 mice via regulating gut microbiota–gut–brain axis
Experimental Brain Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.