Abstract
Metal oxide heterostructures have gained huge attention in the energy storage applications due to their outstanding properties compared to pristine metal oxides. Herein, magnetic Fe2O3@SnO2 heterostructures were synthesized by the sol–gel electrospinning method at calcination temperatures of 450 and 600 °C. XRD line profile analysis indicated that fraction of tetragonal tin oxide phase compared to rhombohedral hematite was enhanced by increasing calcination temperature. FESEM images revealed that hexagonal nanoplatelets of Fe2O3 were hierarchically anchored on SnO2 hollow nanofibers. Optical band gap of heterogeneous structures was increased from 2.06 to 2.40 eV by calcination process. Vibrating sample magnetometer analysis demonstrated that increasing calcination temperature of the samples reduces saturation magnetization from 2.32 to 0.92 emu g-1. The Fe2O3@SnO2-450 and Fe2O3@SnO2-600 nanofibers as active materials coated onto Ni foams (NF) and their electrochemical performance were evaluated in three and two-electrode configurations in 3 M KOH electrolyte solution. Fe2O3@SnO2-600/NF electrode exhibits a high specific capacitance of 562.3 F g-1 at a current density of 1 A g-1 and good cycling stability with 92.8% capacitance retention at a high current density of 10 A g-1 after 3000 cycles in three-electrode system. The assembled Fe2O3@SnO2-600//activated carbon asymmetric supercapacitor device delivers a maximum energy density of 50.2 Wh kg-1 at a power density of 650 W kg-1. The results display that the Fe2O3@SnO2-600 can be a promising electrode material in supercapacitor applications.
Similar content being viewed by others
Introduction
The growing concerns about the consumption of fossil fuels, environmental pollution and their replacement by the development of clean, efficient electricity sources have drawn more attention to the advanced energy storage devices1. Supercapacitors are one of the most importantly researched energy storage devices; depending on the electrode materials can bridge the gap between rechargeable batteries and traditional dielectric capacitors due to their unique properties like long-term cycling stability, high power density, safety and low maintenance cost depending on electrode materials2. Over the past few decades, nanostructured materials with governable morphology and dimensions are designed and ready, which totally different functions are achieved by cutting the form, composition, and assembled structure3. In particular, one-dimensional (1D) nanomaterials such as nanorods4, nanowires5, nanotubes3, nanofibers6, and hollow structures7 possess superior properties like high aspect ratio, small dimension structure and unique device function compared to their microscale and bulk counterparts8. Moreover, semiconductor nanoheterostructures have attracted a lot of interest due to their optoelectrical9, optical10, photocatalytic11 and electrochemical properties12 can be largely improved or amended. Some fantastic characteristics of the heterostructures, like tailoring bandgap, the photo-absorption optimization, structural flexibility and charge carrier mobility enhancement, might give multiple synergistic functions to unravel the problems in environment and energy fields. Semiconductor nanoheterostructures are promising electrode materials for high-rate supercapacitors13. Tubular nanomaterials have received significant study attention in recent years due to their hollow shape, which provides a number of unique features that should be beneficial in a variety of applications such as information storage medium14, catalysts15, electronics16, gas sensors17, and medicine18. Therefore, many methods have been reported for preparing tubular magnetic nanomaterials, including template-assisted electrodeposition19, co-precipitation20, sol-gel21 and solvothermal22 methods. Nonetheless, these methods are frequently hampered by time-consuming procedures and special situations. Fundamental research considers the development of simple and effective methods for producing tubular nanostructures at cheap cost to be a significant issue for future practical applications23. Transition metal oxides such as Co3O4, Fe2O3, and SnO2 can offer excellent specific capacity because of the various element valence states of their reversible reactions; as a result, transition metal oxide electrode materials with excellent pseudocapacitance properties are being developed by researchers24,25.
Hematite (α-Fe2O3) material has been considered as a promising material for application in electrochemical energy storage devices due to their non-toxicity, high ideal theoretical capacitance (3625 F g-1 in ΔV = 1 V)26 and abundant reserves. Nevertheless, the low conductivity of Fe2O3 (~ 10−14 S cm-1) severely limits its further growth in the energy storage fields and its actual capacitance (120–320 F g-1) is very low compared to the theoretical value27,28. Many efforts have been taken to address this issue, including the development of Fe2O3-based composites, nanostructured Fe2O3 and oxygen-deficient Fe2O3. The most operative of these strategies is to build Fe2O3-based composites by utilizing the synergistic effect among different materials25,29. Furthermore, SnO2 nanomaterials are one of the most important typical n-type metal oxide semiconductors due to their excellent electrochemical stability30. Fe2O3 and SnO2 have many attractive features, including environmental friendliness, low cost, and natural abundance; particularly, it displays high discharge time at high current density in Li-ion batteries (LIBs)6,24.
Recently, studies have been conducted on the electrochemical performance of the active materials of Fe2O3, SnO2 and their composites with other materials as electrodes in supercapacitors. Ardakani et al.31 reported a high specific capacitance of 168 F g-1 at 5 mV s-1 for the α-Fe2O3@CeO2 core–shell heterostructure on a stainless steel substrate in 2 M Na2SO4 solution, which was prepared by co-precipitation method. Geerthana et al.32 synthesized ternary α-Fe2O3/MnO2/rGO heterostructures via a solvothermal method and pasted on the nickel (Ni) foam, which performed the maximum specific capacitance value of 447 F g-1 at 1 A g-1 in 6 M KOH solution. Cao et al.33 synthesized the lignin-based multi-channels carbon nanofibers (MCNFs)@SnO2 nanocomposites by the co-electrospinning method, which cast on nickel foam and indicated the high energy storage capacitance of 406 F g-1 at 0.5 A g-1 in 6 M KOH electrolyte solution. Asaithambi et al.34 examined the supercapacitor performance of Ce-SnO2@g-C3N4 composites which deposited on the Ni foam (NF) and reported the high capacitance of 274 F g-1 at 0.5 A g-1 in 2 M KOH, the active materials prepared by hydrothermal method.
In the present work, we have prepared unique hollow heterostructured materials at various calcination temperatures, including two parts, one is the SnO2 hollow nanofibers and the other is the scattered Fe2O3 hexagonal nanoplatelets on the surface of SnO2 hollow fibers. The SnO2 hollow nanofibers are the composites’ skeleton that can enhance the surface area and conductivity of the composites. On the other hand, the scattered Fe2O3 nanoplatelets can be considered muscles that attach to the skeleton and play a supporting role for efficient charge transfer and mass transfer in the charge–discharge process for supercapacitor applications.
Experimental
Materials
Tin (II) chloride dihydrate (SnCl2·2H2O) and potassium hydroxide (KOH) were purchased from Chem-Lab (Belgium). Iron (III) chloride hexahydrate (FeCl3·6H2O), N, N-Dimethylformamide (DMF), carbon black, N-methyl-2-pyrrolidinone (NMP), polyvinylidene difluoride (PVDF) were obtained from Merck (Germany). Polyvinylpyrrolidone (PVP, 1,300,000 g/mol) and pure ethanol (C2H5OH, 99.9%) were purchased from Sigma Aldrich and Samchun companies, respectively.
Preparation of nanofibers
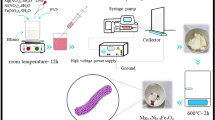
The Fe2O3@SnO2 composite nanofibers were prepared by sol–gel electrospinning method at different calcination temperatures. Firstly, 0.81 g FeCl3·6H2O and 0.34 g SnCl2·2H2O were stirred in 7.5 ml of ethanol and DMF as solvents for 30 min. Then, 0.6 g PVP was gradually added to the mixture and magnetically stirred overnight. The obtained sol was transferred into 10 ml syringe with a needle of 23G which was fed by a syringe pump at the rate of 0.4 ml h-1 and a high voltage of 16.5 kV to produce nanofiber composites. The needle was set at a distance of 10 cm from the aluminum foil collector. The as-spun nanofibers were obtained by drying at 100 °C for 18 h in the oven. Finally, Fe2O3@SnO2–450 and Fe2O3@SnO2–600 composite nanofibers were generated at the calcination temperatures of 450 and 600 °C for 2 h with a ramp rate of 2 °C min-1 under an air furnace, respectively. The synthesis schematic is displayed in Fig. 1.
Characterization
The thermal properties of the as-spun Fe2O3@SnO2 composite were performed by thermogravimetric-derivative thermogravimetry (TGA-DTG) analysis using Bahr model STA 504 thermal analyzer instrument. The structure of the prepared samples was analyzed by X-ray diffraction (X'Pert Pro, Panalytical) using Cu Kα (λ = 1.5406 Å) radiation. Fourier-transform infrared (FTIR) spectra of the samples were recorded by the Bruker Alpha in the range of 400–4000 cm-1. Field emission scanning electron microscopy (FESEM) with an energy dispersive spectroscopy (EDS, MIRA3, TESCAN-XMU) was used to investigate the morphology of the mentioned samples. The absorption spectra of the samples were measured by Varian Cary 100 UV/Visible spectrophotometer. The magnetic parameters of the composites were investigated using a vibrating sample magnetometer (VSM, Magnetic Daghigh Kavir Co., Iran) at room temperature (300 K) with a maximum applied magnetic field of ± 15 kOe.
Electrochemical measurements
The working electrodes were prepared by mixing active materials (Fe2O3@SnO2 composites), carbon black and PVDF as a binder at a weight ratio of 80:10:10 in NMP as a solvent to form a homogeneous slurry. The Ni foam (NF) substrates (2 × 1 cm2) were successively cleaned in aqueous HCl (3 M), deionized water, ethanol, and acetone for removing the NiO layer using an ultrasonic device each for 30 min, subsequently dried in the oven at 65 °C for 1 h. The prepared homogeneous slurries were pasted onto nickel foam (1 × 1 cm2) and dried overnight at 130 °C. The mass of the active materials on electrodes was about 1 mg. The electrochemical measurements of the electrodes were executed in a three-electrode system by Zahner Zennium device, comprised of Fe2O3@SnO2 composite as a working electrode, platinum wire as a counter electrode and Ag/AgCl as a reference electrode in 3 M KOH electrolyte at room temperature. Cyclic voltammetry (CV) was recorded at a potential window from 0 to 0.5 V, galvanostatic charge–discharge (GCD) was tested in the potential between 0 and 0.43 V, and the electrochemical impedance spectroscopy (EIS) was performed at the frequency ranges from 100 kHz to 10 mHz at an open circuit potential of 0.01 V and 5 mV AC amplitude. The impedance spectra were fitted by the equivalent circuit using the Z-view software.
Result and discussion
Thermal analysis
The suitable temperature to decompose PVP, remove of residual compounds and form Fe2O3@SnO2 nanofibers was determined through the thermogravimetric measurement in argon atmosphere from room temperature to 800 °C with a heating rate of 10 °C/min. The TGA and its derivative (DTG) curves for the as-spun nanofibers are shown in Fig. 2, demonstrating the weight loss percentage as a function of temperature. Three distinct stages were observed in weight loss. The first stage with a weight loss of about 12.53% at the range of room temperature to 200 °C could be ascribed to the evaporation of the solvents, including ethanol and DMF in the as-spun nanofibers35. The second weight loss in the temperature range of 200–350 °C corresponds to the decomposition of the metal precursors, which is verified by an endothermic peak centered at 240 °C in the DTG curve36,37. The third stage is the strong weight loss of 28.42% occurred at around 400 °C, which could be ascribed to the formation of metal oxide phases and the decomposition of the polymer side chain35. Above 550 °C in the TGA curve, no change was observed in the mass of the nanofibers, indicating complete combustion of PVP and the formation of crystalline Fe2O3@SnO2 composite.
XRD analysis
The X-ray diffraction (XRD) patterns of the calcined samples at 450 and 600 °C are depicted in Fig. 3. The emerged diffraction peaks can be allotted to rhombohedral Fe2O3 (JCPDS card No. 33-0664, space group: R-3c, 167) and tetragonal SnO2 (JCPDS card No. 41-1445, space group: P42/mnm, 136). Also, there were no traces of the other phases in the XRD patterns. The phase fractions of constituent components in the XRD pattern were evaluated by the Rietveld analysis in HighScore plus (Malvern Panalytical) software and obtained values are displayed in Table 1. The crystallite size and strain of the product were calculated from two reliable approaches, the Williamson-Hall (W–H) equation38 and the Rietveld refinement analysis using PANalytical X'pert HighScore Plus software39. Extracted data from the mentioned analysis revealed that the crystallite size increased and the lattice strain decreased with increasing calcination temperature, respectively. For Fe2O3@SnO2-450 sample, the phase fractions of α-Fe2O3, and SnO2 were estimated to be 52.6 and 47.4%, respectively. Meanwhile, these phases contribute 39.5 and 60.5% to Fe2O3@SnO2-600 sample, respectively. The detailed results are represented in Table 1.
FTIR analysis
The FTIR analysis is used to examine the details in the chemical bond structure of the samples which have shown in Fig. 4. The absorption bands under 700 cm-1 wavenumber are due to the proximity of O–Sn–O and Fe–O absorption band in the range of 470 cm-1; the band at 467 cm-1 can be ascribed to overlapped absorption of O–Sn–O and Fe–O8. The appeared band at 543 cm-1 is allocated to stretching vibration of Fe–O bond and a band that looks like a shoulder at 615 cm-1 may be ascribed to stretching vibration of Sn–O bond. In the case of non-metallic bonds, the one that can be observed at 3427 cm-1 corresponds to OH stretching vibration of water molecules. The bands which are located around ~ 2960 and 2929 cm-1 can be related to asymmetric stretching vibration of CH2 and the one that is located around 2864 cm-1 can be attributed to symmetric stretching vibration of CH2 bond. The two absorptions are seen at 1728 and 1632 cm-1 which are assigned to stretch vibrated C = O and that one is observed at 1281 cm-1 can be related to asymmetric stretching vibration of C-N. The deformed modes of CH and NCH bonds can be identified around 1462 and 1384 cm-1, respectively. Finally, the absorption located at 1127 and 1073 cm-1 can be assigned to rocking vibration and at 840 cm-1 is ascribed to bending vibration of C–H40,41,42. The absorption intensity of non-metallic bands dramatically decreased with increasing temperature up to 600 °C which can be in agreement with the fact that polymeric compounds such as PVP and DMF almost eliminated at high temperatures.
Morphological properties
The FESEM images of the as-spun and calcined Fe2O3@SnO2 nanofibers at different temperatures are revealed in Fig. 5. The as-spun nanofibers (Fig. 5a–c) a smooth and uniform surface with an average diameter of about 349 nm. After the nanofibers were annealed at 450 °C, the average diameter of nanofibers shrunk to 189 nm because of the decomposition of the polymer. The average diameter promotes to 303 nm when the calcined temperature reaches to 600 °C. This phenomenon could be ascribed to the particle growth of the metal oxides35. As shown in Fig. 5d–i, the hexagonal plates have grown hierarchically on the hollow nanofibers, which reduce with increasing calcination temperatures from 450 to 600 °C. The Hollow interiors for nanofibers are distinguished in Fig. 5d, g, which is clearly demonstrated that open-ended nanotubes could be maintained during calcination. These open tubular architectures will largely facilitate the ion migration between active material on the electrode surface and electrolyte in the electrochemical process43. EDS map-scan sum spectra in Fig. 5c, f, i, revealed the presence of C, O, Fe and Sn elements, which carbon was eliminated with increasing calcination temperature up to 600 °C due to complete thermal decomposition of PVP and metal oxidation.
According to the XRD results, the reduction of the hexagonal platelets on the nanofibers by increasing calcination temperature can be associated with a decrease in the fraction of Fe2O3 phase, For further clarification of elemental distribution, EDS point-scan analysis of Fe2O3@SnO2-600 composite were recorded in two regions (A and B), which are marked in the FESEM image (Fig. 6). The EDS spectra indicate that Sn and O elements are the dominant constituent elements of nanofibers (Region A) while the content of Fe and O is superior for the hexagonal platelet (Region B). The results prove the formation of Fe2O3 hexagonal platelets anchored on SnO2 nanofibers. Similar morphology was observed for selenization of electrospun carbon nanofibers, including tris(acetylacetonate) iron (III) with polyacrylonitrile (PAN) polymer, at different temperatures under H2Se gas, which resulted in the decoration of FeSe nanocrystals on the carbon nanofiber surfaces44.
Optical band gap
UV–Vis absorption analysis was carried out to study of optical properties of the products. Tauc plot was employed to estimate the optical band gap of nanofibers. The optical absorption spectra using Tauc's relation45:
where A* is a constant, α is the absorption coefficient, and hν is the photon energy. The absorption coefficient, α was determined from absorption data using the relation46:
where d is the sample thickness which is about equivalent to the quartz cell's path length, and \(A = ln\frac{{I_{0} }}{{I_{t} }}\) is the absorbance. The variation of (αhν)2 vs. photon energy (hν) for relevant composite is shown in the inset of Fig. 7. The direct optical band gap of nanocomposites was calculated via extrapolating the linear part of the (αhν)2 versus (hν) curve to intercept the energy axis (αhν = 0). According to the results by increasing temperature up to 600 °C, the Eg value was increased from 2.06 to 2.40 eV. This band gap widening can be related to the enhancement of SnO2 fraction in composite with an increase in the calcination temperature. The hollow Fe2O3@SnO2-600 nanofiber composite has a lower band gap compared to the Eg of the bulk SnO2 (3.6 eV)47,48 and a higher Eg than the bulk Fe2O3 band gap (2.2 eV)20,49. The deviation of the obtained Eg from the theoretical values (linear combination of bulk band gaps of constituent phases, SnO2 and Fe2O3) can be related to the structural disorder and surface defects, which cause to the optical band gap narrowing50.
Magnetic properties
The M–H curves were recorded using VSM analysis between − 8 to 8 kOe and shown in Fig. 8, confirming the ferromagnetic behavior of the samples. It is clearly seen that the calcination temperature increasing causes the decrease in saturation magnetization (Ms) values of nanofibers from 2.32 to 0.92 emu g-1. Both of the samples have very low Ms compared to similar articles8,51. The small Ms can be ascribed to the diminished effective weight fraction of magnetic core owing to the growth of the non-magnetic SnO2 phase in the calcined nanofibers52. Furthermore, small changes were observed in the coercive field (Hc) and the remnant magnetization (Mr) of the samples. With calcination temperature increasing the coercive field decreased from 165 to 157 Oe and magnetic remanence decreased from 0.373 to 0.131 emu g-1.
Electrochemical properties
To investigate the electrochemical performance of prepared electrodes, the Fe2O3@SnO2-450/NF and Fe2O3@SnO2-600/NF were tested by a three-electrode system including 3 M KOH electrolyte solution. Figure 9a,b displays the CV curves of both nanofiber electrodes at various scan rates from 10 to 80 mV s-1 within the potential window of 0 to 0.5 V. Existence of the two pairs of faradaic redox reaction peaks at the potential of about 0.22/0.33 V is due to the pseudocapacitance behavior of the electrodes in the high scan rates, which implies outstanding electrochemical performance53. Furthermore, the pair of redox peaks are nearly symmetrical, which means the high reversibility of the electrodes. The possible redox reactions for Fe2O3 and SnO2 could be explained by the following equations54,55:
The specific capacitance (Cs) for both electrodes from CV curves was calculated by the following Eq. 56:
where \(\smallint IdV\) is the surface area enclosed by the CV curve, \(v\) is scan rate (V s-1), \({\Delta }V\) is the difference of potential window (V), and m is the mass of active materials on the electrodes (g). The Cs values of Fe2O3@SnO2-450/NF electrode were 299.4, 222.6, 203.8 and 178.8 F g-1 at scan rates of 10, 30, 50 and 80 mV s-1, respectively. Also, the specific capacitances of Fe2O3@SnO2-600/NF electrode 553.5, 450.1, 394.9 and 330.6 F g-1 were achieved at the same scan rates. Increasing the scan rate minimizes the contribution of the electrode's active sites and the diffusion of electrolyte ions on the surface, lowering the Cs values of the electrodes57.
The GCD curves of both electrodes in the potential range between 0 and 0.43 V at different current densities are depicted in Fig. 10a,b. To avoid the water electrolysis (oxygen evolution reaction) during charging process, a smaller potential window than the CV curve was chosen58,59. The pseudocapacitive behavior of the two electrodes was confirmed from potential plateaus in the GCD curves, which correspond to the CV curves in Fig. 9. Also, the IR drop in the GCD curves of both electrodes at the beginning of the discharge time can be due to the internal resistance and energy loss of the electrode materials. The specific capacitance (Cs) is computed from GCD curves using the following equation56:
where m is the mass of active material on the electrode (g), I is discharge current (A), \(\Delta V\) is the potential window (V), and \(\Delta t\) is discharge time (s). The graph of the Cs values at different current densities for Fe2O3@SnO2-(450 and 600)/NF electrodes is shown in Fig. 11a. The maximum values of Cs for Fe2O3@SnO2-600/NF electrode were 562.3, 528.8, 508.1, 459.1 and 397.7 F g-1 at current densities of 1, 3, 5, 7 and 10 A g-1 with 70.7% capability. Also, the specific capacitances for Fe2O3@SnO2-450/NF electrode were 365.3, 258.8, 201.2, 174.2 and 162.8 F g-1 at the same current densities with 44.5% capability. The active sites of the electrode at low current densities can appropriately react with electrolyte ions, but at high current densities, the redox reactions only occur on the surface of the active materials due to the limitation of ion diffusion which leads to a decrease of the Cs values57. Figures 9c and 10c are given to compare the CV (at 10 mV s-1) and GCD (at 1 A g-1) curves for the two electrodes, respectively. As shown in Fig. 11a, with increasing calcination temperature from 450 to 600 °C the specific capacitance is enhanced. It can be due to the reduction of the hematite phase and promotion of the cassiterite phase which leads to more conductivity of the electrode60,61,62. Moreover, Fig. 11b demonstrates the cycling stabilities of Fe2O3@SnO2-450/NF with capacitance retention of 89.9% and Fe2O3@SnO2-600/NF electrode maintained 92.8% of its initial capacitance, which indicates excellent stability of the Fe2O3@SnO2-600/NF electrode. Table 2 compares the values calculated in this work with other reports, including Fe2O3 and SnO2 composites with different materials.
Electrochemical impedance spectroscopy (EIS) of both electrodes is obtained in a frequency range from 100 kHz to 10 mHz at an open circuit potential, which the Nyquist plots of electrodes are demonstrated in Fig. 11c. All the spectra were fitted using the equivalent circuit (as displayed in the inset of Fig. 11c). The x-axis intercept at high frequencies, the depressed semicircle at high-medium frequencies and the linear line at lower frequencies are assigned to solution resistance (Rs), charge transfer resistance (Rct) at the electrodes/electrolyte interface and Warburg impedance (W), respectively6,67. Also, the constant phase element (CPE) denotes the double-layer capacitance in simulating the behavior of imperfect dielectrics. The Rct value reduced from 10.5 to 9.18 Ω, which demonstrates an increase in the conductivity of the electrodes.
To evaluate the practical applications of the Fe2O3@SnO2-(450 and 600) nanofiber composites, an asymmetric supercapacitor was built with the electrodes as a cathode, activated carbon pasted on nickel foam (AC/NF) as an anode electrode, and Whatman filter paper as a separator in 3 M KOH electrolyte. Figure 12a illustrates the CV curves of the individual positive electrodes (Fe2O3@SnO2-450 and 600) within a potential range from 0 to 0.5 V and the single negative electrode (AC/NF) from − 1 to 0 V in a three-electrode system at a scan rate of 10 mV s-1. To achieve the high electrochemical performance of an asymmetric supercapacitor in a two-electrode system, the charge equilibrium (Q+ = Q-) is essential between the two electrodes. Therefore, the mass loading of active materials on the negative and positive electrodes can be inferred by the following equation68:
where C+ (C−) and ΔV+ (ΔV−) are the specific capacitance and working potential window of positive (negative) electrode, respectively. The specific capacitance of positive and negative electrodes can be calculated based on the CV curves using Eq. (6). Figure 12b,c show CV curves of assembled ASCs operated in the voltage range of 0–1.6 V at different scan rates. Figure 12d compares the CV curves for the two ASC devices. The redox peaks represent the pseudocapacitance contributions from the positive electrodes. Also, this pseudocapacitance characteristic is confirmed by GCD curves at various current densities (Fig. 14a,b).
The surface and diffusion controlled charge storage processes could be identified by the power-law relationship69:
where i denotes a current density, ν stands for a scan rate, a and b are adjustable variables. In general, the slope of the plot of log (i) versus log (ν) at a fixed potential determined the b-value. If b≈1, the charge storage mechanism is considered the surface-controlled capacitive process, while b≈0.5 represents the diffusion-controlled performance. As demonstrated in Fig. 13a, the b-values for Fe2O3@SnO2-450 and Fe2O3@SnO2-600 are about 0.55 and 0.67 corresponding to the oxidation reaction peaks, respectively, indicating that the charge storage mechanism is mainly dominated by the ion diffusion-controlled. The contribution of the surface capacitive effect and diffusion-controlled process with the scan rates were determined by Dunn’s equation69:
where i(V) represents the current response at a given potential, ν is a scan rate, k1 and k2 are constants. The slope and intercept of the linear relationship between i(V)/ν1/2 versus ν1/2 give the values of k1 and k2, respectively. The shaded blue regions in Fig. 13b,c indicate the surface-controlled contributions at a scan rate of 10 mV s-1 for the electrodes, which occupied 37.2 and 48.9% of the total region for the Fe2O3@SnO2-450 and Fe2O3@SnO2-600, respectively (Fig. 13d).
According to Eq. (7) and Fig. 14d, the Cs values of Fe2O3@SnO2-450//AC were obtained of 195.8, 173.1, 154.1, 127.1 and 109.7 F g-1 at 1, 3, 5, 7 and 10 A g-1, respectively, with 56% rate capability. Also, the maximum Cs values for Fe2O3@SnO2-600//AC were achieved as 213.9, 191.7, 180.7, 169.3, and 157.7 F g-1 at the same current densities with 73.7% rate capability. Figure 14c compares the GCD curves for the two ASC devices.
One of the most critical metrics in asymmetric supercapacitor devices is cycling stability. As shown in Fig. 14e, the cycling stabilities of the Fe2O3@SnO2-(450 and 600)//AC were recorded at a current density of 10 A g-1 after 3000 cycles. The ASC devices demonstrate 72 and 85% capacitance retention for Fe2O3@SnO2-450//AC and Fe2O3@SnO2-600//AC. The energy density (Es) and power density (Ps) are the two main and comparative parameters used to describe the supercapacitor performance. The Es (Wh kg-1) and Ps (W kg-1) of the Fe2O3@SnO2-(450 and 600)//AC asymmetric supercapacitors are calculated by GCD curves using the following equations70:
The Ragone plot is depicted in Fig. 14f, which relates the energy and power densities of the asymmetric supercapacitors. The maximum Es of 45.95 and 50.2 Wh kg-1 are achieved at a Ps of 650 W kg-1, as well as the minimum energy densities of 25.7 and 37 Wh kg-1 are retained at a higher Ps of 6500 W kg-1 for the Fe2O3@SnO2-450//AC and Fe2O3@SnO2-600//AC ASCs, respectively. These values are superior than most of other reported ASC devices, such as VO-Fe2O3@Sn2O3 (39.1 Wh kg-1 at 1280 W kg-1)29, RGO||α-Fe2O3@CeO2 (15.62 Wh kg-1 at 781 W kg-1)31, Ce-SnO2/g-C3N4//Activated Carbon (39.3 Wh kg-1 at 7425 W kg-1)34, α-Fe2O3/SnO2/rGO (17 Wh kg-1 at 3585 W kg-1)53, Fe-SnO2@CeO2 (32.2 Wh kg-1 at 747 W kg-1)65, SnO2@C (29.4 Wh kg-1 at 418 W kg-1)71, CC-Fe2O3/Na2WO4 NF (50 Wh kg-1 at 514.28 W kg-1)72. The results obtained from Fig. 14 indicate that the Fe2O3@SnO2-600//AC is the most suitable option for ASC device fabrication due to its high specific capacitance and long cycling stability.
The best electrode Fe2O3@SnO2-600 was examined as a power source. As shown in Fig. 15, two cells of the ASC device were connected in series and were able to light up the blue light-emitting diode (LED, 20 mA, 3.6 V) for about 5 min after charging by a power supply. In addition, a mini fan (0.1 W, 3 V) can be rapidly rotated by these cells for about 20 s (see Video S1). From the results, it was revealed that the Fe2O3@SnO2-600//AC ASC device had an outstanding performance in storing energy.
Conclusion
The hollow Fe2O3@SnO2 nanofiber composites were successfully synthesized by the sol–gel electrospinning process at different calcination temperatures of 450 and 600 °C. The composite structures of rhombohedral and tetragonal were confirmed for hematite and cassiterite by XRD analysis, respectively. The phase percentage of SnO2 was increased from 47.4 to 60.5% during calcination. FESEM images showed that the hexagonal nanoplatelets of Fe2O3 are hierarchically anchored on the SnO2 hollow nanofibers, which are reduced during calcination from 450 to 600 °C and verified with XRD and EDS analyses. Increasing the cassiterite phase with calcination temperatures grew the optical band gap from 2.06 to 2.40 eV due to the nature of the SnO2 band gap. VSM results demonstrated that a significant drop in the saturation magnetization from 2.32 to 0.92 emu g-1 during calcination temperatures was due to the reduction of the Fe2O3 phase. The electrochemical performance of the Fe2O3@SnO2-450 and 600 active materials pasted on the Ni foams indicated that the prepared Fe2O3@SnO2-600/NF electrode has a maximum specific capacitance of 562.3 F g-1 at a current density of 1 A g-1, a remarkable rate capability (70.7%) and excellent retention (92.8%) after 3000 cycles. Increasing the capacitance contribution from 37.2 to 48.9% during calcination distinguishes the Fe2O3@SnO2-600/NF electrode from another electrode. Furthermore, the assembled Fe2O3@SnO2-600//AC ASC device delivers a maximum energy density of 50.2 Wh kg-1 at a power density of 650 W kg-1. Overall, this study provides a promising strategy for the production of new hollow nanofiber electrode materials that encounter high power and energy density provisions for supercapacitor applications. The hexagonal platelets Fe2O3 decorated on SnO2 hollow nanofiber is an admirable candidate for electrode material in electrochemical energy storage devices.
Data availability
All data generated or analyzed during this study are included in this published article, and the datasets used/ or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nie, G. et al. Hierarchical α-Fe2O3@MnO2 core-shell nanotubes as electrode materials for high-performance supercapacitors. Electrochim. Acta 231, 36–43 (2017).
Raj, B. G. S. et al. A novel Fe2O3-decorated N-doped CNT porous composites derived from tubular polypyrrole with excellent rate capability and cycle stability as advanced supercapacitor anode materials. Electrochim. Acta 334, 135627 (2020).
Li, F. et al. Rational design and controllable synthesis of multishelled Fe2O3@SnO2@C nanotubes as advanced anode material for lithium-/sodium-ion batteries. ACS Appl. Mater. Interfaces 11, 36949–36959 (2019).
Nagaraju, Y. S. et al. Self-templated one-step hydrothermal synthesis of hierarchical actinomorphic flower-like SnO2-ZnO nanorods for high-performance supercapacitor application. J. Electroanal. Chem. 900, 115741 (2021).
Wang, L. et al. Construction of 1D SnO2-coated ZnO nanowire heterojunction for their improved n-butylamine sensing performances. Sci. Rep. 6, 35079 (2016).
Zan, F. et al. SnO2/Fe2O3 hybrid nanofibers as high performance anodes for lithium-ion batteries. Nanotechnology 31, 185402 (2020).
Chen, L., Wan, J., Fan, L., Wei, Y. & Zou, J. Construction of CoNi2S4 hollow cube structures for excellent performance asymmetric supercapacitors. Appl. Surf. Sci. 570, 151174 (2021).
Dhal, J. P., Mishra, B. G. & Hota, G. Fe2O3–SnO2 composite nanorods: Facile synthesis and sorption properties. J. Environ. Chem. Eng. 2, 2188–2198 (2014).
Xu, B., Zhou, G. & Wang, X. Rational synthesis and the structure-property relationships of nanoheterostructures: a combinative study of experiments and theory. NPG Asia Mater. 7, e164–e164 (2015).
Bai, X., Purcell-Milton, F., Kehoe, D. K. & Gun’ko, Y. K. Photoluminescent, “ice-cream cone” like Cu–In–(Zn)–S/ZnS nanoheterostructures. Sci. Rep. 12, 5787 (2022).
Singh, J. & Soni, R. K. Enhanced sunlight driven photocatalytic activity of In2S3 nanosheets functionalized MoS2 nanoflowers heterostructures. Sci. Rep. 11, 15352 (2021).
Yang, X. et al. High efficient photo-fenton catalyst of α-Fe2O3/MoS2 hierarchical nanoheterostructures: Reutilization for supercapacitors. Sci. Rep. 6, 31591 (2016).
Jiao, Y. et al. Hybrid α-Fe2O3@NiO heterostructures for flexible and high performance supercapacitor electrodes and visible light driven photocatalysts. Nano Energy 10, 90–98 (2014).
Kabeel, A. E., Harby, K., Abdelgaied, M. & Eisa, A. Augmentation of a developed tubular solar still productivity using hybrid storage medium and CPC: An experimental approach. J. Energy Storage 28, 101203 (2020).
Cui, H. et al. Template- and catalyst-free synthesis{,} growth mechanism and excellent field emission properties of large scale single-crystalline tubular β-SiC. Chem. Commun. https://doi.org/10.1039/B914846A (2009).
Yang, R. et al. A first-principles study of structural, electronic and optical properties of $\upalpha$-Te tubular nanostructures modulated by uniaxial strain. New J. Phys. 24, 53037 (2022).
Zeng, B., Zhang, L., Wu, L., Su, Y. & Lv, Y. Enclosed hollow tubular ZnO: Controllable synthesis and their high performance cataluminescence gas sensing of H2S. Sens. Actuators B Chem. 242, 1086–1094 (2017).
Massaro, M., Lazzara, G., Milioto, S., Noto, R. & Riela, S. Covalently modified halloysite clay nanotubes: synthesis{,} properties{,} biological and medical applications. J. Mater. Chem. B 5, 2867–2882 (2017).
Yu, T. et al. Exchange bias coupling in NiO/Ni bilayer tubular nanostructures synthetized by electrodeposition and thermal oxidation. J. Magn. Magn. Mater. 429, 74–78 (2017).
Kushwaha, P. & Chauhan, P. Synthesis of spherical and Rod-Like EDTA assisted α-Fe2O3 nanoparticles via Co-precipitation method. Mater. Today Proc. 44, 3086–3090 (2021).
Hassanzadeh-Afruzi, F., Amiri-Khamakani, Z., Bahrami, S., Ahghari, M. R. & Maleki, A. Assessment of catalytic and antibacterial activity of biocompatible agar supported ZnS/CuFe2O4 magnetic nanotubes. Sci. Rep. 12, 4503 (2022).
González-Rivera, J. et al. Magnetothermally-responsive nanocarriers using confined phosphorylated halloysite nanoreactor for in situ iron oxide nanoparticle synthesis: A MW-assisted solvothermal approach. Colloids Surf. A Physicochem. Eng. Asp. 635, 128116 (2022).
Zhao, J. et al. Magnetic and electrochemical properties of CuFe2O4 hollow fibers fabricated by simple electrospinning and direct annealing. CrystEngComm 14, 5879–5885 (2012).
Wang, X. M. et al. Excellent cyclic performance of Fe2O3@C/SnO2 controlled by Fe2O3@C and SnO2/C hybrid structures for lithium-ion batteries. J. Phys. Chem. Solids 132, 130–137 (2019).
Zhang, K., Cen, Z., Yang, F. & Xu, K. Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors. Prog. Nat. Sci. Mater. Int. 31, 19–24 (2021).
Chen, X., Chen, K., Wang, H. & Xue, D. Composition design upon iron element toward supercapacitor electrode materials. Mater. Focus 4, 78–80 (2015).
Ding, Y. et al. Iron oxides nanobelt arrays rooted in nanoporous surface of carbon tube textile as stretchable and robust electrodes for flexible supercapacitors with ultrahigh areal energy density and remarkable cycling-stability. Sci. Rep. 10, 11023 (2020).
Nithya, V. D. & Arul, N. S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 327, 297–318 (2016).
Tian, Y. et al. Defects engineering of Fe2O3@Sn2O3 nanosheet arrays for high-performance hybrid supercapacitor. J. Energy Storage 42, 103123 (2021).
Zhang, D., Sun, Y., Li, P. & Zhang, Y. Facile Fabrication of MoS2-Modified SnO2 Hybrid Nanocomposite for Ultrasensitive Humidity Sensing. ACS Appl. Mater. Interfaces 8, 14142–14149 (2016).
Mazloum-Ardakani, M., Sabaghian, F., Yavari, M., Ebady, A. & Sahraie, N. Enhance the performance of iron oxide nanoparticles in supercapacitor applications through internal contact of α-Fe2O3@CeO2 core-shell. J. Alloys Compd. 819, 152949 (2020).
Geerthana, M., Prabhu, S. & Ramesh, R. Hierarchical α-Fe2O3/MnO2/rGO ternary composites as an electrode material for high performance supercapacitors application. J. Energy Storage 47, 103529 (2022).
Cao, M., Cheng, W., Ni, X., Hu, Y. & Han, G. Lignin-based multi-channels carbon nanofibers @ SnO2 nanocomposites for high-performance supercapacitors. Electrochim. Acta 345, 136172 (2020).
Asaithambi, S. et al. The bifunctional performance analysis of synthesized Ce doped SnO2/g-C3N4 composites for asymmetric supercapacitor and visible light photocatalytic applications. J. Alloys Compd. 866, 158807 (2021).
Safartoobi, A., Mazloom, J. & Ghodsi, F. E. Electrochemical and optical properties of magnetic CuFe2O4 nanofibers grown by PVP and PVA-assisted sol–gel electrospinning. Appl. Phys. A 128, 13 (2021).
Akia, M., Mkhoyan, K. A. & Lozano, K. Synthesis of multiwall α-Fe2O3 hollow fibers via a centrifugal spinning technique. Mater. Sci. Eng. C 102, 552–557 (2019).
Phuoc, P. H. et al. One-step fabrication of SnO2 porous nanofiber gas sensors for sub-ppm H2S detection. Sens. Actuators A Phys. 303, 111722 (2020).
Khorsand Zak, A., Abd Majid, W. H., Abrishami, M. E. & Yousefi, R. X-ray analysis of ZnO nanoparticles by Williamson-Hall and size–strain plot methods. Solid State Sci. 13, 251–256 (2011).
Kaur, J. et al. Rietveld refinement study of GeSb2Te4 bulks prepared through distinct melting profiles. Mater. Today Proc. 4, 9524–9528 (2017).
Shokri, A., Shayesteh, S. F. & Boustani, K. The role of Co ion substitution in SnFe2O4 spinel ferrite nanoparticles: Study of structural, vibrational, magnetic and optical properties. Ceram. Int. 44, 22092–22101 (2018).
Botsa, S. M. et al. Flower like SnO2-Fe2O3-rGO ternary composite as highly efficient visible light induced photocatalyst for the degradation of organic pollutants from contaminated water. J. Mater. Res. Technol. 9, 12461–12472 (2020).
Guo, J., Chen, L., Wang, G., Zhang, X. & Li, F. In situ synthesis of SnO2–Fe2O3@polyaniline and their conversion to SnO2–Fe2O3@C composite as fully reversible anode material for lithium-ion batteries. J. Power Sources 246, 862–867 (2014).
Zhong, Y. et al. Controllable synthesis of TiO2@Fe2O3 Core-shell nanotube arrays with double-wall coating as superb lithium-ion battery anodes. Sci. Rep. 7, 40927 (2017).
Cho, J. S., Park, J.-S. & Kang, Y. C. Preparation of hollow Fe2O3 nanorods and nanospheres by nanoscale kirkendall diffusion, and their electrochemical properties for use in lithium-ion batteries. Sci. Rep. 6, 38933 (2016).
Tauc, J., Grigorovici, R. & Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. status solidi 15, 627–637 (1966).
Mazloom, J. & Zamani, H. Photocatalytic and photo-induced disinfection activities of sol-gel synthesized CeO2–SnO2:TM (TM= Co, Ni and Mn) nanocomposites: Relation between physical properties and their performance. J. Alloys Compd. 754, 238–246 (2018).
Matysiak, W., Tański, T., Smok, W. & Polishchuk, O. Synthesis of hybrid amorphous/crystalline SnO2 1D nanostructures: investigation of morphology, structure and optical properties. Sci. Rep. 10, 14802 (2020).
Pazouki, S. & Memarian, N. Effects of Hydrothermal temperature on the physical properties and anomalous band gap behavior of ultrafine SnO2 nanoparticles. Optik (Stuttg). 246, 167843 (2021).
Baig, F., Hameed Khattak, Y., Jemai, S., Marí Soucase, B. & Beg, S. Hydrothermal syntheses of Vanadium doped α-Fe2O3 cubic particles with enhanced photoelectrochemical activity. Sol. Energy 182, 332–339 (2019).
Burstein, E. Anomalous optical absorption limit in InSb. Phys. Rev. 93, 632–633 (1954).
Biju, C. S., Raja, D. H. & Padiyan, D. P. Fabrication of α-Fe2O3 hexagonal disc/SnO2 nanoparticle semiconductor nanoheterostructures and its properties. Chem. Phys. Lett. 619, 1–6 (2015).
Mi, Y. et al. Synthesis of hierarchical Fe2O3/SnO2 hollow heterostructures and their improved photocatalytic properties. Mater. Chem. Phys. 143, 311–321 (2013).
Geerthana, M. et al. Design and preparation of ternary α-Fe2O3/SnO2/rGO nanocomposite as an electrode material for supercapacitor. J. Mater. Sci. Mater. Electron. https://doi.org/10.1007/s10854-021-06128-6 (2021).
Wu, X. et al. Fe2O3 nanowire arrays on Ni-coated yarns as excellent electrodes for high performance wearable yarn-supercapacitor. J. Alloys Compd. 866, 158156 (2021).
Yang, G. et al. Rational construction of well-defined hollow double shell SnO2/mesoporous carbon spheres heterostructure for supercapacitors. J. Alloys Compd. 873, 159810 (2021).
Zhu, S. et al. Hydrothermal synthesis of graphene-encapsulated 2D circular nanoplates of α-Fe2O3 towards enhanced electrochemical performance for supercapacitor. J. Alloys Compd. 775, 63–71 (2019).
Safari, M. & Mazloom, J. Electrochemical performance of spindle-like Fe2Co-MOF and derived magnetic yolk-shell CoFe2O4 microspheres for supercapacitor applications. J. Solid State Electrochem. 25, 2189–2200 (2021).
Poudel, M. B., Karki, H. P. & Kim, H. J. Silver nanoparticles decorated molybdenum sulfide/tungstate oxide nanorods as high performance supercapacitor electrode. J. Energy Storage 32, 101693 (2020).
Ojha, G. P. et al. Three-dimensionally assembled manganese oxide ultrathin nanowires: Prospective electrode material for asymmetric supercapacitors. Energy 188, 116066 (2019).
Jiang, H. et al. Hybrid α-Fe2O3@Ni(OH)2 nanosheet composite for high-rate-performance supercapacitor electrode. Sci. Rep. 6, 31751 (2016).
Arun, T., Prabakaran, K., Udayabhaskar, R., Mangalaraja, R. V. & Akbari-Fakhrabadi, A. Carbon decorated octahedral shaped Fe3O4 and α-Fe2O3 magnetic hybrid nanomaterials for next generation supercapacitor applications. Appl. Surf. Sci. 485, 147–157 (2019).
Babu, B., Talluri, B., Gurugubelli, T. R., Kim, J. & Yoo, K. Effect of annealing environment on the photoelectrochemical water oxidation and electrochemical supercapacitor performance of SnO2 quantum dots. Chemosphere 286, 131577 (2022).
Yan, Y., Tang, H., Wu, F., Wang, R. & Pan, M. One-step self-assembly synthesis α-Fe2O3 with carbon-coated nanoparticles for stabilized and enhanced supercapacitors electrode. Energies 10(9), 1296 (2017).
Shi, T.-Z., Feng, Y.-L., Peng, T. & Yuan, B.-G. Sea urchin-shaped Fe2O3 coupled with 2D MXene nanosheets as negative electrode for high-performance asymmetric supercapacitors. Electrochim. Acta 381, 138245 (2021).
Asaithambi, S. et al. Preparation of Fe-SnO2@CeO2 nanocomposite electrode for asymmetric supercapacitor device performance analysis. J. Energy Storage 36, 102402 (2021).
Li, Y. et al. One-step hydrothermal synthesis of hybrid core-shell Co3O4@SnO2–SnO for supercapacitor electrodes. Ceram. Int. 46, 15793–15800 (2020).
Deng, J. et al. Bulk-like SnO2-Fe2O3@Carbon composite as a high-performance anode for lithium ion batteries. Nanomaterials 10(2), 249 (2020).
Xu, Y., Jiao, Y., Shen, L., Chen, J. & Lin, H. Ultrathin graphene layer activated dendritic α-Fe2O3 for high performance asymmetric supercapacitors. J. Alloys Compd. 780, 212–219 (2019).
Wang, J., Polleux, J., Lim, J. & Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 111, 14925–14931 (2007).
Guo, R., Dang, L., Liu, Z. & Lei, Z. Incorporation of electroactive NiCo2S4 and Fe2O3 into graphene aerogel for high-energy asymmetric supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 602, 125110 (2020).
Rani, M. U. et al. In-situ formation of mesoporous SnO2@C nanocomposite electrode for supercapacitors. Electrochim. Acta 365, 137284 (2021).
Nourani, N., Dashtian, K., Ghaedi, M., Shahbazi, S. & Hajati, S. Hierarchical Fe2O3/Na2WO4 nanofibers supported on conductive carbon cloth as a high-performance supercapacitor. Energy Fuels 35, 11551–11562 (2021).
Acknowledgements
The authors gratefully acknowledge the University of Guilan Research Council for financial support.
Author information
Authors and Affiliations
Contributions
M.S.: Investigation, Data curation, Writing - original draft. J.M.: Supervision, Methodology, Validation, Writing - review and editing. K.B. and A.M.: Methodology, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Safari, M., Mazloom, J., Boustani, K. et al. Hierarchical Fe2O3 hexagonal nanoplatelets anchored on SnO2 nanofibers for high-performance asymmetric supercapacitor device. Sci Rep 12, 14919 (2022). https://doi.org/10.1038/s41598-022-18840-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18840-2
This article is cited by
-
Photo-driven interfacial electron transfer reduction of cis-[Co(phen)2Cl2]Cl complexes with heterojunction CeO2/SnO2 nanocomposite and functional insights into supercapacitance
Ionics (2024)
-
Optimized electrospun MnFe2O4 nanofibers as promising electrode materials for supercapacitor applications: physical and electrochemical properties
Journal of Materials Science: Materials in Electronics (2024)
-
Enhanced electrochemical energy storage devices utilizing a one-dimensional (1D) α-MnO2 nanocomposite encased in onion-like carbon
Journal of Materials Science (2024)
-
Boosted electrochemical performance of magnetic caterpillar-like Mg0.5Ni0.5Fe2O4 nanospinels as a novel pseudocapacitive electrode material
Scientific Reports (2023)
-
Ni/Mn metal–organic framework decorated bacterial cellulose (Ni/Mn-MOF@BC) and nickel foam (Ni/Mn-MOF@NF) as a visible-light photocatalyst and supercapacitive electrode
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.