Abstract
Inonotus hispidus is a valuable and rare edible and medicinal mushroom with extremely high nutritional and medicinal value. However, there is no holistic insight to elucidate the molecular basis of the differentiated usage and accurate annotation of physiological maturity to fluctuating yields and quality. This study aimed to figure out the fruiting bodies' metabolites change regulation and potential maturating indicators to distinguish different quality I. hispidus. We applied non-targeted ultra-high performance liquid chromatography and high-resolution mass spectrometry combined and with multivariate analysis and analyzed cultivated and wild mushroom I. hispidus in different growth periods (budding, mature and aging). With the fruiting bodies maturating, 1358 metabolites were annotated, 822 and 833 metabolites abundances changed greater than or equal to 1 time from the budding period to the aging period in abundance in cultivated and wild, the total polysaccharides, crude fat, total flavonoids, and total terpenes increased at first and then decreased. Total amino acids, crude protein, and total polyphenols decreased, while the total steroids increased linearly. The change of metabolites showed certain regularity. Metabolic pathways enrichment analysis showed that these metabolites are involved in glycolysis, biosynthesis of amino acids, organic acid metabolism, glycine-serine-and-threonine metabolism, tricarboxylic acid cycle, purine metabolism, and pyrimidine metabolism. In addition, ergosterol peroxide and (22E)-ergosta-4,6,8(14),22-tetraen-3-one can be used as indicator compounds, and their contents increase linearly with the fruiting bodies of I. hispidus’ physiological maturation. This comprehensive analysis will help to evaluate the edible values and facilitate exploitation in mushroom I. hispidus.
Similar content being viewed by others
Introduction
The mushroom Inonotus hispidus (Bull.: Fr.) P. Karst. (I. hispidus) is an edible and medicinal mushroom, and the fruiting body is not only used as a food material but commonly soaked in water as a health drink in China1. It is a traditional Chinese medicine "Sanghuang" that was described in ancient Chinese Materia medica books in China, such as Shennong's Classic of Materia Medica and Compendium of Materia Medica. It is used in folk culture as an astringent, a diuretic, and for the treatment of mouth ulcers and inflammation2. Novel scientific investigations revealed anti-tumor3,4, improving human immunity5, anti-inflammatory6, antioxidant7, antiviral8, and hypolipidemic activity9, and it has great potential application value and development potential in the field of functional food and medicine. The appearance of the fruiting bodies of I. hispidus is golden in the budding period, which can be used as delicious food, but the yield is low. The fruiting bodies in the mature period appears yellowish-brown, while the fruiting body in the aging period contains a large amount of melanin10, which is grayish-black, and the yield is relatively high, but the harvest time is longer than the budding period. As to the fruiting bodies of I. hispidus in different growth periods, the pharmacological effects are also different.
At present, in the research and commercial production of functional food related to I. hispidus, there is a lack of research on the variation of metabolite abundance in different growth periods of I. hispidus. Because of the lack of measurable indicators that can characterize the physiological maturity of I. hispidus, which leads to the subjective evaluation of the physiological maturity and the fluctuation of the yield and quality of I. hispidus and the difference in production efficiency of different producers. The quality of I. hispidus can be understood in this way, the fruiting bodies with various metabolic components that can meet the industrial needs of pharmaceutical production are considered to be high-quality I. hispidus samples. For example, in industrial production, the main purpose is to obtain the total amino acid components in the fruiting bodies of I. hispidus, then the fruiting body with a relatively high content of total amino acids is considered to be of high quality. The high quality of I. hispidus fruiting bodies can’t be effectively selected in functional food, medical research and production practice, which has become one of the important factors restricting industrial production. Therefore, there is an urgent need for an indicator of fruiting bodies' maturation, to provide a basis for production and research.
Metabolomics is a new discipline that has developed rapidly following genomics, transcriptomics, and proteomics11,12,13. It aims to detect the overall trajectory of endogenous metabolites in organisms or cells under specific conditions to reflect the pathological and physiological processes of organisms, and some differential metabolites detected have become potential markers that characterize the pathological and physiological states of organisms14. Metabolomics has been widely used in many fields, such as animal and plant metabolism15,16, microbial metabolism17,18, disease diagnosis19,20,21, and drug development22. In recent years, metabolomics technology has been gradually applied to the field of edible and medicinal mushrooms to study metabolic profiling23,24,25. Those studies were carried out scientifically by the method of metabolomics, such as analyzing the metabolic profiling changes in mycelia collected at Pleurotus tuoliensis day 0, and day 35 under different temperatures (17 and 29 °C) by using GC–MS-based metabolomics26, and targeted metabolic profiling annotate changes in the lenticular edodes mycelial metabolome under high-temperature stress27.
So far, the metabolic profiling of different periods of I. hispidus mushroom has largely remained unexplored, and the grading has been confused by producers, traders, consumers, researchers, and related regulatory bodies. Almost all of them in the market were objective for discrimination of growth period based on phenotypic information and physical properties such as individual weight, tissue size, color, water content, etc. We hypothesized that the different qualities of I. hispidus differ in their metabolomic profiles, and they could be distinguished by their chemical constituents.
This study aimed to figure out the fruiting bodies' metabolites change regulation and potential maturating indicators to distinguish different quality I. hispidus. Through the potential indicator compounds of physiological maturity of it as a reference, to determine the best harvest time and guide the production practice, to maximize the yield and quality. We found that two metabolites were closely related to their physiological maturity, indicating that they have the potential to be used as markers to distinguish different quality grades. The result was helpful to evaluate I. hispidus fruiting body quality and facilitate exploitation in its consumption and processing.
Results
Principal component analysis of cultivated and wild Inonotus hispidus fruiting bodies in different growth periods
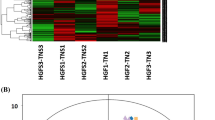
First, unsupervised Principal Component Analysis (PCA) was applied to assess the overall distribution among all samples and the stability of the entire analysis process. As shown in (Fig. 1B), all the quality control samples are clustered together, showing good analytical stability and experimental reproducibility. In our principal component analysis model, 6 groups of samples are well separated. Among them, the samples of wild I. hispidus in the budding period (WB), wild I. hispidus in the mature period (WM), artificial cultivated I. hispidus in the budding period (AB), and cultivated I. hispidus in the mature period (AM) are clustered together closely, while the samples of wild I. hispidus in aging period (WA) and cultivated I. hispidus in aging period (AA) deviate slightly. These results are consistent with our previous conclusion that there are great differences in the types of metabolites in different growth periods of the fruiting bodies of I. hispidus, and there are great differences in the abundance of metabolites between the fruiting bodies in the aging period and the other two periods. The wild and cultivated I. hispidus mushroom in different growth periods as shown in Fig. 1A.
The Inonotus hispidus samples and principal component analysis score chart. (A) The fruiting bodies of Inonotus hispidus in different growth periods. (B) Principal component analysis score chart. (C) Partial Least-Squares Discriminant Analysis (PLS-DA) score plots from metabolite profiles for fruiting bodies and a 200 times permutation test of PLS-DA models for it. Score plots from metabolite profiles for fruiting bodies and a 200 times permutation test of PLS-DA models for it. Note: AB, AM, and AA are samples of fruiting bodies of cultivated Inonotus hispidus in budding, mature and aging growth periods, respectively. WB, WM, and WA are samples of fruiting bodies of wild Inonotus hispidus in budding, mature and aging growth periods, respectively.
Partial least-squares discriminant analysis
To determine the budding, mature, and aging fruiting bodies of cultivated and wild I. hispidus, and the metabolic differences among 6 groups of samples, the supervised partial least square discriminant analysis model was used to further optimize the population separation of fruiting bodies. The comparison between paired AB, AM, and AA samples of Partial Least-Squares Discriminant Analysis (PLS-DA) fruiting bodies showed that there were significant differences in metabolism among different categories in each pairwise comparison of the first component (Fig. 1C). The partial least squares model has high R2Y and Q2 values, which indicated an excellent fit and satisfactory predictive power. A permutation test was used to evaluate the possible overfitting of the PLS-DA model. In this study, a 200-time permutation test was performed. R2 intercept of fruiting body AB and WB samples is 0.90 and R2 intercept of WM samples is 0.94. R2 intercept of AA and WA samples is 0.90, while Q2 intercept is − 0.72, − 0.70, and − 0.76, respectively. Figure 1C indicates that the PLS-DA model showed no overfitting and was credible. The annotation of differential metabolites between pairs of samples was performed using VIP values and confirmed by the non-parametric Mann–Whitney U test.
Comparative analysis of differential metabolites
With the fruiting bodies maturating, 1358 metabolites were annotated, 822 and 833 metabolites' abundances changed greater than or equal to 1 time from the budding period to the aging period in abundance in cultivated and wild (as shown in Supplementary Materials S1). Taking the expression of all metabolites annotated as data, the samples were compared by single-factor analysis of variance (one-way ANOVA). Corrected by the Benjamini–Hochberg method, the metabolites were screened for differential expression with P-value 0.05 as the threshold, and the metabolites were classified according to the expression between samples. The heat-map of different metabolites of cultivated and wild mushroom I. hispidus can be seen in (Fig. 2A,B). Vertical clustering is the clustering of samples, and horizontal clustering is the clustering of metabolites. The shorter the clustering branches are, the higher the similarity is. Through horizontal comparison, we can see the relationship of metabolite abundance clustering between groups. The results of the heat map showed that the abundance of metabolites varied greatly from the budding period to the mature period and then to the aging period, no matter whether in cultivated or wild ones. Because the abundance of metabolites is different in different growth periods, the pharmacological activity and value of I. hispidus are different, which shows that it is necessary to use scientific methods to accurately judge the best harvest time. In addition, the volcano plot of differential metabolites showed that there were great differences in metabolites between cultivated and wild mushroom I. hispidus in the same period. Volcano plot indicating upregulated and downregulated (Fig. 2C,D). In different growth periods (budding, mature and aging), the Venn diagram of the differential metabolites in the fruiting bodies of cultivated and wild I. hispidus showed the overlapping and differential parts of the metabolites. In this study, the Venn diagram analysis of the differential metabolites of the three comparative combinations is shown in Fig. 3 (Venn diagram in positive ion mode as shown in Supplementary Materials S2, and in negative ion mode as shown in Supplementary Materials S3).
Metabolite profiling of different growth periods of fruiting bodies of cultivated and wild Inonotus hispidus are compared at budding, mature and aging stages (the data used in heatmaps are Z-score of expressions). Note: AB, AM, and AA are samples of fruiting bodies of cultivated Inonotus hispidus in budding, mature and aging growth periods, respectively. WB, WM, and WA are samples of fruiting bodies of wild Inonotus hispidus in budding, mature and aging growth periods, respectively. Heat map of cultivated Inonotus hispidus in positive ion mode (A), negative ion mode (B). Each fruiting bodies sample is visualized in a single column and each metabolite is represented by a single row. blue colors indicate lower metabolite concentration, while red colors show enhanced metabolite levels. Volcano plot in positive ion mode (C), negative ion mode (D). Each point in the Volcano plot represents a metabolite, with significantly up-regulated metabolites represented by red dots and significantly down-regulated metabolites represented by green dots.
Venn diagram analysis of differential metabolites of cultivated and wild Inonotus hispidus in different growth periods comparative combinations. (A) Under the positive ion mode and (B) under the negative ion mode. Note: AB, AM, and AA are samples of fruiting bodies of cultivated Inonotus hispidus in budding, mature and aging growth periods, respectively. WB, WM, and WA are samples of fruiting bodies of wild Inonotus hispidus in budding, mature and aging growth periods, respectively.
Sugars, amino acids metabolites, and oligopeptides were annotated in Inonotus hispidus
In this study, 20 sugars,12 amino acids with different configurations (including 4 essential amino acids and 8 non-essential amino acids), and 24 oligopeptides were annotated in mushroom I. hispidus. With the physiological maturity of the fruiting body, most of the sugars and amino acid content of cultivated I. hispidus decreased. The abundance of arginine increased at first and then decreased. The abundance of proline decreased at first and then increased. However, with the physiological maturity of the fruiting body, the abundance of phenylalanine, proline, aspartic acid, and tyrosine increased at first and then decreased, and the abundance of other amino acids decreased in the wild I. hispidus. These changes are worth thinking about and studying, and the collection of these substances is not only the characteristic components of I. hispidus, but also the material basis to play a unique biological function. The detailed changes of amino acids and oligopeptides are shown in Table 1.
Sterols, phenols, flavonoids, and terpenoids metabolites were annotated in Inonotus hispidus
With the physiological maturity of the I. hispidus fruiting body, most steroids and phenols metabolites increased over the whole period. Most flavonoid metabolites increased at first and then decreased. Among sterols and their derivatives metabolites, the abundance of ergosta-5,7,9(11),22-tetraen-3-beta-ol increased at first and then decreased in cultivated I. hispidus. But in the wild I. hispidus increased with the fruiting body physiological maturity. However, the abundance of ergosterol peroxide of cultivated I. hispidus decreased in the whole period. The abundance of ouabain of wild I. hispidus increased at first and then decreased, but in wild samples, it decreased first and then increased. These changes will directly affect the quality of I. hispidus in different growth periods. Detailed changes about sterols, phenols, flavonoids, and terpenoids metabolites in mushroom I. hispidus are shown in Table 2.
Changes of total polysaccharides, total amino acids, crude protein, crude fat, total sterols, total polyphenols, total flavonoids, and total terpenes in fruiting bodies of Inonotus hispidus in different growth periods
With the physiological maturity of the I. hispidus fruiting body, the metabolites showed a regular change trend, and this changing trend occurred not only in cultivated I. hispidus, but also in wild. The results showed that the fruiting body of I. hispidus is in the process from the budding period to the mature period and then to the aging period, the abundance of total polysaccharides, crude fat, total flavonoids, and total terpenes increased at first and then decreased. Interestingly, total amino acids, crude protein, and total polyphenols decreased during the growth periods, while the total steroids increased linearly. The analysis of the content and change regular of the metabolites showed distinct changes in abundance in cultivated and wild. From an overall point of view, the changes of metabolites in cultivated were more stable and the content of metabolites higher than the wild ones. Therefore, we speculate that the cultivated I. hispidus may be more suitable for the function food or medicines. The 8 kinds of primary and secondary metabolites changed trend were shown in Fig. 4.
Metabolic pathway analysis
In order to further understand the differences in metabolic networks among samples, the annotated differential metabolites were submitted to the KEGG website for metabolic pathway enrichment analysis. The enrichment pathway of differential metabolites between cultivated and wild I. hispidus in the bud stage, mature stage, and aging stage (Fig. 5). It mainly includes eight main metabolic pathways, such as steroid biosynthesis, biosynthesis of amino acids, organic acid metabolism, glycine, serine and threonine metabolism, tricarboxylic acid cycle, glycolysis, purine metabolism, and pyrimidine metabolism, as well as some secondary metabolic pathways. Although artificial cultivation and wild I. hispidus have the same kinds of metabolites, the enrichment degree of metabolites is different in different physiological maturity stages.
KEGG enrichment bubble diagram of metabolites of cultivated and wild Inonotus hispidus in different growth periods. (A) AB vs. WB under the positive ion mode, (B) AB vs. WB under the negative ion mode, (C) AM vs. WM under the positive ion mode, (D) AM vs. WM under the negative ion mode, (E) AA vs. WA under the positive ion mode, (F) AA vs. WA under the negative ion mode. Note: AB, AM, and AA are samples of fruiting bodies of cultivated Inonotus hispidus in budding, mature and aging growth periods, respectively. WB, WM, and WA are samples of fruiting bodies of wild Inonotus hispidus in budding, mature and aging growth periods, respectively.
Ergosterol peroxide and (22E)-ergosta-4,6,8(14),22-tetraen-3-one content in Inonotus hispidus samples collected at different physiological maturity stages
Among the annotated metabolites, ergosterol peroxide attracted our attention because of the high fold-change difference in its abundance in different grow period samples. The abundance of ergosterol peroxide increased significantly, which prompted us to consider whether this metabolite can be used as an indicator of the physiological maturity of fruiting bodies. Therefore, the fruiting body samples were collected on days 10, 20, 30, 40, and 50, and the contents of ergosterol peroxide were determined by High Performance Liquid Chromatography (HPLC) according to the method reported in the literature. During the period of day 1 to day 20, the fruiting body of I. hispidus was golden in the budding period, mature on days 20 to day 50, yellowish-brown in the fruiting body, and gray-black in the aging period after day 50. During the whole process of growth and development, the metabolites with continuous decrease or increase in the abundance of metabolites may become an indicator of fruiting body maturity. It was found that the content of ergosterol peroxide was low in the budding period and could hardly be detected, but it was higher in the aged fruiting body. It is speculated that ergosterol peroxide can be used as a potential indicator to identify whether the fruiting body has reached physiological maturity. The content of ergosterol peroxide was 0.008% on days 10 of the fruiting body of cultivated I. hispidus, 0.024% on day 30 of maturity, 0.048% on day 50 of aging, and with the extension of the fruiting body growth period, the content of ergosterol peroxide increased and showed an upward trend (Fig. 6A).
In addition, the results of HPLC showed that the content of (22E)-ergosta-4,6,8(14),22-tetraen-3-one gradually increased with the physiological maturity of I. hispidus mushroom. The compound (22E)-ergosta-4,6,8(14),22-tetraen-3-one has strong fluorescence under 365 nm ultraviolet lamp, so it was easy to be identified and determined. The content was 0.001% at the budding period on day 10 of the cultivated fruiting body, 0.002% on day 30 of maturity, and only 0.003% on day 50 aging period. In this study, the contents of ergosterol peroxide, and (22E)-ergosta-4,6,8(14),22-tetraen-3-one in the fruiting bodies of wild and cultivated I. hispidus showed some regular and linear changes in the process of physiological maturation (Fig. 6B).
Discussion
This work investigated the fruiting bodies' metabolites change regulation and potential maturating indicators to distinguish different quality I. hispidus. The results showed that there were significant differences in the composition of metabolites in the fruiting bodies of I. hispidus in different growth periods, which would lead to different medicinal values. Therefore, different growth periods have a significant impact on the quality of it, and it is necessary to further study and clarify the differences. In our analysis, a total of 1358 metabolites were annotated from I. hispidus fruiting bodies. Based on the metabolic profile analysis of the KEGG database (https://www.genome.jp/kegg/pathway.html) and LIPIDMaps database (http://www.lipidmaps.org/), including 20 sugars, 12 amino acids, 24 oligopeptides, 23 steroids, 31 phenols, 12 flavones, 4 isoflavones, and 10 flavonoids and 11 terpenes were annotated. There are significant differences in metabolites in the process of physiological maturation of mushroom I. hispidus, which is mainly reflected in the change of abundance rather than in kinds.
Among the metabolites of I. hispidus, sugars, amino acids, oligopeptides, steroids, polyphenols, flavonoids, and terpenoids have been reported to have pharmacology activity. Therefore, these metabolites are mainly listed in this paper. Sugars are one of the effective components in I. hispidus, modern pharmacological studies have shown that I. hispidus sugars have protective effects on acute alcoholic liver injury in mice1. Amino acids as one of the important indicators of food nutritional value evaluation28,29, and have many pharmacological activities, such as L-leucine is used in the clinical treatment of liver disease30. Modern pharmacological studies show that serine plays a role in relieving chronic low-back and knee pain in adults31, leucine can by promoting insulin and Glucagon-Like Peptide 1 (GLP-1) secretion. With physiological maturity, the relative abundance of amino acids such as lysine, threonine, asparagine, serine and glutamic acid and histidine in the fruiting body decreased gradually. The oligopeptide is a biochemical substance between amino acid and protein, which is composed of 2 to 15 amino acids. In this study, it is found that the content of oligopeptides is abundant, especially with the physiological maturity of the fruiting body, the abundance of Glu-Val-Phe increases significantly. On the contrary, the abundance of Tyr-Tyr decreases significantly with the physiological maturity of the fruiting body. Steroids and phenols in I. hispidus are important active components, which have antioxidants2, anti-inflammatory6, and bacteriostatic activities6. In recent years, flavonoids and terpenoids as two important kinds of metabolites in I. hispidus have been widely concerned and studied2. Modern pharmacological studies have shown that flavonoids have antioxidant potential and antimicrobial activity32. Some terpenoids have the effects of antioxidation, liver protection, immunity enhancement, anti-tumor, and so on. Such as betulin, which has anti-inflammatory33, antiviral34, and other activities35. With physiological maturity, the relative abundance of betulin in the fruiting body decreased gradually. These findings suggest that the abundance of metabolites showed some regular changes with the fruiting bodies maturating of I. hispidus. When using these fruiting bodies as raw materials for functional foods and drugs, special attention should be paid to the changes of the abundance of metabolites in the fruiting bodies of I. hispidus.
From the perspective of primary metabolites and secondary metabolites, the changes of eight main metabolites of wild and cultivated I. hispidus mushroom during different growth stages were compared and analyzed. The result showed that the abundance of total polysaccharides, crude fat, total flavonoids, and total terpenes increased at first and then decreased. Interestingly, total amino acids, crude protein, and total polyphenols decreased during the growth periods, while the total steroids increased linearly. In this study, it is worth noting that the content of 8 kinds of primary and secondary metabolites of cultivated I. hispidus mushroom is significantly higher than that of wild, which may be related to the fact that water, temperature, nutrients, and other factors in the artificial cultivation environment are more suitable for the growth of it. Compared with the growth environment conditions of wild I. hispidus, cultivated has more stable growth environment conditions, and is more stable and unified in soil nutritional status, temperature, humidity, precipitation, and so on. These may be some important reasons for the high quality of cultivated. Metabolite profiles are highly affected by environmental conditions, but it needs to be explained by further research. On the one hand, the changes of metabolites in cultivated were more stable and the content of metabolites higher than the wild ones, which is more suitable for the function of food and medicine. On the other hand, cultivated I. hispidus is easier to control the growth environment conditions, standardized production, and easy to obtain high yield. These results suggested that cultivated I. hispidus may be more suitable for the needs of industrial products. From the perspective of primary metabolites and secondary metabolites, the relative contents of total amino acids and total polysaccharides decrease gradually with the physiological maturity of fruiting bodies, if the sugars or amino acids in I. hispidus are used in industry, then the maximum benefit may be obtained in the bud stage, of course, the yield needs to be considered. If steroids are used in industry, and the content of steroids increases with the physiological maturity of the I. hispidus fruiting bodies, then the aging I. hispidus fruiting bodies may be more suitable. This study showed that there were significant differences in the relative abundance of primary and secondary metabolites in the fruiting bodies of I. hispidus in different growth periods. This study provides a scientific basis for selecting the best I. hispidus fruiting bodies in the growing period according to the demand.
In addition, a lack of measurable indicators that can characterize the physiological maturity of I. hispidus, leads to the subjective evaluation of the physiological maturity and the fluctuation of the yield and quality of I. hispidus and the difference of production efficiency of different producers. Therefore, there is an urgent need for an indicator of fruiting body maturation, to provide a basis for production and research. In our study, the content of ergosterol peroxide and (22E)-ergosta-4,6,8(14),22-tetraen-3-one in fruiting bodies showed a regular change during physiological maturation. Almost no ergosterol peroxide and (22E)-ergosta-4,6,8(14),22-tetraen-3-one were detected in fruiting bodies collected on day 0 of the wild and cultivated I. hispidus, but its content was extremely high in physiologically mature fruiting bodies. We deduced that ergosterol peroxide and (22E)-ergosta-4,6,8(14),22-tetraen-3-one may be used as potential indicators to identify whether fruiting bodies have reached physiological maturity. However, the utility of ergosterol peroxide and (22E)-ergosta-4,6,8(14),22-tetraen-3-one content as an indicator of the physiological maturation of mycelia was not determined, and this question merits additional fruiting experiments.
In summary, this study showed that with the fruiting bodies maturating, the abundance of metabolites showed some regular changes. The cultivated and wild I. hispidus mushroom has the same 1358 metabolites, but the metabolites showed distinct changes in abundance in cultivated and wild. It is worth noting that mass spectrometry can’t detect all the metabolites, not because the mass spectrometry is not sensitive enough, but because mass spectrometry can only detect ionized substances, but some metabolites can’t be ionized in the mass spectrometer. Nuclear magnetic resonance (NMR) is needed to make up for the lack of chromatography in the future, and further research is needed36,37,38. Through the metabonomic and natural product research combined, the content of ergosterol peroxide and (22E)-ergosta-4,6,8(14),22-tetraen-3-one showed a linear trend increase, which indicated the feasibility of their use as an indicator for fruiting body maturation, but whether this indicator can be applied for the commercial production of mushroom I. hispidus requires the verification of large-scale fruiting experiences. The metabolites of I. hispidus fruiting bodies were different in different growth stages, so the quality was different. However, the maturity of the fruiting body can be determined by peroxidation of ergosterol and (22E)-ergosta-4,6,8(14),22-tetraen-3-one, and the quality of the fruiting body can be screened indirectly. Our research provided an atheoretical basis for quality evaluation and comprehensive utilization of the mushroom I. hispidus from cultivated and wild.
Materials and methods
Chemicals
All chemicals used in this study were of chromatographic grade for liquid chromatography. Acetonitrile, methanol, and formic acid were purchased from Merck (Darmstadt, Germany). Acetic acid and methyl alcohol were from Tedia (Tedia Co., Ohio, USA). Deionized water was purified by a Milli-Q water purification system (Millipore, Billerica, MA, USA).
Samples
Wild I. hispidus mushrooms were collected from the ancient mulberry protection forest in Xiajin County, Dezhou City, Shandong Province, China (36°59′ N, 115°11′ E). Samples were randomly collected and immediately frozen in liquid nitrogen. The cultivated I. hispidus mushroom was collected from Xiajin Sanghuang Biotechnology Co., Ltd. 6 replicate samples (sampling position: middle part) were collected at each time point and frozen in liquid nitrogen immediately. All samples are stored at − 80 °C until metabolomics analysis. The specimens (No. HMJAU58767) are deposited in the Key Laboratory of Medicinal Fungal Resources and Development and Utilization, Jilin agricultural university.
Metabolites extraction
Crushed tissues (100 mg) were placed in the Eppendorf tube (EP tube) and resuspended with prechilled 80% methanol and 0.1% formic acid. After the vortex shock, the samples were incubated on ice for 5 min and then were centrifuged at 15,000×g, 4 °C for 20 min. To reduce the matrix effect, better detect metabolites and optimize the peak shape, 300 μL supernatant and 150 μL LC–MS grade water. were diluted to 53% methanol content. The samples were subsequently transferred to a fresh Eppendorf tube and then were centrifuged at 15,000×g, 4 °C for 20 min. Finally, the supernatant was injected into the UHPLC-MS/MS system analysis39.
UHPLC-MS/MS analysis
UHPLC-MS/MS analysis refers to previously methods40. UHPLC-MS/MS analyses were performed using a Vanquish UHPLC system (Thermo Fisher, Germany) coupled with an Orbitrap Q Exactive HF mass spectrometer (Thermo Fisher, Germany). Samples were injected onto a Hypesil Gold column (100 × 2.1 mm, 1.9 μm) using a 17 min linear gradient at a flow rate of 0.2 mL/min. The eluents for the positive polarity mode were eluent A (0.1% FA in Water) and eluent B (Methanol). The eluents for the negative polarity mode were eluent A (5 mM ammonium acetate, pH 9.0) and eluent B (Methanol). The solvent gradient was set as follows: 2% B, 1.5 min; 2–100% B, 12.0 min; 100% B, 14.0 min; 100–2% B, 14.1 min; 2% B, 17 min. Q Exactive HF mass spectrometer was operated in positive/negative polarity mode with a spray voltage of 3.2 kV, capillary temperature of 320 °C, sheath gas flow rate of 40 arb, and aux gas flow rate of 10 arb.
Total polysaccharides, total amino acids, crude proteins, total sterols, total polyphenols, total flavonoids, terpenes, and crude fat were determined in fruiting bodies of Inonotus hispidus during different growth periods
Through the analysis of the metabolic profiling of the fruiting bodies of wild and cultivated I. hispidus in different growth periods, the complex chemical composition and its change trend line were shown, but the practical application may involve a macroscopic overall quantitative analysis. Therefore, the contents of total polysaccharides, total amino acids, crude protein, total steroids, polyphenols, total flavonoids, terpenoids, and crude fat in wild and cultivated I. hispidus fruiting bodies were analyzed in this study.
Data processing and metabolite annotation
Data processing and metabolite annotation refer to previously methods23. The raw data files generated by UHPLC-MS/MS were processed using the Compound Discoverer 3.1 (CD3.1, Thermo Fisher, USA) to perform peak alignment, peak picking, and quantitation for each metabolite. The main parameters were set as follows: retention time tolerance, 0.2 min; actual mass tolerance, 5 ppm; signal intensity tolerance, 30%; signal/noise ratio, 3; and minimum intensity, 100,000. After that, peak intensities were normalized to the total spectral intensity. According to the formal definition of metabolites annotation and annotation developed by the Chemical Analysis Working Group of the Metabolomics Standards Initiative (MSI) working Group on Chemical Analysis41, this study annotates metabolites detected by non-targeted metabonomic scans. The normalized data were used to predict the molecular formula based on additive ions, molecular ion peaks, and fragment ions. And then peaks were matched with the mzCloud (https://www.mzcloud.org/), mzVault, and MassList database to obtain the accurate qualitative and relative quantitative results. Statistical analyses were performed using the statistical software R (R version R-3.4.3), Python (Python 2.7.6 version), and CentOS (CentOS release 6.6), When data were not normally distributed, normal transformations were attempted using of area normalization method.
Metabonomic data analysis
Data were normalized for internal consistency by processing the constant weight of the solvent per volume of each sample. The internal consistency of the data is normalized data were scaled to the median value for each compound, then missing values were imputed with the minimum detected value for that compound. Moreover, a variety of curation procedures were carried out to ensure that a high-quality data set was made available for statistical analysis and data interpretation. The QC and curation processes were designed to ensure accurate and consistent annotation of true chemical entities and to remove those representing system artifacts, misassignments, and background noise. Library matches for each compound were checked for each sample and corrected if necessary. Metabonomic data analysis refers to previously methods24,26,42,43,44. These metabolites were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.genome.jp/kegg/pathway.html), Human Metabolome Database (HMDB) (https://hmdb.ca/metabolites), and LIPIDMaps database (http://www.lipidmaps.org/).
The data analysis of PCA and PLS-DA were performed using software python (Python-3.5.0), and the charts were drawn by software R (R-3.4.3). We applied univariate analysis (t-test) to calculate the statistical significance (P-value). The metabolites with VIP > 1 and P-value < 0.05 and fold change ≥ 2 or FC ≤ 0.5 were considered to be differential metabolites. Volcano plots were used to filter metabolites of interest-based on log2(FoldChange) and -log10(p-value) of metabolites.
For clustering heat maps, the data were normalized using z-scores of the intensity areas of differential metabolites and were plotted by the Pheatmap package in R language. A statistically significant correlation between differential metabolites was calculated by R language. P-value < 0.05 was considered as statistically significant and correlation plots were plotted by corrplot package in R language. The metabolic pathways enrichment of differential metabolites was performed, when the ratio was satisfied by x/n > y/N, metabolic pathways were considered as enrichment, when the P-value of metabolic pathway < 0.05, metabolic pathways were considered as statistically significant enrichment. For line charts and tables, the data were normalized using Microsoft Office Excel 2019 software, and the chemical structure formula was made by ChemDraw 15 software.
References
Liu, X. et al. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 129, 41–49 (2019).
Yousfi, M. et al. Isolation and characterization of a new hispolone derivative from antioxidant extracts of Pistacia atlantica. Phytother. Res. 23, 1237–1242 (2009).
Wang, T., Bao, H. Y., Bau, T. & Li, Y. Antitumor effect of solid state fermentation powder of Inonotus hispidus on H22 bearing mice. Zhong Yao Cai 39, 389–394 (2016).
Yang, S., Bao, H., Wang, H. & Li, Q. Anti-tumour effect and pharmacokinetics of an active ingredient isolated from Inonotus hispidus. Biol. Pharm. Bull. 42, 10–17 (2019).
Grundemann, C. et al. Effects of Inonotus hispidus extracts and compounds on human immunocompetent cells. Planta Med. 82, 1359–1367 (2016).
Kou, R. W. et al. Phenolic and steroidal metabolites from the cultivated edible Inonotus hispidus mushroom and their bioactivities. J. Agric. Food Chem. 69, 668–675 (2021).
Zan, L. F. et al. Antioxidant hispidin derivatives from medicinal mushroom Inonotus hispidus. Chem. Pharm. Bull. (Tokyo) 59, 770–772 (2011).
Awadh Ali, N. A., Mothana, R. A., Lesnau, A., Pilgrim, H. & Lindequist, U. Antiviral activity of Inonotus hispidus. Fitoterapia 74, 483–485 (2003).
Benarous, K. et al. Harmaline and hispidin from Peganum harmala and Inonotus hispidus with binding affinity to Candida rugosa lipase: In silico and in vitro studies. Bioorg. Chem. 62, 1–7 (2015).
Hou, R. et al. Characterization of the physicochemical properties and extraction optimization of natural melanin from Inonotus hispidus mushroom. Food Chem. 277, 533–542 (2019).
Trivedi, D. K., Hollywood, K. A. & Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. New Horiz. Transl. Med. 3, 294–305 (2017).
Tripp, B. A. et al. Targeted metabolomics analysis of postoperative delirium. Sci. Rep. 11, 1521 (2021).
Harville, E. W. et al. Untargeted analysis of first trimester serum to reveal biomarkers of pregnancy complications: A case-control discovery phase study. Sci. Rep. 11, 3468 (2021).
Kaushik, A. K. & DeBerardinis, R. J. Applications of metabolomics to study cancer metabolism. Biochim. Biophys. Acta Rev. Cancer 1870, 2–14 (2018).
He, C. et al. Characterization of lipid profiling in three parts (muscle, head and viscera) of tilapia (Oreochromis niloticus) using lipidomics with UPLC-ESI-Q-TOF-MS. Food Chem. 347, 129057 (2021).
Lorini, A. et al. Metabolic profile of olive leaves of different cultivars and collection times. Food Chem. 345, 128758 (2021).
Li, S. N., Tang, S. H., Ren, R., Gong, J. X. & Chen, Y. M. Metabolomic profile of milk fermented with Streptococcus thermophilus cocultured with Bifidobacterium animalis ssp. lactis, Lactiplantibacillus plantarum, or both during storage. J. Dairy Sci. 104, 8493–8505 (2021).
Jang, Y. O. et al. High-fiber diets attenuate emphysema development via modulation of gut microbiota and metabolism. Sci. Rep. 11, 7008 (2021).
Rachieriu, C. et al. Lipidomic signatures for colorectal cancer diagnosis and progression using UPLC-QTOF-ESI(+)MS. Biomolecules 11, 417 (2021).
Rhee, S. Y. et al. Plasma amino acids and oxylipins as potential multi-biomarkers for predicting diabetic macular edema. Sci. Rep. 11, 9727 (2021).
Zimmermann, M. et al. Metabolomics-based analysis of miniature flask contents identifies tobacco mixture use among the ancient Maya. Sci. Rep. 11, 1590 (2021).
Zhu, H. et al. The dual roles of ginsenosides in improving the anti-tumor efficiency of cyclophosphamide in mammary carcinoma mice. J. Ethnopharmacol. 265, 113271 (2021).
Luo, F. et al. Metabolomic differential analysis of interspecific interactions among white rot fungi Trametes versicolor, Dichomitus squalens and Pleurotus ostreatus. Sci. Rep. 7, 5265 (2017).
Tang, L. et al. Untargeted metabolite profiling of antimicrobial compounds in the brown film of Lentinula edodes mycelium via LC-MS/MS analysis. ACS Omega 5, 7567–7575 (2020).
Gao, Z. et al. The characteristic, antioxidative and multiple organ protective of acidic-extractable mycelium polysaccharides by Pleurotus eryngii var. tuoliensis on high-fat emulsion induced-hypertriglyceridemic mice. Sci. Rep. 8, 17500 (2018).
Du, F., Zou, Y., Hu, Q., Jing, Y. & Yang, X. Metabolic profiling of pleurotus tuoliensis during mycelium physiological maturation and exploration on a potential indicator of mycelial maturation. Front. Microbiol. 9, 3274 (2018).
Zhao, X. et al. GC(-)MS-based nontargeted and targeted metabolic profiling identifies changes in the lentinula edodes mycelial metabolome under high-temperature stress. Int. J. Mol. Sci. 20, 2330 (2019).
Dala-Paula, B. M., Deus, V. L., Tavano, O. L. & Gloria, M. B. A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem. 343, 128397 (2021).
Zhao, L. et al. Simultaneous determination of 49 amino acids, B vitamins, flavonoids, and phenolic acids in commonly consumed vegetables by ultra-performance liquid chromatography-tandem mass spectrometry. Food Chem. 344, 128712 (2021).
Dos Santos, A. L. S. & Anastacio, L. R. The impact of L-branched-chain amino acids and L-leucine on malnutrition, sarcopenia, and other outcomes in patients with chronic liver disease. Expert Rev. Gastroenterol. Hepatol. 15, 181–194 (2021).
Sasahara, I. et al. L-serine and EPA relieve chronic low-back and knee pain in adults: A randomized, double-blind, placebo-controlled trial. J. Nutr. 150, 2278–2286 (2020).
Cascaes, M. M. et al. Flavonoids, antioxidant potential and antimicrobial activity of Myrcia rufipila mcvaugh leaves (myrtaceae). Nat. Prod. Res. 35, 1717–1721 (2021).
Qi, Z., Guo, Y., Zhang, H., Yu, Q. & Zhang, P. Betulin attenuates pneumolysin-induced cell injury and DNA damage. J. Appl. Microbiol. 130, 843–851 (2021).
Heidary Navid, M., Laszczyk-Lauer, M. N., Reichling, J. & Schnitzler, P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 21, 1273–1280 (2014).
Tang, J. J. et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 13, 44–56 (2011).
Gnocchi, D. et al. (1)H-NMR metabolomics reveals a multitarget action of Crithmum maritimum ethyl acetate extract in inhibiting hepatocellular carcinoma cell growth. Sci. Rep. 11, 1259 (2021).
Comella-Del-Barrio, P. et al. Urine NMR-based TB metabolic fingerprinting for the diagnosis of TB in children. Sci. Rep. 11, 12006 (2021).
O’Shea-Stone, G. et al. (1)H NMR based metabolic profiling distinguishes the differential impact of capture techniques on wild bighorn sheep. Sci. Rep. 11, 11308 (2021).
Want, E. J. et al. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 8, 17–32 (2013).
Park, Y. J. et al. Spatial (cap & stipe) metabolomic variations affect functional components between brown and white beech mushrooms. Food Res. Int. 102, 544–552 (2017).
Sumner, L. W. et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 3, 211–221 (2007).
Kanehisa, M. et al. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 49, D545–D551 (2021).
Acknowledgements
The authors are grateful for the Special Fund for the National Natural Science Foundation of China (No. 32070021).
Author information
Authors and Affiliations
Contributions
H.B. and Z.L. designed the study. Z.L., conducted the experiments. Z.L., C.H., and M.S. analyzed the data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Z., Bao, H., Han, C. et al. The regular pattern of metabolite changes in mushroom Inonotus hispidus in different growth periods and exploration of their indicator compounds. Sci Rep 12, 14354 (2022). https://doi.org/10.1038/s41598-022-18631-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18631-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.